Changes in the Quality Parameters and Antimicrobial Activity of Ozonated Virgin and Pomace Olive Oils Under Different Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
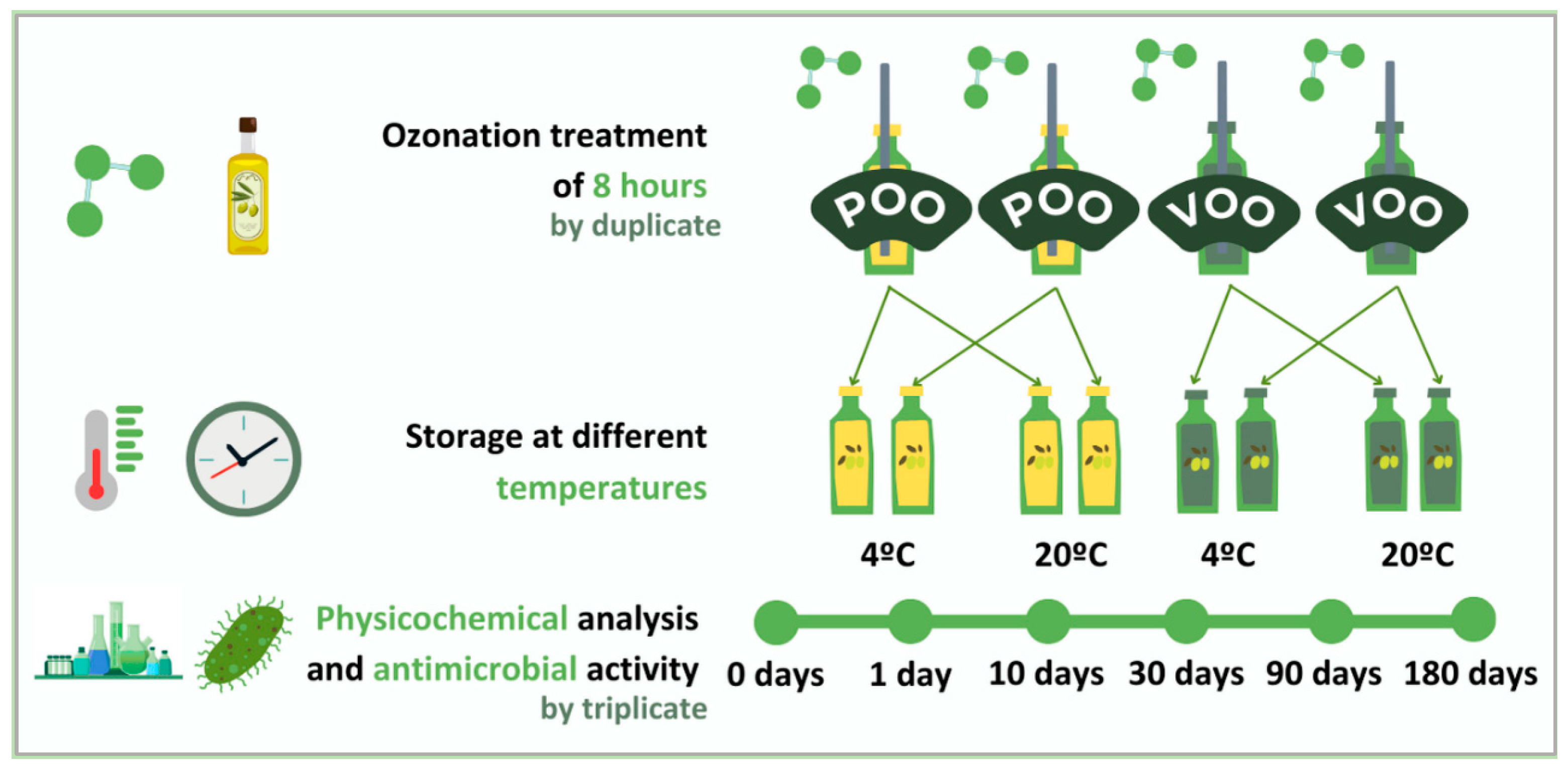
2.2. Ozonation and Storage Conditions
2.3. Reagents
2.4. Quality Parameters
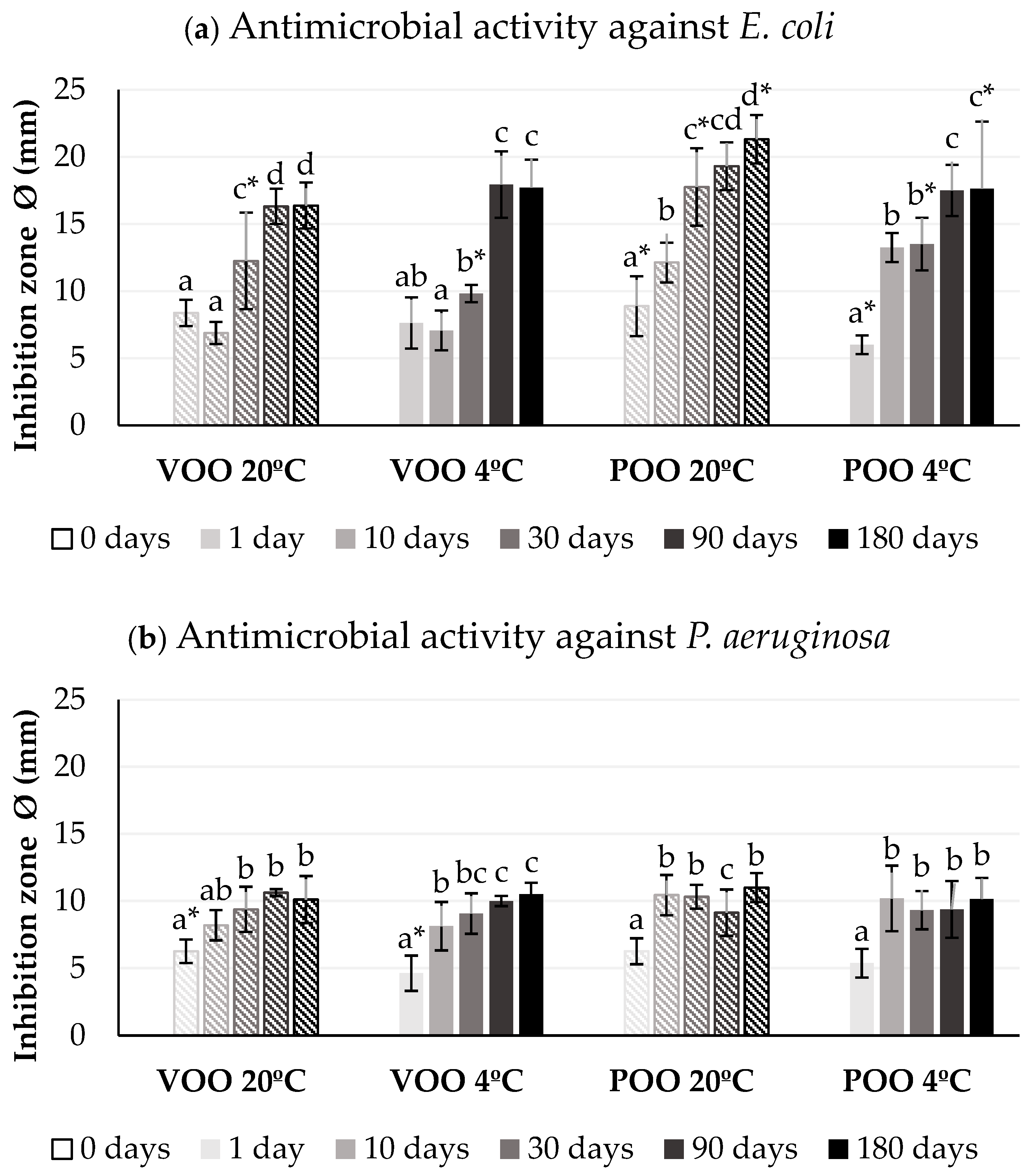
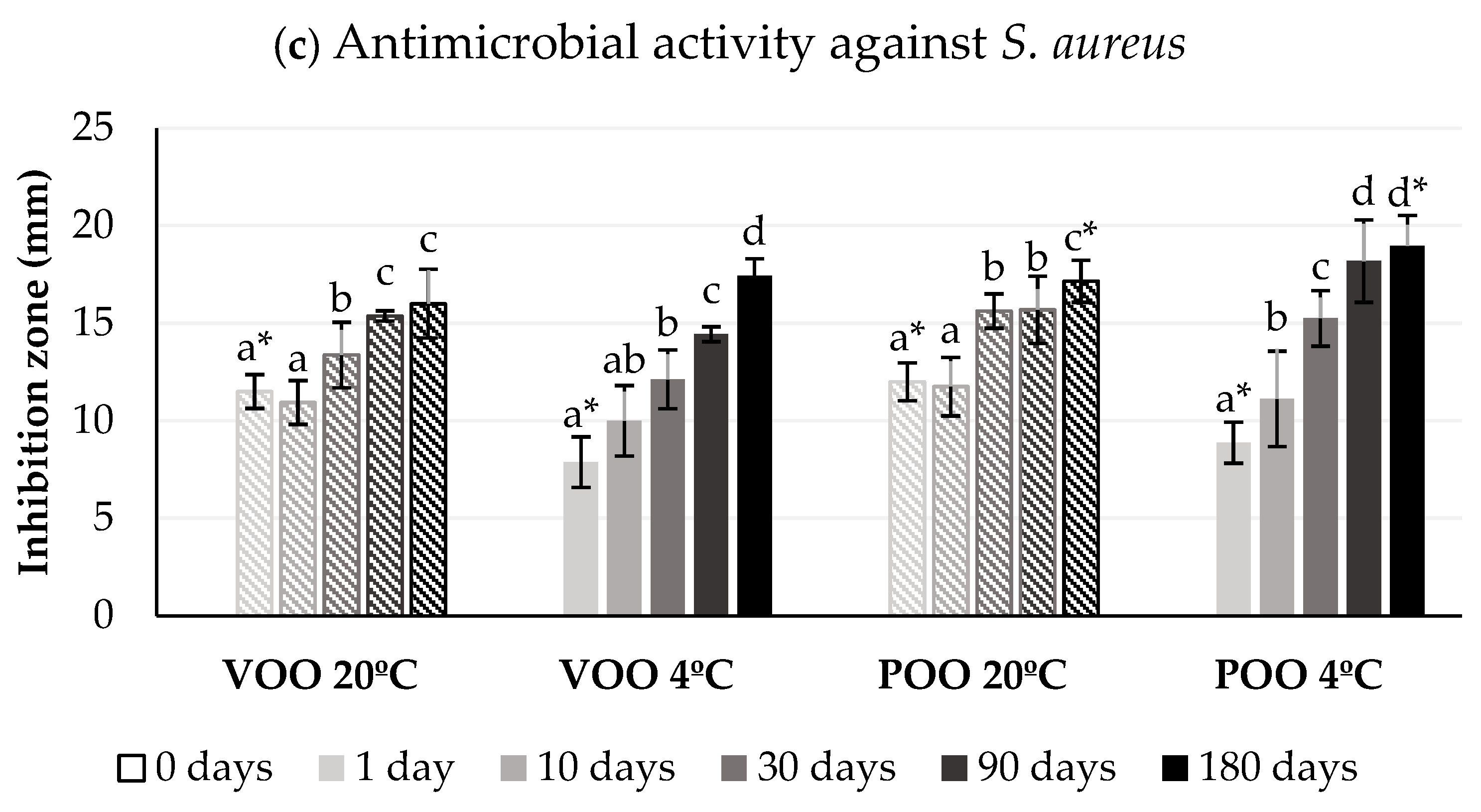
2.5. Antimicrobial Activity Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Storage Conditions on Quality Parameters of Ozonated Olive Oils
3.2. Effect of Storage Conditions on Antimicrobial Efficiency of Ozonated Olive Oils
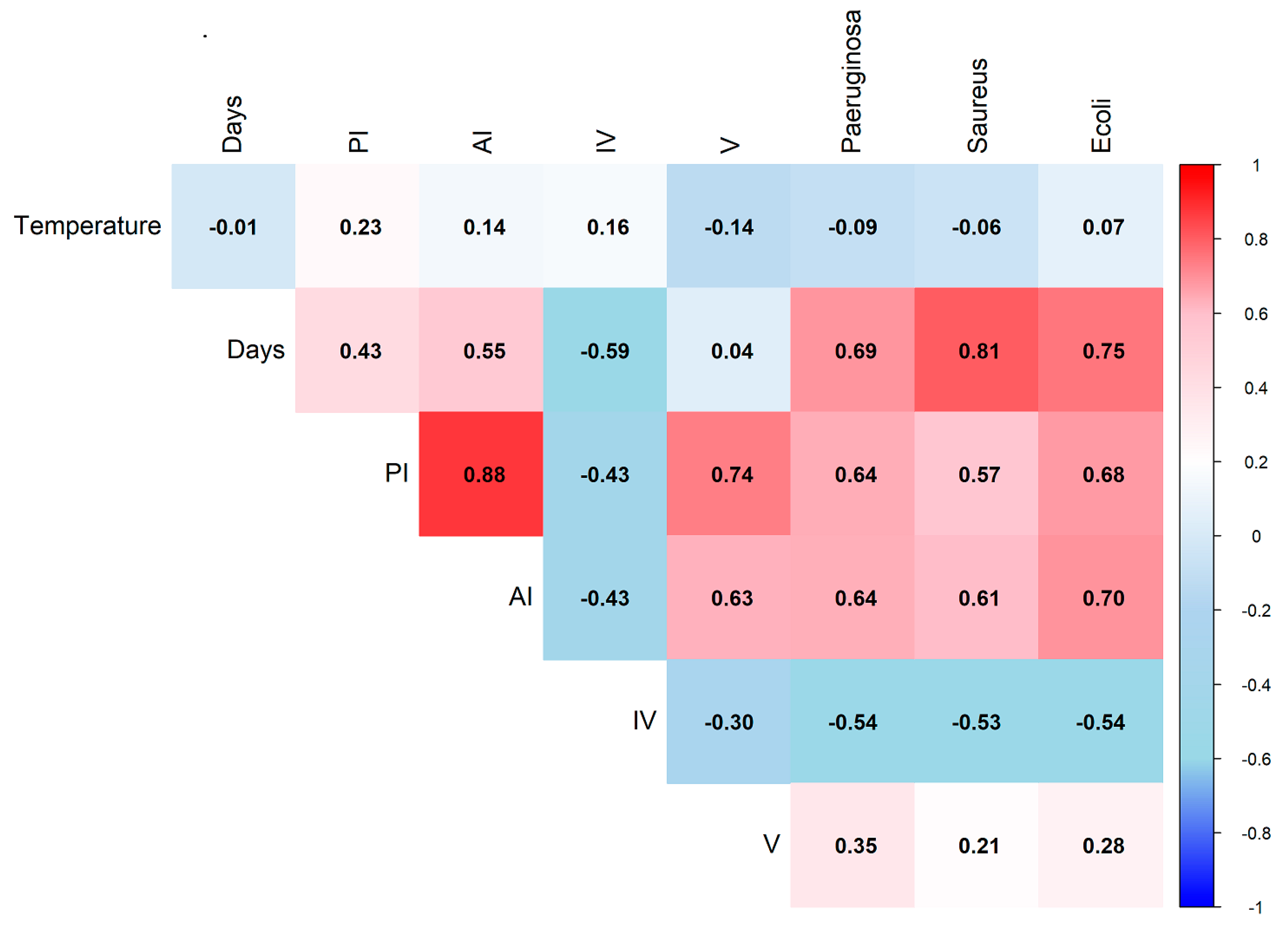
3.3. Correlation Among Storage Variables, Quality Parameters, and Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocci, V. Physical-Chemical Properties of Ozone—Natural Production of Ozone: The Toxicology of Ozone. In OZONE; Springer: Dordrecht, The Netherlands, 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone Application in Different Industries: A Review of Recent Developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Almeida, N.; Beatriz, A.; Arruda, E.; de Lima, D.; Oliveira, L.; Micheletti, A. Ozonized Vegetable Oils: Production, Chemical Characterization and Therapeutic Potential. In Vegetable Oil: Properties, Uses and Benefits; Nova Science Publisher: Hauppauge, NY, USA, 2016; pp. 129–160. [Google Scholar]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Günaydın, Y.; Sevim, H.; Tanyolaç, D.; Gürpınar, Ö.A. Ozonated Olive Oil with a High Peroxide Value for Topical Applications: In-Vitro Cytotoxicity Analysis with L929 Cells. Ozone Sci. Eng. 2018, 40, 37–43. [Google Scholar] [CrossRef]
- Bouzid, D.; Merzouki, S.; Boukhebti, H.; Zerroug, M.M. Various Antimicrobial Agent of Ozonized Olive Oil. Ozone Sci. Eng. 2021, 43, 606–612. [Google Scholar] [CrossRef]
- Almeida, N.R.; Beatriz, A.; Micheletti, A.C.; Arruda, E.J. de Ozonized Vegetable Oils and Therapeutic Properties: A Review. Orbital Electron. J. Chem. 2013, 4, 313–326. [Google Scholar] [CrossRef]
- Borges, G.Á.; Elias, S.T.; da Silva, S.M.M.; Magalhães, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In Vitro Evaluation of Wound Healing and Antimicrobial Potential of Ozone Therapy. J. Cranio-Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone Therapy: An Overview of Pharmacodynamics, Current Research, and Clinical Utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar] [CrossRef]
- Zeng, J.; Lu, J. Mechanisms of Action Involved in Ozone-Therapy in Skin Diseases. Int. Immunopharmacol. 2018, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sehim, A.E.; Abd Elghaffar, R.Y.; Emam, A.M.; El-Desouky, T.A. Evaluation of the Efficacy of Ozonated Olive Oil for Controlling the Growth of Alternaria Alternata and Its Toxins. Heliyon 2023, 9, e17885. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A.; Scapin, R.M.; Zannoni, S.; Contiero, B.; Catellani, P.; Giaccone, V. Evaluation of Quality and Safety of Beef Hamburgers Fortified with Ozonated Extra Virgin Olive Oil. LWT 2022, 170, 114100. [Google Scholar] [CrossRef]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial Activity of Ozonized Sunflower Oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef]
- Guadalupe Armas, G.D.C.; Martel Benítez, C.J.; Alayón-Afonso, R.; Clavo, B.; Benítez, A.; González-Martín, J.; Torres-Mata, L.; Barrasa, J.L. In Vitro Antimicrobial Activity of Ozonated Sunflower Oil in Milk against Escherichia coli: Comparative Study in Cow, Goat and Sheep. J. Appl. Anim. Res. 2022, 50, 715–724. [Google Scholar] [CrossRef]
- Soto Beltran, J.; Jiménez Edeza, M.; Amézquita-López, B.; Martínez-Rodriguez, C.; Chaidez-Quiroz, C. Antibacterial Activity of Ozonized Olive (Olea europaea L.) and Venadillo (Swietenia humilis zucc.) Oils Against Escherichia coli and Staphylococcus aureus. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 947–949. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-Chemical Investigation and Antimicrobial Efficacy of Ozonated Oils: The Case Study of Commercial Ozonated Olive and Sunflower Seed Refined Oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef]
- Radzimierska-Kaźmierczak, M.; Śmigielski, K.; Sikora, M.; Nowak, A.; Plucińska, A.; Kunicka-Styczyńska, A.; Czarnecka-Chrebelska, K.H. Olive Oil with Ozone-Modified Properties and Its Application. Molecules 2021, 26, 3074. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, G.; De Ascentiis, A.; Tortini, P. Stability Studies of Ozonized Sunflower Oil and Enriched Cosmetics with a Dedicated Peroxide Value Determination. Ozone Sci. Eng. 2012, 34, 293–299. [Google Scholar] [CrossRef]
- Jbara, S.; Shehadeh, A.S.; Trefi, S.; Bitar, Y. Assessing the Quantity and Quality of Ozonated Olive Oil and Studying Its Shelf-Life Stability. Bull. Pharm. Sci. Assiut 2022, 45, 249–268. [Google Scholar] [CrossRef]
- Lima, M.D.; Ramos, R.S. Influence of Storage Conditions on the Stability of Ozonized Vegetable Oils. Res. Soc. Dev. 2024, 13, e133131247796. [Google Scholar] [CrossRef]
- Moureu, S.; Violleau, F.; Ali Haimoud-Lekhal, D.; Calmon, A. Influence of Storage Temperature on the Composition and the Antibacterial Activity of Ozonized Sunflower Oil. Ozone Sci. Eng. 2016, 38, 143–149. [Google Scholar] [CrossRef]
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive Pomace Oil versus High Oleic Sunflower Oil and Sunflower Oil: A Comparative Study in Healthy and Cardiovascular Risk Humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- IOC Standards, Methods and Guides. 2023. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 20 February 2025).
- Picazo, J.J.; García Rodríguez, J.A.; Cantón, R.; García Sánchez, J.E.; Goméz-Lus, M.L.; Martínez Martínez, L.; Rodríguez-Avial, C.; Vila, J. Clinical Microbiology Procedures: Recomendations of the Spanish Society of Infectious Diseases and Clinical Microbiology. 2020. Available online: https://www.seimc.org/documentos-cientificos/procedimientos-microbiologia (accessed on 20 February 2025).
- Cobzaru, C.; Bordeianu, G.; Apostolescu, G.A.; Marinoiu, A.; Cernatescu, C. Quality Evolution of the Olive Oil during Storage Time. Rev. Roum. Chim. 2016, 8–9, 705–710. [Google Scholar]
- Sadowska, J.; Johansson, B.; Johannessen, E.; Friman, R.; Broniarz-Press, L.; Rosenholm, J.B. Characterization of Ozonated Vegetable Oils by Spectroscopic and Chromatographic Methods. Chem. Phys. Lipids 2007, 151, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Guerra Blanco, P.; Chairez, I.; Tatyana, P.; Brito-Arias, M. Kinetic Analysis of Ozonation Degree Effect on the Physicochemical Properties of Ozonated Vegetable Oils. Ozone Sci. Eng. 2021, 43, 546–561. [Google Scholar] [CrossRef]
- Balea, A.; Ciotlaus, I.; Pojar-Fenesan, M.-F.; Carpa, R. Comparative Chemical and Antimicrobial Characterization of Non-Ozonated and Ozonated Vegetable Oils. Stud. Univ. Babeș-Bolyai Chem. 2023, 68, 285–301. [Google Scholar] [CrossRef]
- Audran, G.; Marque, S.R.A.; Santelli, M. Ozone, Chemical Reactivity and Biological Functions. Tetrahedron 2018, 74, 6221–6261. [Google Scholar] [CrossRef]
- Díaz, M.F.; Hernández, R.; Martínez, G.; Vidal, G.; Gómez, M.; Fernández, H.; Garcés, R. Comparative Study of Ozonized Olive Oil and Ozonized Sunflower Oil. J. Braz. Chem. Soc. 2006, 17, 403–407. [Google Scholar] [CrossRef]
- Slavinskienė, G.; Grigonis, A.; Ivaškienė, M.; Sinkevičienė, I.; Andrulevičiūtė, V.; Ivanauskas, L.; Juodžentė, D.; Ramanauskienė, K.; Daunoras, G. A Comparative Study of the Chemical Properties and Antibacterial Activity of Four Different Ozonated Oils for Veterinary Purposes. Vet. Sci. 2024, 11, 161. [Google Scholar] [CrossRef]

| Peroxide Index Values | ||||||
| Sample | 0 days | 1 day | 10 days | 30 days | 90 days | 180 days |
| VOO 20 °C | 13.03 ± 5.92 Aa | 590.43 ± 40.03 Ba | 634.87 ± 41.23 Ba | 741.44 ± 32.16 Ca | 689.73 ± 47.81 Ca | 604.23 ± 10.09 Da |
| VOO 4 °C | 13.03 ± 5.92 Aa | 350.25 ± 36.64 Bb | 430.21 ± 21.16 Cb | 534.78 ± 49.62 Db | 524.84 ± 19.01 Eb | 500.68 ± 35.21 Eb |
| POO 20 °C | 119.53 ± 9.22 Aa | 875.19 ± 74.35 Ba | 1032.74 ± 76.73 Ca | 1053.40 ± 70.97 Ca | 1036.07 ± 83.50 Ca | 906.62 ± 14.69 Da |
| POO 4 °C | 119.53 ± 9.22 Aa | 674.33 ± 54.31 Bb | 868.74 ± 70.23 Cb | 1067.23 ± 56.56 Da | 1004.78 ± 43.90 Da | 982.36 ± 23.36 Db |
| Acidity Index Values | ||||||
| Sample | 0 days | 1 day | 10 days | 30 days | 90 days | 180 days |
| VOO 20 °C | 1.08 ± 0.02 Aa | 2.38 ± 0.07 Ba | 2.93 ± 0.18 Ca | 3.49 ± 0.14 Da | 3.33 ± 0.19 Da | 3.80 ± 0.05 Ea |
| VOO 4 °C | 1.08 ± 0.02 Aa | 1.62 ± 0.15 Bb | 2.23 ± 0.40 Cb | 3.08 ± 0.21 Db | 3.18 ± 0.12 Da | 3.40 ± 0.07 Eb |
| POO 20 °C | 3.03 ± 0.26 Aa | 4.67 ± 0.34 Ba | 5.43 ± 0.28 Ca | 5.81 ± 0.22 Da | 6.14 ± 0.22 DEa | 6.32 ± 0.14 Ea |
| POO 4 °C | 3.03 ± 0.26 Aa | 3.55 ± 0.46 Bb | 4.25 ± 0.36 Cb | 5.02 ± 0.31 Db | 5.72 ± 0.26 Eb | 5.83 ± 0.18 Eb |
| Iodine Values | ||||||
| Sample | 0 days | 1 day | 10 days | 30 days | 90 days | 180 days |
| VOO 20 °C | 94.33 ± 3.07 Aa | 55.99 ± 7.12 Ba | 50.67 ± 10.65 BCa | 47.93 ± 2.42 BCa | 48.75 ± 4.13 BCa | 44.37 ± 2.90 Ca |
| VOO 4 °C | 94.33 ± 3.07 Aa | 54.62 ± 8.27 Ba | 44.04 ± 4.25 Ca | 43.38 ± 5.20 Ca | 41.26 ± 5.19 Ca | 36.46 ± 5.71 Ca |
| POO 20 °C | 87.08 ± 2.73 Aa | 58.33 ± 3.81 Ba | 48.10 ± 6.45 Ca | 41.59 ± 2.60 CDa | 41.20 ± 2.47 CDa | 37.38 ± 3.19 Da |
| POO 4 °C | 87.08 ± 2.73 Aa | 52.87 ± 4.75 Ba | 47.21 ± 4.20 Ca | 48.74 ± 5.87 Ca | 45.11 ± 2.09 Ca | 39.81 ± 0.94 Ca |
| Viscosity Values | ||||||
| Sample | 0 days | 1 day | 10 days | 30 days | 90 days | 180 days |
| VOO 20 °C | 60.22 ± 0.77 Aa | 343.20 ± 14.80 Ba | 335.28 ± 2.96 Ba | 318.56 ± 0.16 Ca | 290.99 ± 0.77 Da | 232.19 ± 9.05 Ea |
| VOO 4 °C | 60.22 ± 0.77 Aa | 358.45 ± 3.30 Ba | 365.26 ± 18.89 Ba | 372.82 ± 6.56 Bb | 355.83 ± 24.98 Bb | 309.52 ± 2.80 Cb |
| POO 20 °C | 64.90 ± 0.76 Aa | 665.75 ± 7.92 Ba | 605.40 ± 0.12 Ca | 573.35 ± 1.23 Da | 445.60 ± 38.46 Ea | 401.42 ± 2.83 Fa |
| POO 4 °C | 64.90 ± 0.76 Aa | 627.75 ± 28.14 Ba | 597.66 ± 3.15 Bb | 651.77 ± 0.32 Cb | 624.18 ± 5.72 BDb | 602.45 ± 3.12 Db |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez Lacueva, P.; Corella Guillamón, P.; Cantalejo Díez, M.J. Changes in the Quality Parameters and Antimicrobial Activity of Ozonated Virgin and Pomace Olive Oils Under Different Storage Conditions. Foods 2025, 14, 999. https://doi.org/10.3390/foods14060999
Dominguez Lacueva P, Corella Guillamón P, Cantalejo Díez MJ. Changes in the Quality Parameters and Antimicrobial Activity of Ozonated Virgin and Pomace Olive Oils Under Different Storage Conditions. Foods. 2025; 14(6):999. https://doi.org/10.3390/foods14060999
Chicago/Turabian StyleDominguez Lacueva, Paula, Paula Corella Guillamón, and María J. Cantalejo Díez. 2025. "Changes in the Quality Parameters and Antimicrobial Activity of Ozonated Virgin and Pomace Olive Oils Under Different Storage Conditions" Foods 14, no. 6: 999. https://doi.org/10.3390/foods14060999
APA StyleDominguez Lacueva, P., Corella Guillamón, P., & Cantalejo Díez, M. J. (2025). Changes in the Quality Parameters and Antimicrobial Activity of Ozonated Virgin and Pomace Olive Oils Under Different Storage Conditions. Foods, 14(6), 999. https://doi.org/10.3390/foods14060999








