Structure and Characterizations of Different Hydrophobically Modified Phytoglycogen Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Phytoglycogen
2.3. Alkenyl Succinic Anhydride Modification
2.4. Determination of DS of Modified PG
2.5. FT-IR
2.6. Zeta-Potential and Particle Size Distribution
2.7. Turbidity Analysis
2.8. Water Solubility of Modified PG
2.9. Molecular Weight Distribution Profiles
2.10. Flow Behavior Analysis
2.11. In Vitro Digestion
2.12. Thermogravimetric Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Modified PG
3.1.1. FT-IR
3.1.2. The DS of Modified PG
3.2. Structure of Modified PG
3.2.1. Molecular Weight Distribution Profiles of PG and Modified PG
3.2.2. Solution Properties of PG and Modified PG
3.3. Physiochemical Properties of Modified PG
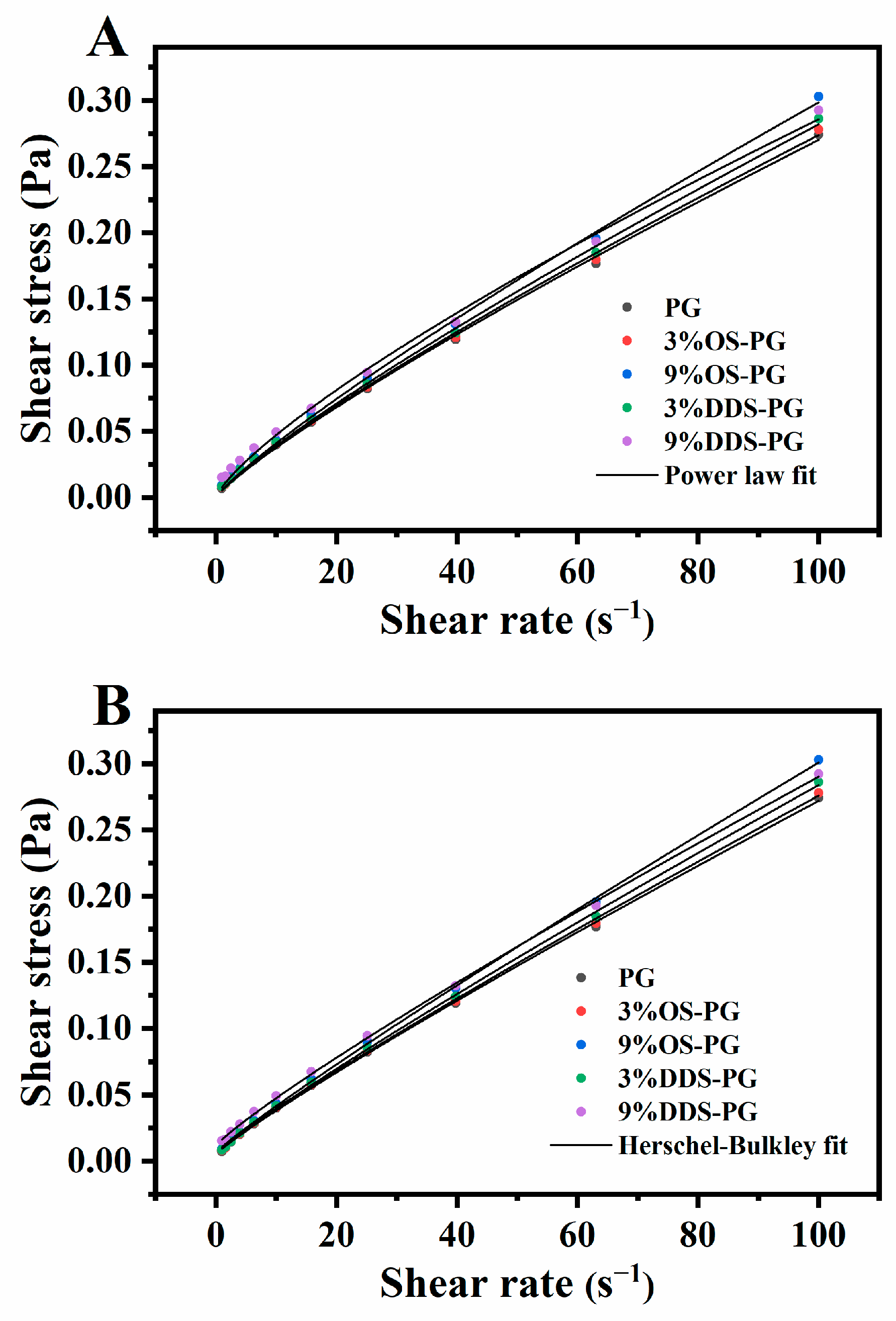
3.3.1. Rheological Behavior of PG and Modified PG
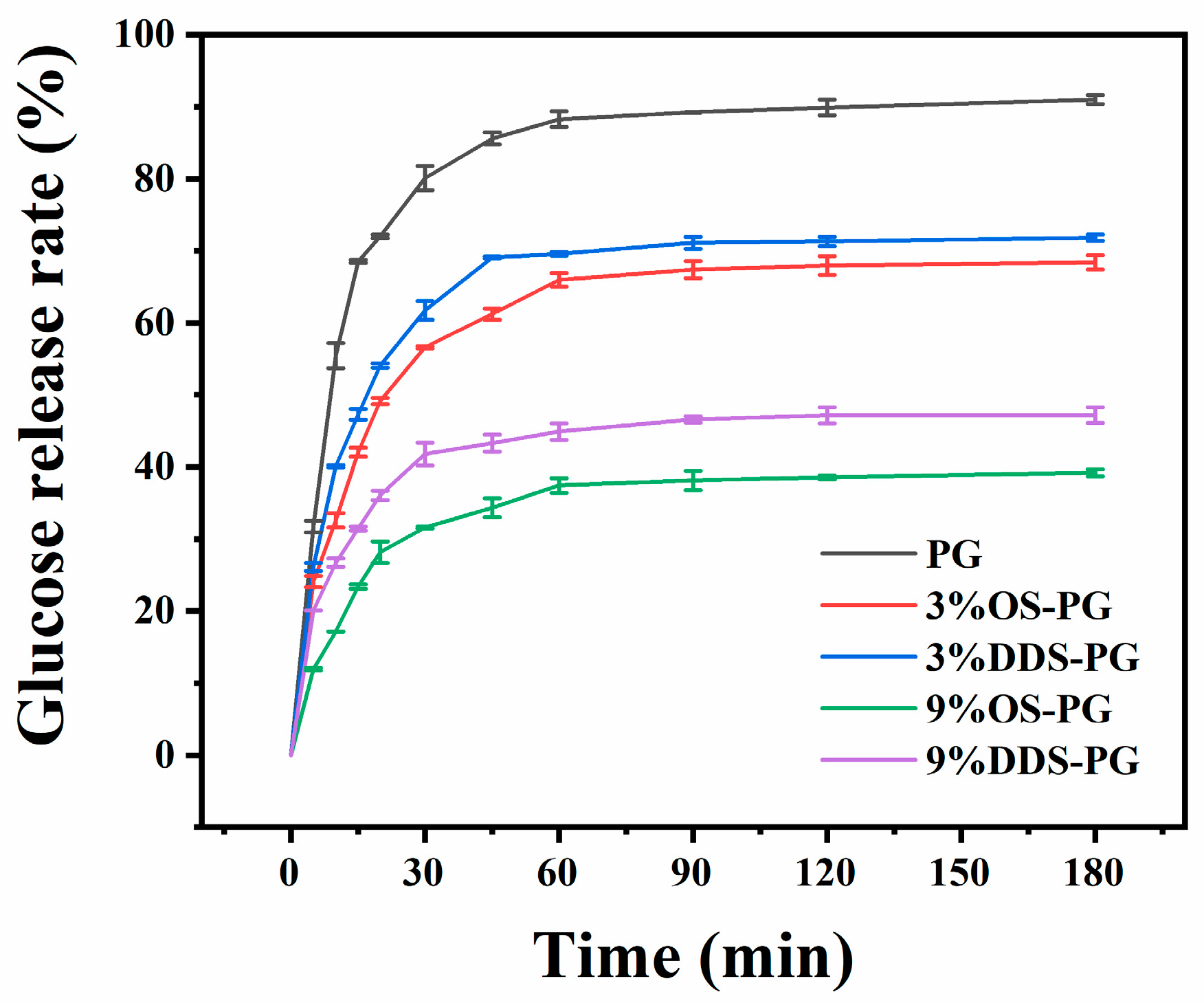
3.3.2. In Vitro Digestion of PG and Modified PG
3.3.3. Thermal Properties of PG and Modified PG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, F.; Miao, M.; Lu, K.; Jiang, B.; Li, X.; Cui, S.W. Structure and Physicochemical Properties for Modified Starch-Based Nanoparticle from Different Maize Varieties. Food Hydrocoll. 2017, 67, 37–44. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, N.; McClements, D.J.; Hamaker, B.R.; Miao, M. Advanced Dendritic Glucan-derived Biomaterials: From Molecular Structure to Versatile Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4107–4146. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Y. Particulate Structure of Phytoglycogen Nanoparticles Probed Using Amyloglucosidase. Carbohydr. Polym. 2011, 83, 1665–1671. [Google Scholar] [CrossRef]
- Xiang, L.; Lu, S.; Quek, S.Y.; Liu, Z.; Wang, L.; Zheng, M.; Tang, W.; Yang, Y. Exploring the Effect of OSA-Esterified Waxy Corn Starch on Naringin Solubility and the Interactions in Their Self-Assembled Aggregates. Food Chem. 2021, 342, 128226. [Google Scholar] [CrossRef]
- Putaux, J.-L.; Potocki-Ve, G.; Buleon, A. R-D-Glucan-Based Dendritic Nanoparticles Prepared by in Vitro Enzymatic Chain Extension of Glycogen. Biomacromolecules 2006, 7, 1720–1728. [Google Scholar] [CrossRef]
- Izawa, H.; Nawaji, M.; Kaneko, Y.; Kadokawa, J. Preparation of Glycogen-Based Polysaccharide Materials by Phosphorylase-Catalyzed Chain Elongation of Glycogen. Macromol. Biosci. 2009, 9, 1098–1104. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and Physicochemical Properties of Octenyl Succinic Anhydride Modified Starches: A Review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and Characterization of Octenyl Succinic Anhydride Modified Starches for Food Applications. A Review of Recent Literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Han, J.-A.; BeMiller, J.N. Preparation and Physical Characteristics of Slowly Digesting Modified Food Starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Liang, S.; Du, J.; Hong, Y.; Cheng, L.; Gu, Z.; Li, Z.; Li, C. Octenyl Succinate Anhydride Debranched Starch-Based Nanocarriers for Curcumin with Improved Stability and Antioxidant Activity. Food Hydrocoll. 2023, 135, 108118. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Hemar, Y.; Yang, Z.; Singh, H. Self-Assembled Micelles Based on OSA-Modified Starches for Enhancing Solubility of β-Carotene: Effect of Starch Macromolecular Architecture. J. Agric. Food Chem. 2019, 67, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, S.; Ratcliffe, I.; Williams, P.A. The Interfacial, Emulsification and Encapsulation Properties of Hydrophobically Modified Inulin. Carbohydr. Polym. 2018, 194, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. A Functional Pickering Emulsion Coating Based on Octadecenylsuccinic Anhydride Modified γ-Cyclodextrin Metal-Organic Frameworks for Food Preservation. Food Hydrocoll. 2024, 150, 109668. [Google Scholar] [CrossRef]
- Xue, J.; Luo, Y. Properties and Applications of Natural Dendritic Nanostructures: Phytoglycogen and Its Derivatives. Trends Food Sci. Technol. 2021, 107, 432–444. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, Y. Octenylsuccinate Hydroxypropyl Phytoglycogen Enhances the Solubility and In-Vitro Antitumor Efficacy of Niclosamide. Int. J. Pharm. 2018, 535, 157–163. [Google Scholar] [CrossRef]
- Scheffler, S.L.; Wang, X.; Huang, L.; San-Martin Gonzalez, F.; Yao, Y. Phytoglycogen Octenyl Succinate, an Amphiphilic Carbohydrate Nanoparticle, and ε-Polylysine to Improve Lipid Oxidative Stability of Emulsions. J. Agric. Food Chem. 2010, 58, 660–667. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Huang, C.; Ye, F.; Jiang, B.; Zhang, T. Structural Modification and Characterisation of a Sugary Maize Soluble Starch Particle after Double Enzyme Treatment. Carbohydr. Polym. 2015, 122, 101–107. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of Ferulic Acid in High Internal Phase Pickering Emulsions Stabilized Using Nonenyl Succinic Anhydride (NSA) and Octenyl Succinic Anhydride (OSA) Modified Quinoa and Maize Starch Nanoparticles. Food Chem. 2023, 429, 136748. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Liu, Z.; Luo, S.; Liu, C.; Hu, X. Preparation and Characterization of Octenyl Succinate β-Limit Dextrin. Carbohydr. Polym. 2020, 229, 115527. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, K.; Hu, X.; Jin, Z.; Miao, M. Structure, Properties and Potential Applications of Phytoglycogen and Waxy Starch Subjected to Carboxymethylation. Carbohydr. Polym. 2020, 234, 115908. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- Zhang, B.; Mei, J.-Q.; Chen, B.; Chen, H.-Q. Digestibility, Physicochemical and Structural Properties of Octenyl Succinic Anhydride-Modified Cassava Starches with Different Degree of Substitution. Food Chem. 2017, 229, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Morphological, Structural and Digestibility Properties of RS4 Enriched Octenyl Succinylated Sweet Potato, Banana and Lentil Starches. Food Hydrocoll. 2018, 82, 219–229. [Google Scholar] [CrossRef]
- Shah, N.N. Modification of Proteins and Polysaccharides Using Dodecenyl Succinic Anhydride: Synthesis, Properties and Applications—A Review. Int. J. Biol. Macromol. 2018, 107, 2224–2233. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Synthesis of Dodecenyl Succinic Anhydride (DDSA) Corn Starch. Food Res. Int. 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Interactions between Octenyl-Succinic-Anhydride-Modified Starches and Calcium in Oil-in-Water Emulsions. Food Hydrocoll. 2018, 77, 30–39. [Google Scholar] [CrossRef]
- Torres, L.G.; Iturbe, R.; Snowden, M.J.; Chowdhry, B.Z.; Leharne, S.A. Preparation of o/w Emulsions Stabilized by Solid Particles and Their Characterization by Oscillatory Rheology. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 439–448. [Google Scholar] [CrossRef]
- Shamana, H.; Grossutti, M.; Papp-Szabo, E.; Miki, C.; Dutcher, J.R. Unusual Polysaccharide Rheology of Aqueous Dispersions of Soft Phytoglycogen Nanoparticles. Soft Matter 2018, 14, 6496–6505. [Google Scholar] [CrossRef]
- Xue, J.; Li, Z.; Duan, H.; He, J.; Luo, Y. Chemically Modified Phytoglycogen: Physicochemical Characterizations and Applications to Encapsulate Curcumin. Colloids Surf. B Biointerfaces 2021, 205, 111829. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Rheological Properties in Relation to Molecular Structure of Quinoa Starch. Int. J. Biol. Macromol. 2018, 114, 767–775. [Google Scholar] [CrossRef]
- Midmore, B.R. Effect of Aqueous Phase Composition on the Properties of a Silica-Stabilized W/O Emulsion. J. Colloid Interface Sci. 1999, 213, 352–359. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, J.; Zhang, G. Slowly Digestible Waxy Maize Starch Prepared by Octenyl Succinic Anhydride Esterification and Heat−Moisture Treatment: Glycemic Response and Mechanism. Biomacromolecules 2008, 9, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Venkateshaiah, A.; Havlíček, K.; Timmins, R.L.; Röhrl, M.; Wacławek, S.; Nguyen, N.H.A.; Černík, M.; Padil, V.V.T.; Agarwal, S. Alkenyl Succinic Anhydride Modified Tree-Gum Kondagogu: A Bio-Based Material with Potential for Food Packaging. Carbohydr. Polym. 2021, 266, 118126. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, Q.; Zhang, Y.; Weng, H.; Wang, S.; Chen, F.; Xiao, A. A Comparative Study on the Gel Transition, Structural Changes, and Emulsifying Properties of Anhydride-Esterified Agar with Varied Degrees of Substitution and Carbon Chain Lengths. Food Hydrocoll. 2023, 141, 108690. [Google Scholar] [CrossRef]
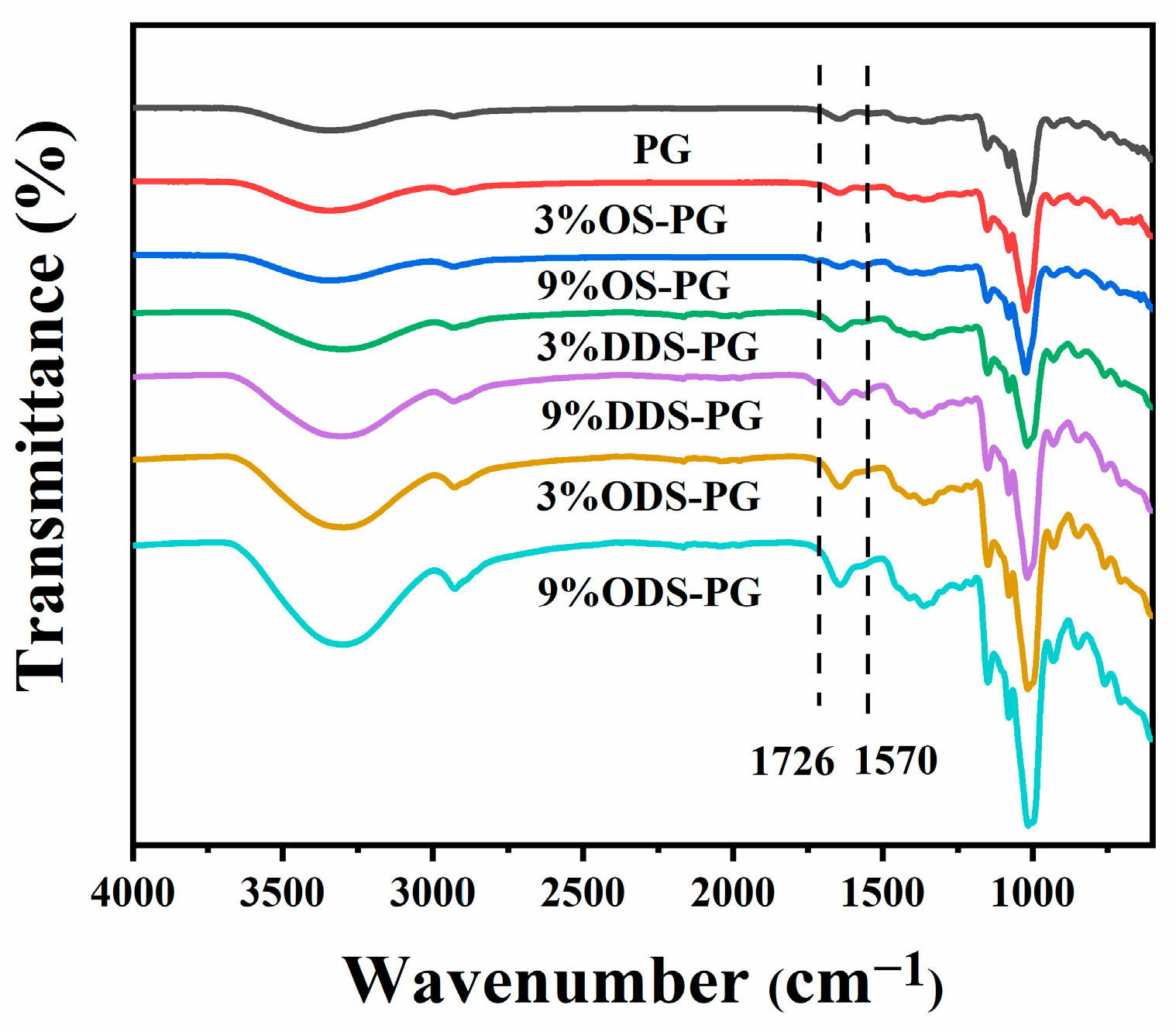

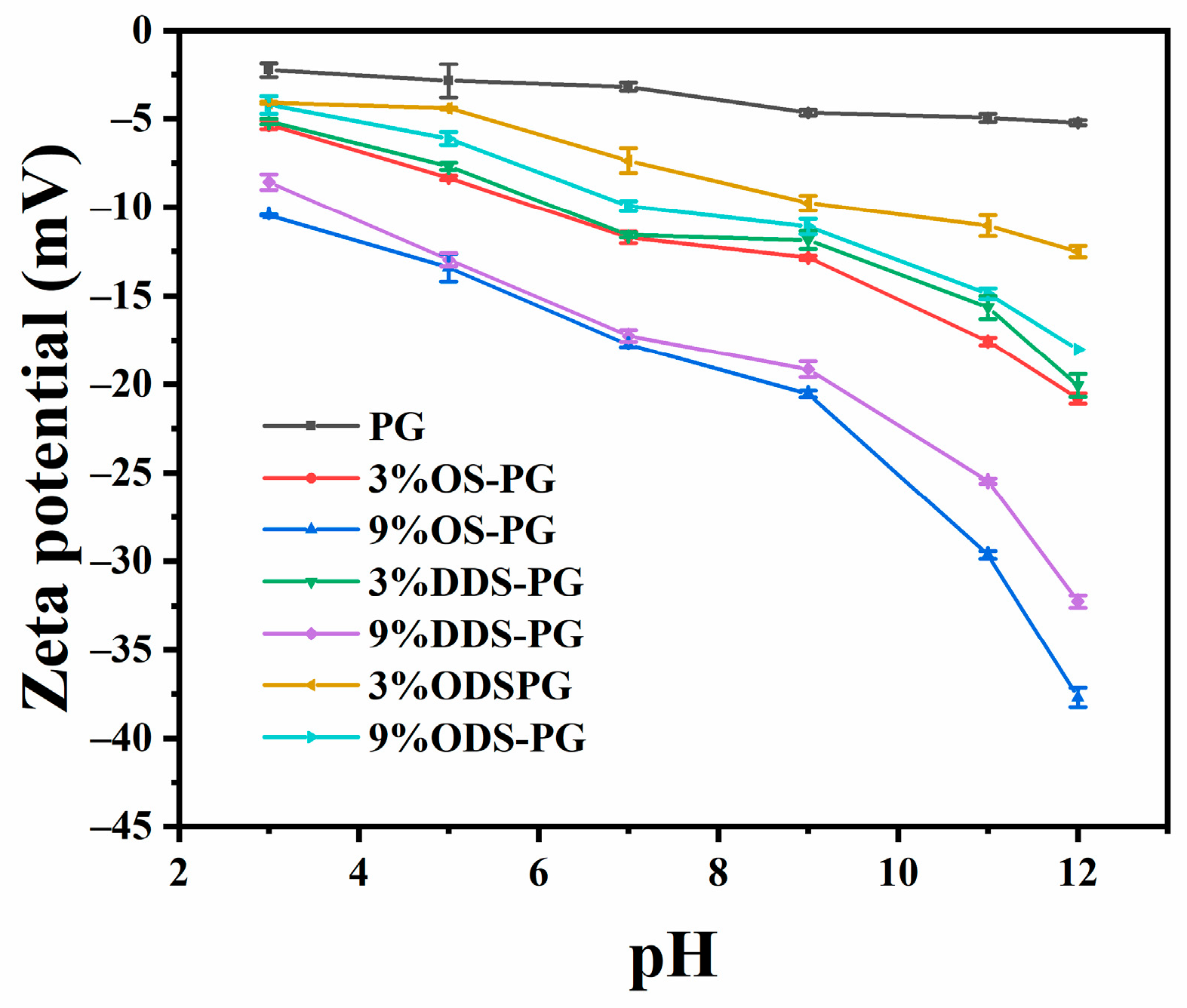

| DS (10−2) | Mw (107 g/mol) | Rz (nm) | Particle Size(nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|---|---|
| PG | 0 | 1.64 ± 0.02 e | 15.85 ± 0.49 a | 32.27 ± 0.35 a | 0.15 ± 0.00 a | −3.70 ± 0.23 d |
| 3%OS-PG | 1.53 ± 0.02 c | 1.89 ± 0.01 f | 16.75 ± 0.21 a | 34.42 ± 0.04 b | 0.18 ± 0.01 ab | −11.03 ± 0.45 b |
| 9%OS-PG | 4.32 ± 0.08 e | 1.86 ± 0.05 f | 19.10 ± 0.71 b | 35.13 ± 0.06 b | 0.19 ± 0.02 b | −17.33 ± 0.39 a |
| 3%DDS-PG | 1.07 ± 0.07 b | 1.50 ± 0.02 c | 16.00 ± 0.13 a | 34.80 ± 0.64 b | 0.17 ± 0.01 ab | −10.34 ± 0.20 b |
| 9%DDS-PG | 2.79 ± 0.02 d | 1.44 ± 0.07 b | 18.95 ± 0.64 b | 36.91 ± 0.61 c | 0.19 ± 0.01 b | −16.17 ± 0.55 a |
| 3%ODS-PG | 0.75 ± 0.02 a | 1.560 ± 0.02 d | 15.65 ± 0.34 a | 38.42 ± 0.14 d | 0.22 ± 0.01 c | −7.26 ± 0.33 c |
| 9%ODS-PG | 1.00 ± 0.03 b | 1.35 ± 0.03 a | 19.75 ± 0.21 b | 41.09 ± 0.67 e | 0.26 ± 0.01 d | −9.95 ± 1.34 b |
| Power Law | Herschel–Bulkley | ||||||
|---|---|---|---|---|---|---|---|
| K (Pa·sn) | n | R2 | σ0 (Pa) | K (Pa·sn) | n | R2 | |
| PG | 0.00525 | 0.856 | 0.998 | 0.00516 | 0.00405 | 0.909 | 0.999 |
| 3%OSA-PG | 0.00537 | 0.854 | 0.998 | 0.00570 | 0.00404 | 0.912 | 0.999 |
| 9%OSA-PG | 0.00562 | 0.862 | 0.998 | 0.00652 | 0.00416 | 0.925 | 0.999 |
| 3%DDSA-PG | 0.00544 | 0.857 | 0.998 | 0.00567 | 0.00414 | 0.914 | 0.999 |
| 9%DDSA-PG | 0.00780 | 0.782 | 0.996 | 0.0117 | 0.00460 | 0.891 | 0.999 |
| Weight Loss at Different Stages | |||||||
|---|---|---|---|---|---|---|---|
| RDS | SDS | RS | Stage 1 (%) | Stage 2 (%) | Stage 3 (%) | Solid Residue (%) | |
| PG | 69.50 ± 0.25 e | 17.89 ± 0.84 b | 12.61 ± 1.10 a | 7.76 ± 0.18 c | 56.11 ± 0.18 a | 20.38 ± 0.27 e | 15.72 ± 0.32 a |
| 3%OS-PG | 46.54 ± 0.46 c | 18.81 ± 0.85 b | 34.65 ± 1.31 c | 6.10 ± 0.08 b | 56.78 ± 0.09 ab | 18.04 ± 0.10 d | 19.06 ± 0.17 b |
| 9%OS-PG | 25.45 ± 1.48 a | 10.39 ± 1.76 a | 64.16 ± 0.27 e | 5.38 ± 0.10 a | 57.69 ± 0.03 b | 9.00 ± 0.40 b | 27.86 ± 0.21 d |
| 3%DDS-PG | 51.60 ± 0.32 d | 17.22 ± 0.97 b | 31.18 ± 0.66 b | 6.44 ± 0.22 b | 61.92 ± 1.00 c | 12.73 ± 0.75 c | 18.90 ± 0.31 b |
| 9%DDS-PG | 33.51 ± 0.66 b | 11.10 ± 0.47 a | 55.39 ± 1.13 d | 5.61 ± 0.08 a | 64.57 ± 0.08 d | 5.83 ± 0.32 a | 23.99 ± 0.47 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Miao, M. Structure and Characterizations of Different Hydrophobically Modified Phytoglycogen Nanoparticles. Foods 2025, 14, 1351. https://doi.org/10.3390/foods14081351
Wang H, Miao M. Structure and Characterizations of Different Hydrophobically Modified Phytoglycogen Nanoparticles. Foods. 2025; 14(8):1351. https://doi.org/10.3390/foods14081351
Chicago/Turabian StyleWang, Huixuan, and Ming Miao. 2025. "Structure and Characterizations of Different Hydrophobically Modified Phytoglycogen Nanoparticles" Foods 14, no. 8: 1351. https://doi.org/10.3390/foods14081351
APA StyleWang, H., & Miao, M. (2025). Structure and Characterizations of Different Hydrophobically Modified Phytoglycogen Nanoparticles. Foods, 14(8), 1351. https://doi.org/10.3390/foods14081351







