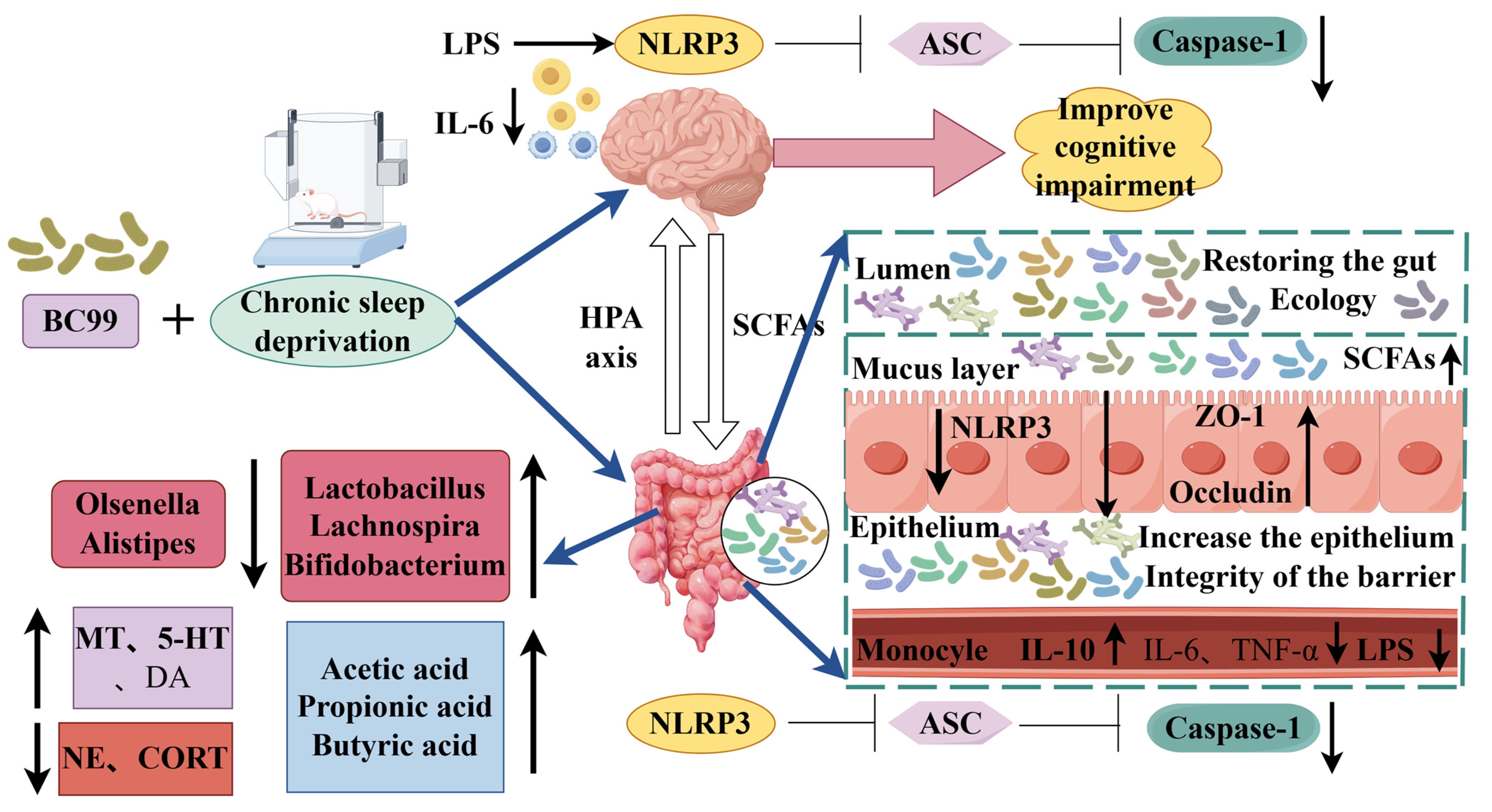
Weizmannia coagulans BC99 Improve Cognitive Impairment Induced by Chronic Sleep Deprivation via Inhibiting the Brain and Intestine’s NLRP3 Inflammasome
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Methods
Preparation of Probiotic Suspension
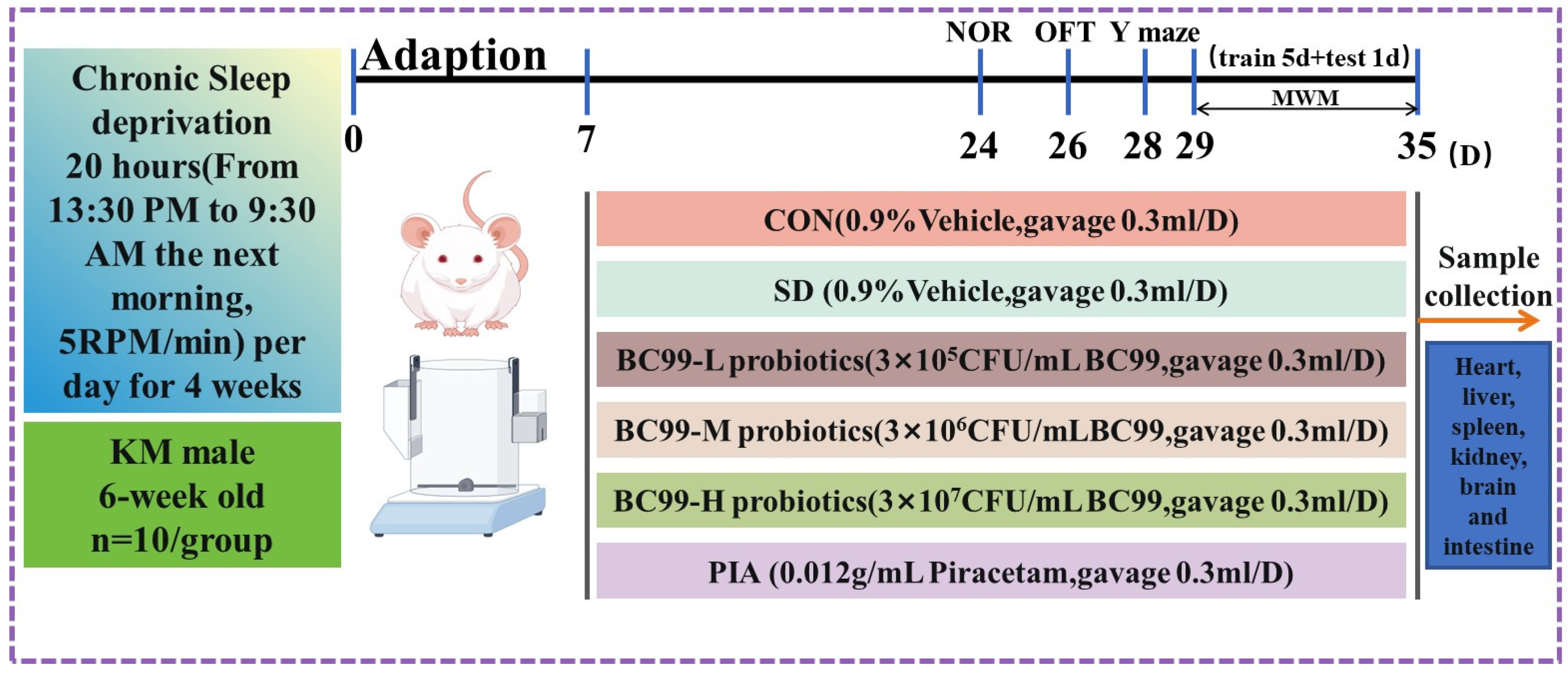
2.2. Design of Animal Experiments
2.3. Chronic Sleep Deprivation
2.4. Probiotic Therapy
2.5. Morris Water Maze (MWM) Test
2.6. Novel Object Recognition Test
2.7. Measurement of Plasma Inflammatory Response
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Assessment of Oxidative Stress in Brain Tissue
2.10. Measurement of Fecal SCFAs
2.11. Real-Time Fluorescence Quantitative PCR
2.12. 16S rRNA Microbiota Assessment and Bioinformatics
2.13. Statistical Analysis
3. Results
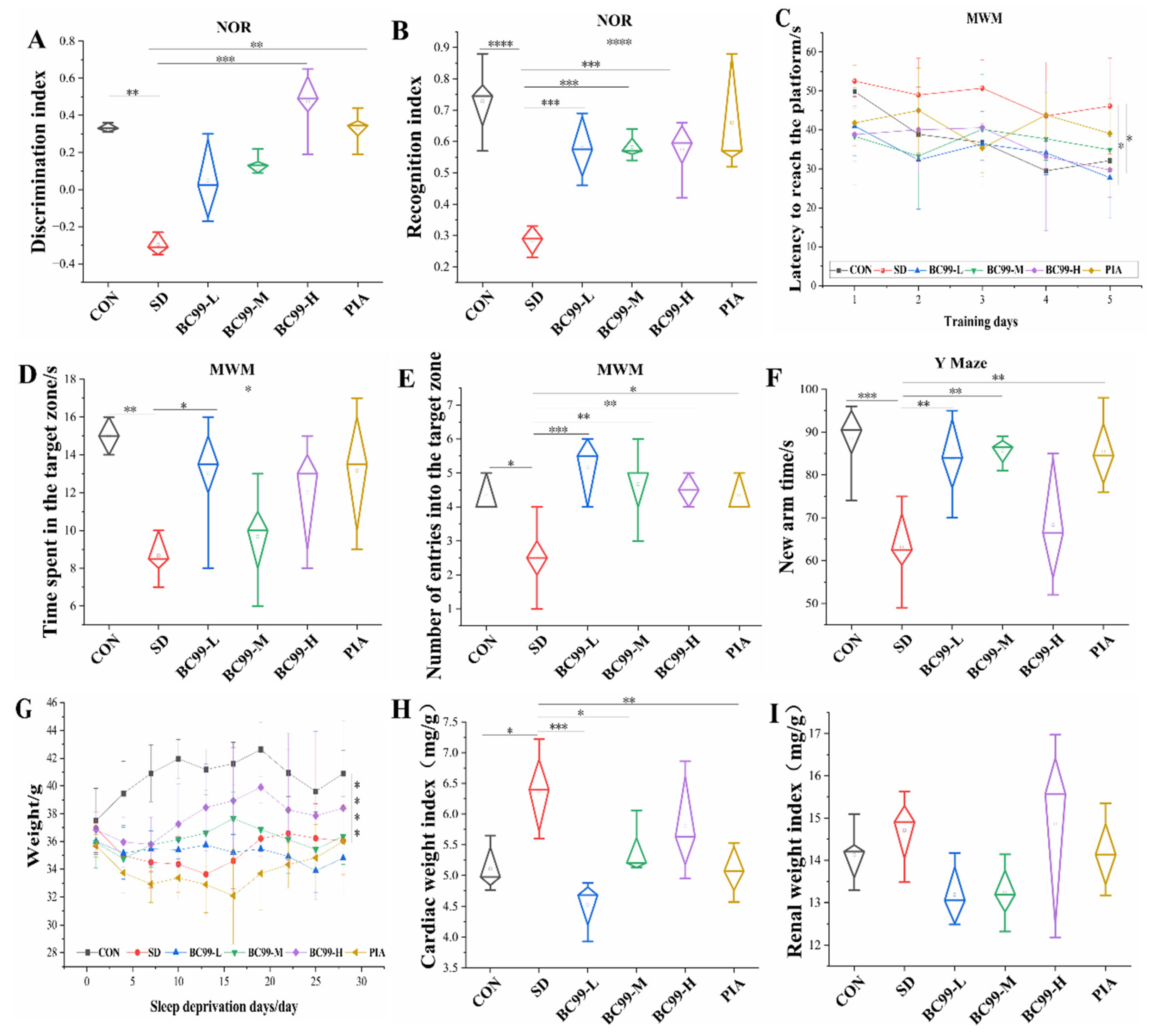
3.1. Effects of W. coagulans BC99 on Behavioral and Physiological Indices of Chronic Sleep Deprivation Mice
3.2. Effect of W. coagulans BC99 on Biochemical Indices in Chronic Sleep Deprivation Mice
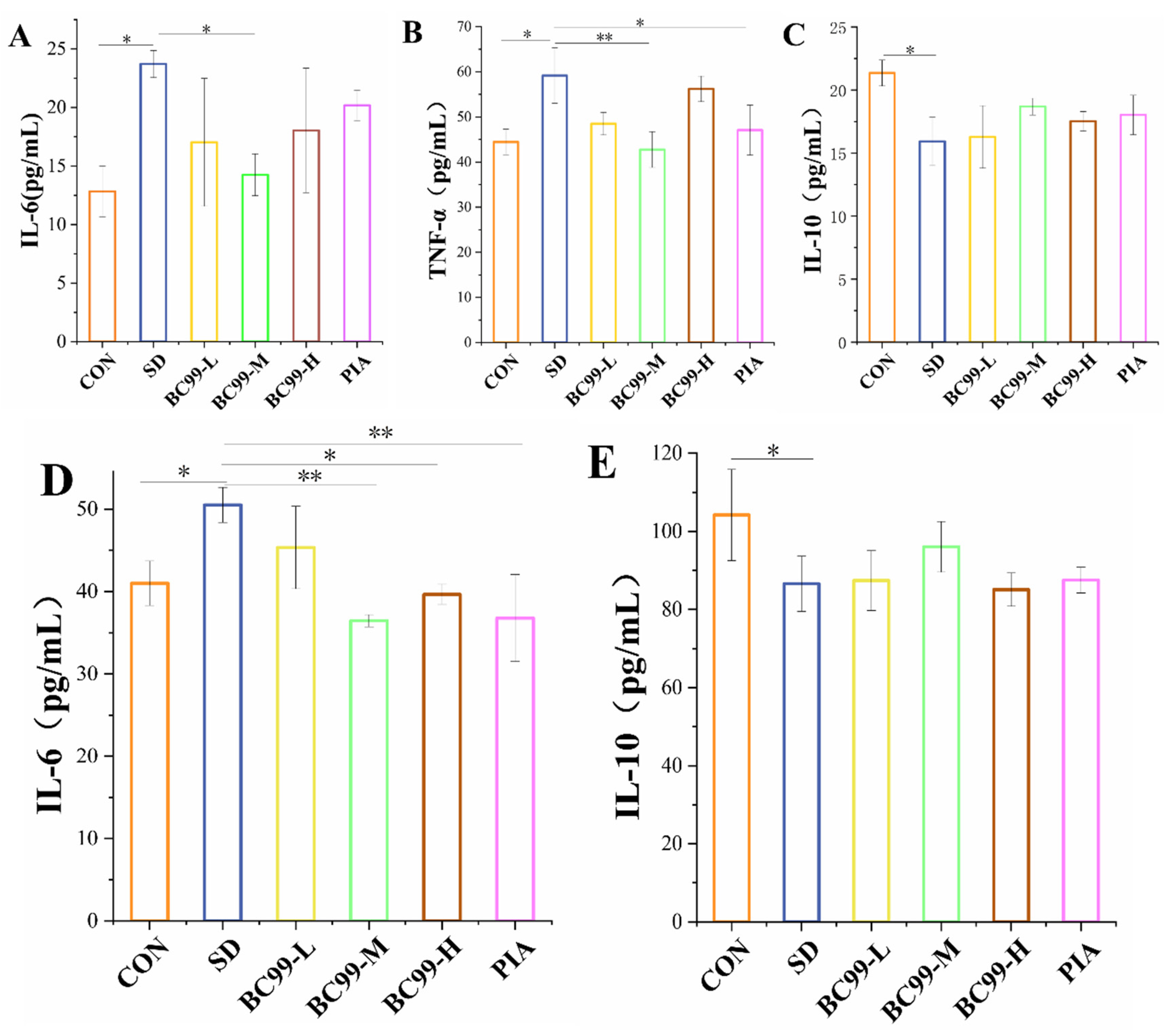
3.3. Effect of W. coagulans BC99 on Brain and Plasma Inflammation in Chronic Sleep Deprivation Mice
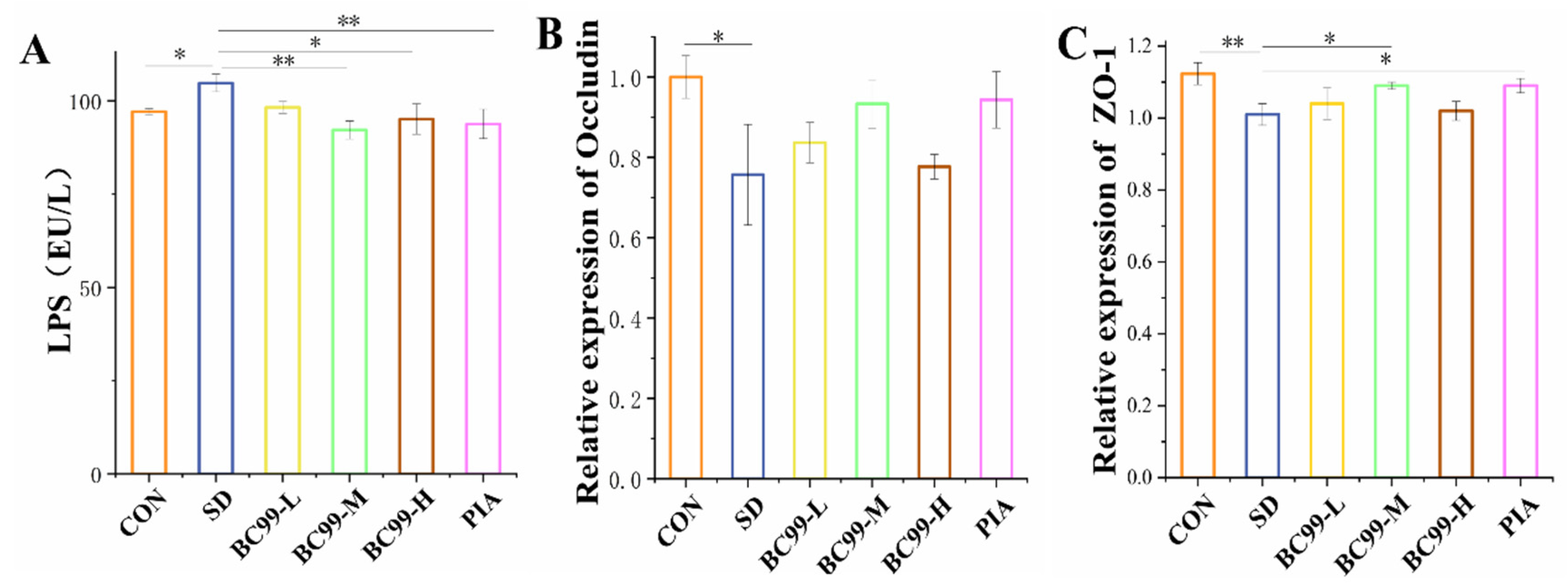
3.4. Effect of W. coagulans BC99 on Intestinal Barrier in Chronic Sleep Deprivation Mice
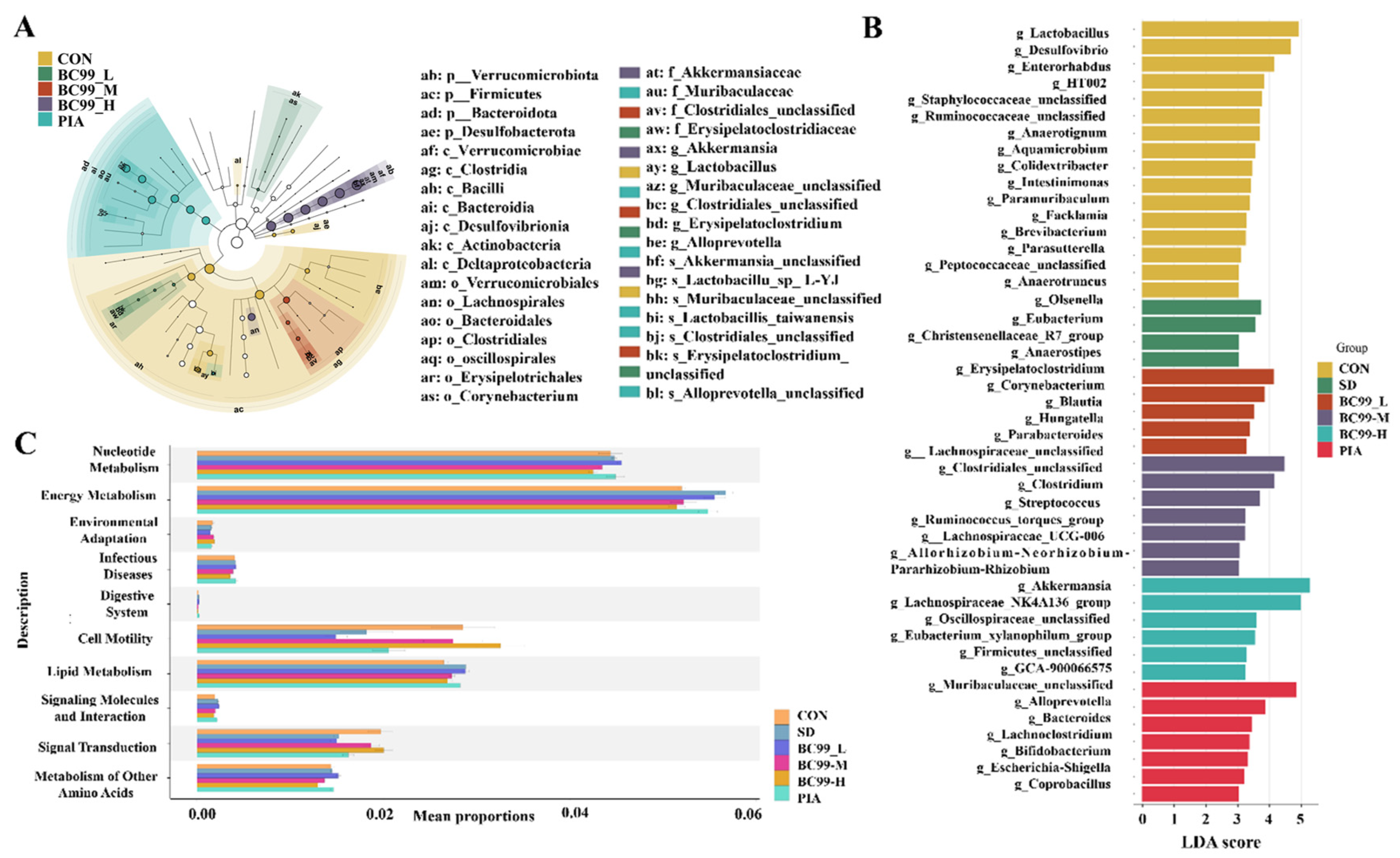
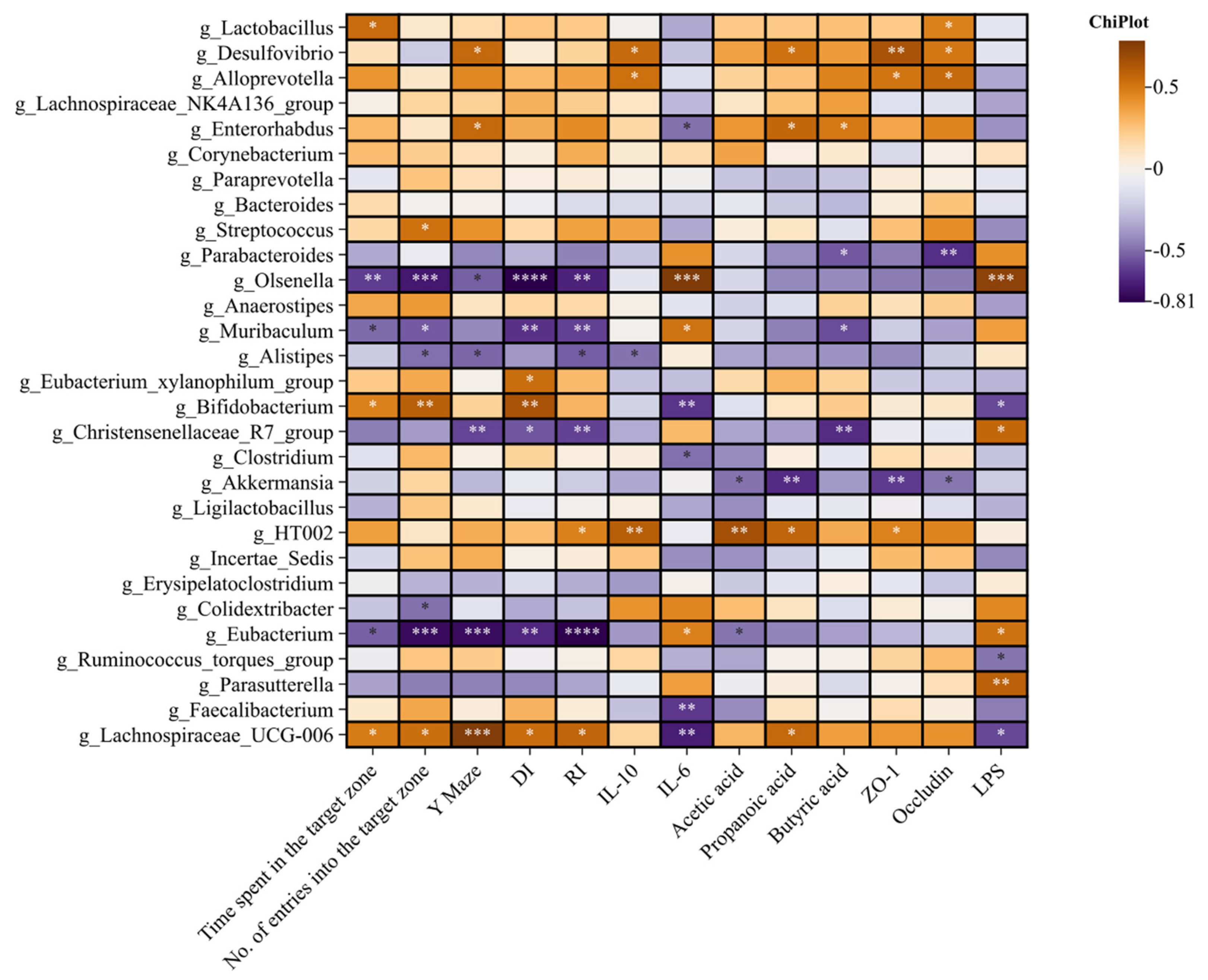
3.5. Effects of BC99 on Intestinal Flora in Chronic Sleep Deprivation Mice
3.6. Differential Effects of W. coagulans BC99 on LEfSe and Prediction of PICRUSt2 Function in Chronic Sleep Deprivation Mice
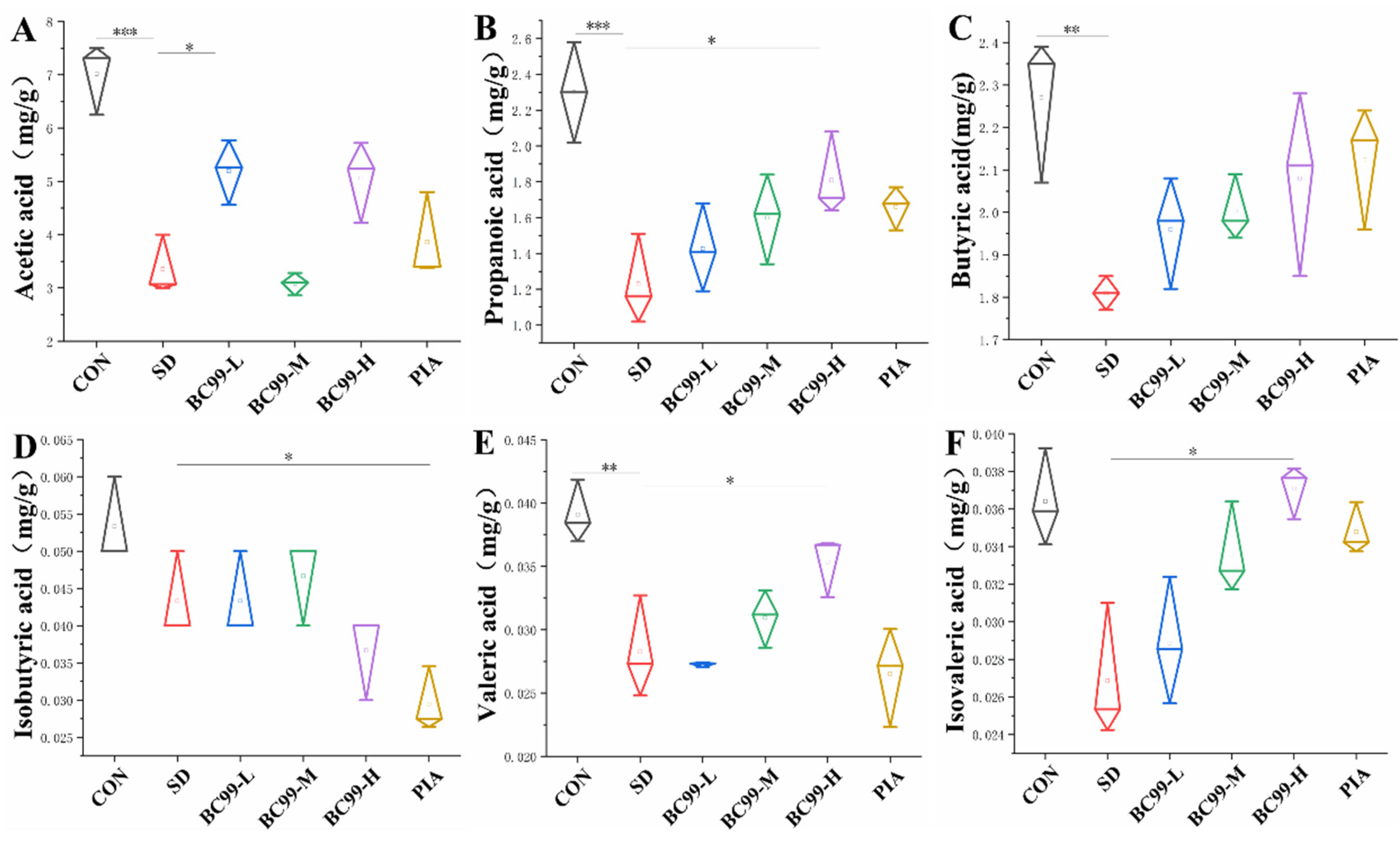
3.7. Effects of W. coagulans BC99 on Short-Chain Fatty Acids in Chronic Sleep Deprivation Mice
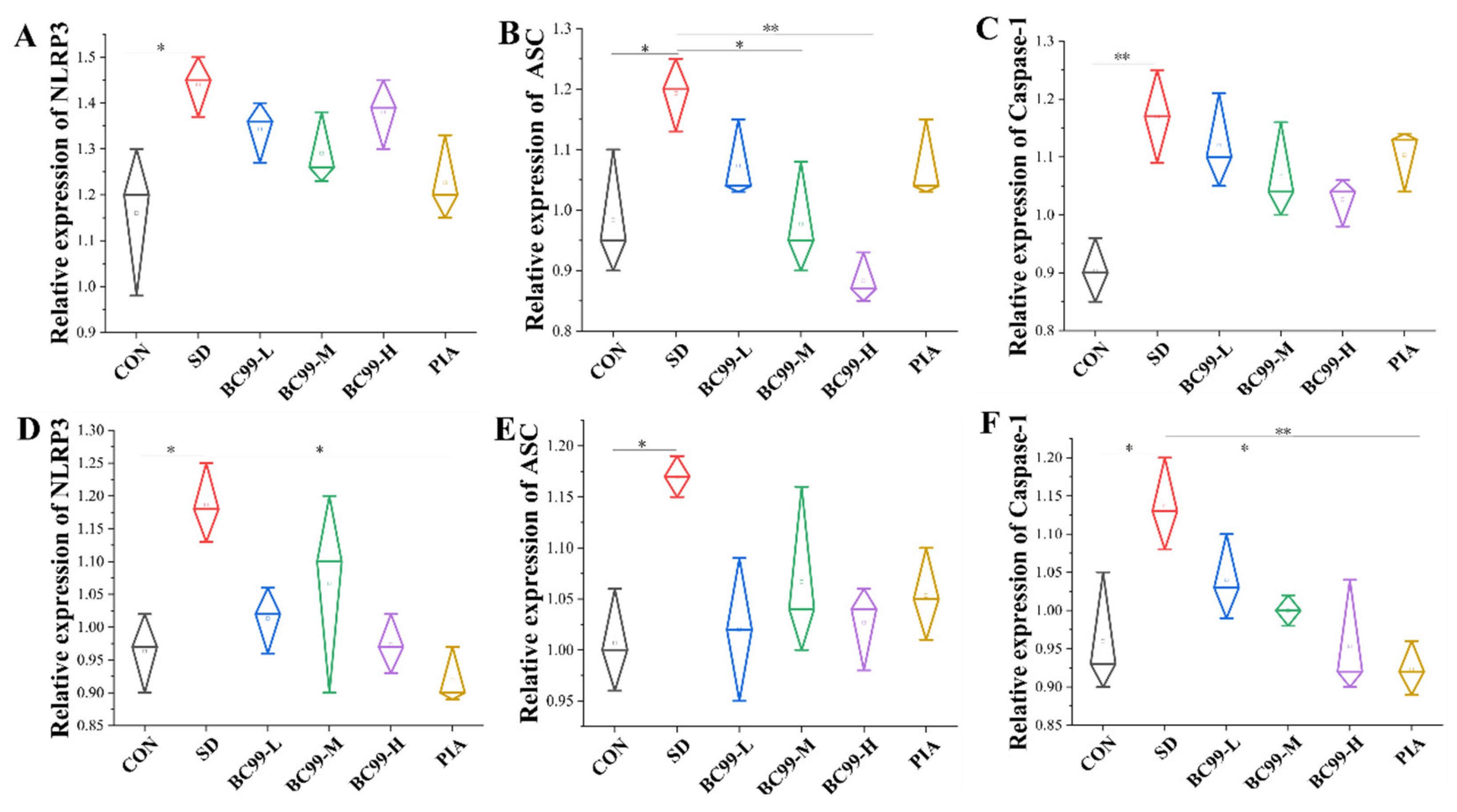
3.8. Effect of W. coagulans BC99 on NLRP3 Inflammasome Signaling Pathway in the Brain and Jejunum of Chronic Sleep Deprivation Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 24, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 16, 246–259. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Wong, L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- An, Q.; Li, C.; Chen, Y.; Yang, Y.; Song, R.; Zhou, L.; Li, J.; Tong, A.; Luo, Y. Scaffold hopping of agomelatine leads to enhanced antidepressant effects by modulation of gut microbiota and host immune responses. Pharmacol. Biochem. Behav. 2020, 192, 172910. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.Y.; Wang, F.; Qu, H.; Zhang, M.; Luo, T.; Wang, H.Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice. Sci. Rep. 2019, 20, 19575. [Google Scholar] [CrossRef]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Raparelli, V.; Basili, S.; Carnevale, R.; Napoleone, L.; Del Ben, M.; Nocella, C.; Bartimoccia, S.; Lucidi, C.; Talerico, G.; Riggio, O.; et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017, 65, 571–581. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 14, 35405. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods 2024, 28, 3851. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Zhao, Y.; Jin, Q.; Li, S. Protective Effects of Bacillus coagulans JA845 against D-Galatose/AlCl3Iduced Cognitive Decline, Oxidative Stress and Neuroinflammation. J. Microbiol. Biotechnol. 2022, 28, 32. [Google Scholar]
- Liu, Y.; Lang, H.; Zhou, M.; Huang, L.; Hui, S.; Wang, X.; Chen, K.; Mi, M. Pterostil bene improves exercise intolerance induced by sleep restriction in mice by activating AMPK/SIRT1 signaling and promoting mitochondrial biosynthesis. J. Third Mil. Med. Univ. 2020, 42, 259–266. [Google Scholar]
- Qi, L.; Cheng, Y.; Sun, S.; Wan, H. The administration of rhBmal1 reduces sleep deprivation-induced anxiety and cognitive impairment in mice. World J. Biol. Psychiatry 2024, 25, 43–53. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 2021, 1, 666–676. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000, 7, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Steere, J.C.; Hunt, R.D. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry 1996, 53, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.P.; Arnsten, A.F. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007, 113, 523–536. [Google Scholar] [CrossRef]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C. Hippocampal 5-HT1A Receptor and Spatial Learning and Memory. Front. Pharmacol. 2015, 10, 289. [Google Scholar] [CrossRef]
- Meunie, C.N.; Chameau, P.; Fossier, P.M. Modulation of Synaptic Plasticity in the Cortex Needs to Understand All the Players. Front. Synaptic Neurosci. 2017, 1, 2. [Google Scholar] [CrossRef]
- Barajas, C.R.; Israel, C.; Florán, B. Dopamine Receptors and Neurodegeneration. Aging Dis. 2015, 6, 349–368. [Google Scholar]
- Seamans, J.K.; Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004, 74, 1–58. [Google Scholar] [CrossRef]
- Li, Y.C.; Gao, W.J. GSK-3β activity and hyperdopamine-dependent behaviors. Neurosci. Biobehav. Rev. 2011, 35, 645–654. [Google Scholar] [CrossRef]
- Masoud, S.T.; Vecchio, L.M.; Bergeron, Y.; Hossain, M.M.; Nguyen, L.T.; Bermejo, M.K.; Kile, B.; Sotnikova, T.D.; Siesser, W.B.; Gainetdinov, R.R.; et al. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol. Dis. 2015, 74, 66–75. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; et al. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease. J. Pineal Res. 2021, 71, 12774. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.S.; Zortea, M.; Souza, A.; Santos, V.; Biazús, J.V.; Torres, I.L.S.; Fregni, F.; Caumo, W. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2020, 17, e0231379. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Bakker, A.C.; Vanhaesebroeck, B.; Beyaert, R.; Jacob, W.A.; Fiers, W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 1992, 267, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.M.; Opp, M.R.; Krueger, J.M. Sleep and immune function: Glial contributions and consequences of aging. Curr. Opin. Neurobiol. 2013, 23, 806–811. [Google Scholar] [CrossRef]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol. Cell. Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014, 19, 27–31. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimers Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Monti, B.; Gatta, V.; Piretti, F.; Raffaelli, S.S.; Virgili, M.; Contestabile, A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: Involvement of alpha-synuclein. Neurotox. Res. 2010, 17, 130–141. [Google Scholar] [CrossRef]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell Res. 2020, 1, 111772. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.G.; Takeo Sato, F.; Curi, R.; Vinolo, M.A.R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 2016, 785, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Van Esch Bcam Henricks, P.A.J.; Garssen, J.; Folkerts, G. Time and Concentration Dependent Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α- Induced Endothelial Activation. Front. Pharmacol. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Baldwin, A.G.; Brough, D.; Freeman, S. Inhibiting the inflammasome: A chemical perspective. J. Med. Chem. 2016, 59, 1691–1710. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Inflammasome involvement in Alzheimer’s disease. J. Alzheimers Dis. 2016, 54, 45–53. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Marcellino, D.; Suárez-Boomgaard, D.; Sánchez-Reina, M.D.; Aguirre, J.A.; Yoshitake, T.; Yoshitake, S.; Hagman, B.; Kehr, J.; Agnati, L.F.; Fuxe, K.; et al. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: Studies with the P2X(7) receptor antagonist A-438079. J. Neural Transm. 2010, 117, 681–687. [Google Scholar] [CrossRef]
- Jiang, J.; Pan, H.; Shen, F.; Tan, Y.; Chen, S. Ketogenic Diet Alleviates Cognitive Dysfunction and Neuroinflammation in APP/PS1 Mice via the Nrf2/HO-1 and NF-κB Signaling Pathways. Neural Regen. Res. 2023, 18, 2767–2772. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Cordeira, J.; Kolluru, S.S.; Rosenblatt, H.; Kry, J.; Strecker, R.E.; McCarley, R.W. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav. Brain Res. 2018, 26, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology 2022, 137, 105641. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Diling, C.; Jian, Y.; Ting, L.; Guoyan, H.; Hualun, L.; Tang, X.; Lai, G.; Shuai, O.; Zheng, C.; et al. Effects of Oligosaccharides From Morinda Officinalis on Gut Microbiota and Metabolome of APP/PS1 Transgenic Mice. Front. Neurol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Milner, M.T.; Maddugoda, M.; Götz, J.; Burgener, S.S.; Schroder, K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr. Opin. Immunol. 2021, 68, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef]


| Gene | Primer Sequence |
|---|---|
| Occludin | F: CTCGGTACAGCAGCAATGGT R: TCATAGTGGTCAGGGTCCGT |
| ZO-1 | F: ATTCAGGTCGCTCGCATGAC R: ACTGCGTGGAATGATCGGAG |
| NLRP3 | F: CCAGGAGTTCTTTGCGGCTA R: GCCTTTTTCGAACTTGCCGT |
| ASC | F: AGACCACCAGCCAAGACAAG R: CTCCAGGTCCATCACCAAGT |
| Caspase-1 | F: AACCACTCGTACACGTCTTGCC R: CCAGATCCTCCAGCAGCAACTT |
| β-Actin | F: CTGTGTTTTGGTCTTACGGTAC R: AAAAAGCCTGTCTGTGATTCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Fan, J.; Zhao, L.; Qu, Z.; Dong, Y.; Wu, Y.; Gu, S. Weizmannia coagulans BC99 Improve Cognitive Impairment Induced by Chronic Sleep Deprivation via Inhibiting the Brain and Intestine’s NLRP3 Inflammasome. Foods 2025, 14, 989. https://doi.org/10.3390/foods14060989
Sun Q, Fan J, Zhao L, Qu Z, Dong Y, Wu Y, Gu S. Weizmannia coagulans BC99 Improve Cognitive Impairment Induced by Chronic Sleep Deprivation via Inhibiting the Brain and Intestine’s NLRP3 Inflammasome. Foods. 2025; 14(6):989. https://doi.org/10.3390/foods14060989
Chicago/Turabian StyleSun, Qiaoqiao, Jiajia Fan, Lina Zhao, Zhen Qu, Yao Dong, Ying Wu, and Shaobin Gu. 2025. "Weizmannia coagulans BC99 Improve Cognitive Impairment Induced by Chronic Sleep Deprivation via Inhibiting the Brain and Intestine’s NLRP3 Inflammasome" Foods 14, no. 6: 989. https://doi.org/10.3390/foods14060989
APA StyleSun, Q., Fan, J., Zhao, L., Qu, Z., Dong, Y., Wu, Y., & Gu, S. (2025). Weizmannia coagulans BC99 Improve Cognitive Impairment Induced by Chronic Sleep Deprivation via Inhibiting the Brain and Intestine’s NLRP3 Inflammasome. Foods, 14(6), 989. https://doi.org/10.3390/foods14060989






