Scald Fermentation Time as a Factor Determining the Nutritional and Sensory Quality of Rye Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
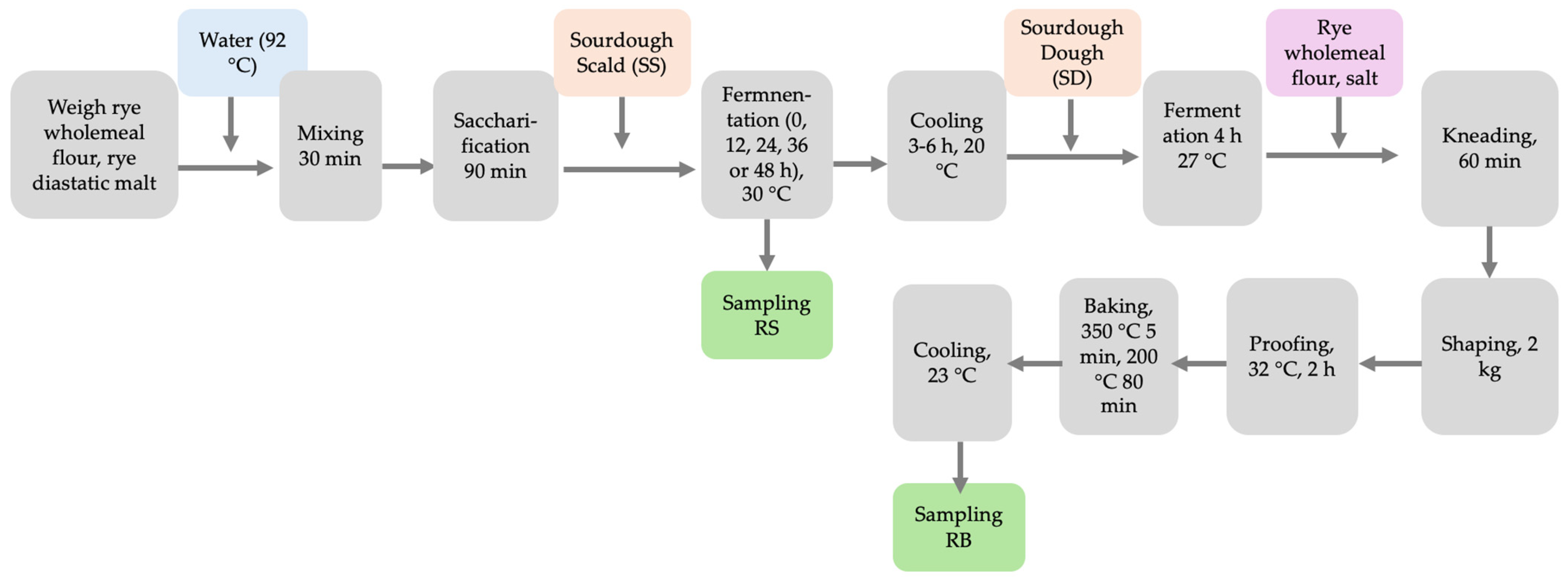
2.2. Preparation and Fermentation of Rye Scald Samples
2.3. Dough Preparation and Bread Baking
2.4. Lyophilization of Scald and Bread Samples
2.5. LAB and Yeast Identification in Sourdough Scald and Fermented Scald
2.6. Lactic Acid Bacteria and Yeast Enumeration in Scald
2.7. Determination of Physicochemical Parameters of Scald and Bread
2.8. Determination of Sugar Profile in Scald and Bread Samples
2.9. Determination of Organic Acids in Scald and Bread Samples
2.10. Quantification of Phytic Acid
2.11. Quantification of Fructans
2.12. Sensory Evaluation of Bread
2.13. Statistical Analysis
3. Results
3.1. Effect of Fermentation Time on Scald and Bread Properties
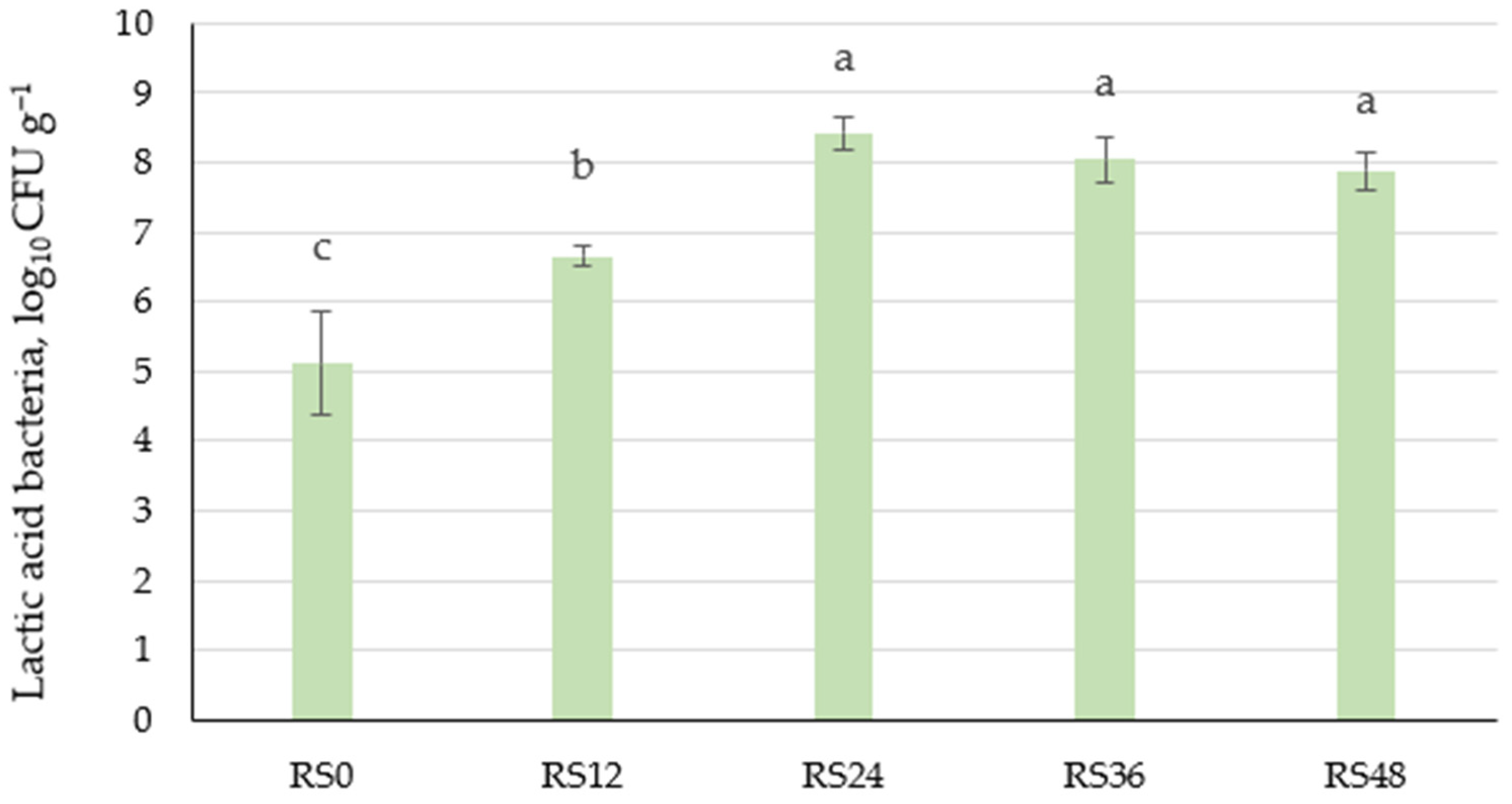
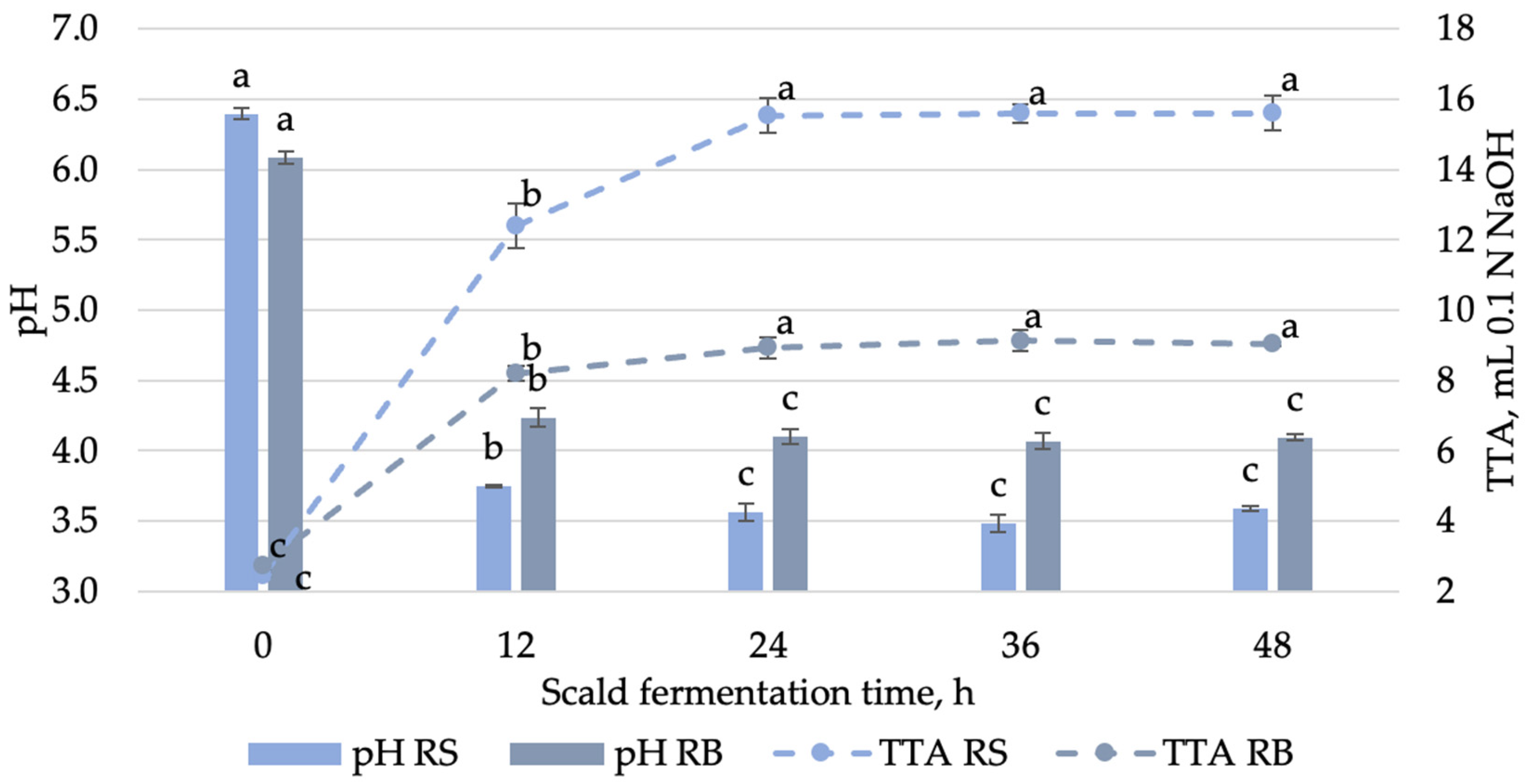
3.1.1. Changes in Microbial and Physicochemical Characteristics of Scald During Fermentation
3.1.2. Effect of Fermentation Time on Organic Acid Profile in Rye Scald and Rye Bread
3.1.3. Effect of Fermentation Time on Rye Scald and Rye Bread Sugar Profile
3.1.4. Effect of Fermentation Time on Phytic Acid Content in Rye Scald and Rye Bread
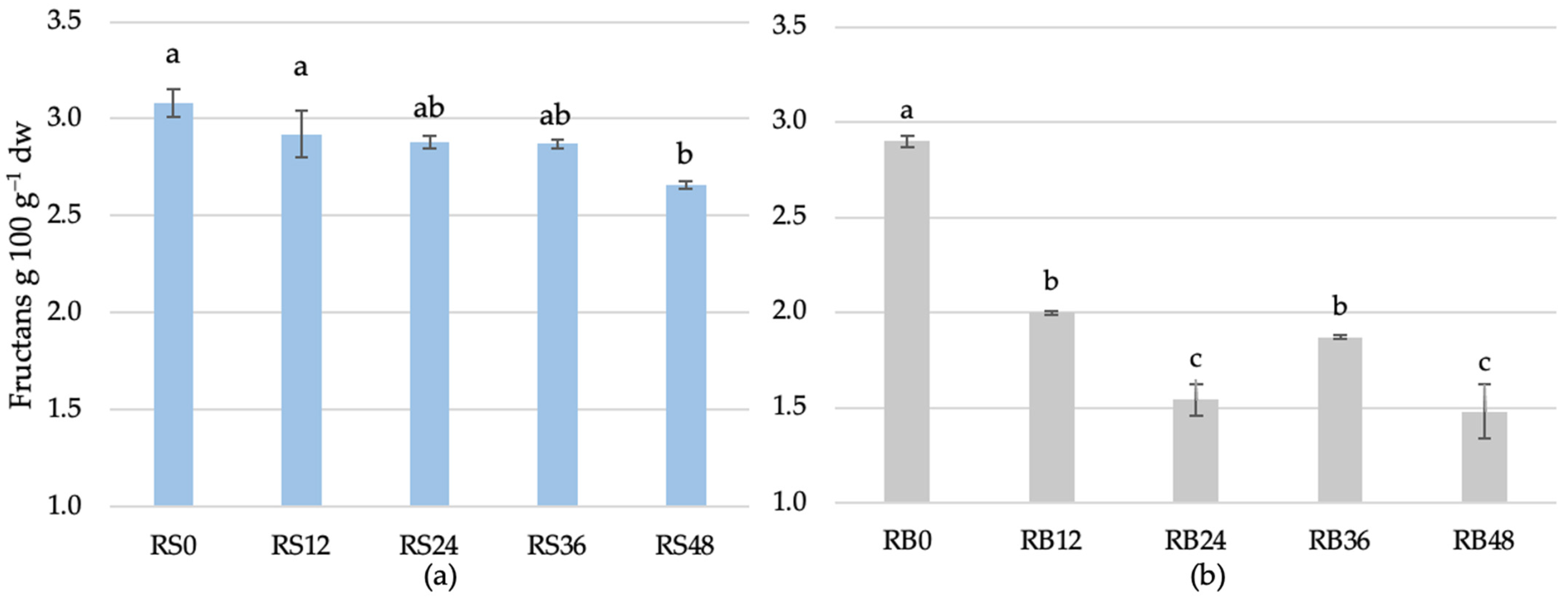
3.1.5. Effect of Fermentation Time on Fructan Content in Rye Scald and Rye Bread
3.2. Sensory Evaluation of Bread with Scald Fermented for Different Durations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonsson, K.; Andersson, R.; Bach Knudsen, K.E.; Hallmans, G.; Hanhineva, K.; Katina, K.; Kolehmainen, M.; Kyrø, C.; Langton, M.; Nordlund, E.; et al. Rye and Health—Where Do We Stand and Where Do We Go? Trends Food Sci. Technol. 2018, 79, 78–87. [Google Scholar] [CrossRef]
- Poutanen, K.; Katina, K.; Heiniö, R.-L. Rye. In Bakery Products Science and Technology; Zhou, W., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 75–87. ISBN 9781118792001. [Google Scholar]
- Kamal-Eldin, A.; Åman, P.; Zhang, J.X.; Bach Knudsen, K.E.; Poutanen, K. Rye Bread and Other Rye Products. In Technology of Functional Cereal Products; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 233–260. ISBN 9781845691776. [Google Scholar]
- Deleu, L.J.; Lemmens, E.; Redant, L.; Delcour, J.A. The Major Constituents of Rye (Secale cereale L.) Flour and Their Role in the Production of Rye Bread, a Food Product to Which a Multitude of Health Aspects Are Ascribed. Cereal Chem. 2020, 97, 739–754. [Google Scholar] [CrossRef]
- Klava, D.; Kunkulberga, D.; Duka, G.; Murniece, R. Traditional Latvian Sourdough Rye Bread; Garcia-Vaguero, M., Pastor, K., Orhun, G.E., McElhatton, A., Rocha, J.M.F., Eds.; Springer Nature AG: Cham, Switzerland, 2023; ISBN 9783031233517. [Google Scholar]
- Murniece, R.; Reidzane, S.; Radenkovs, V.; Matisons, R.; Dabina-Bicka, I.; Klava, D.; Galoburda, R. Impact of Rye Malt with Various Diastatic Activity on Wholegrain Rye Flour Rheology and Sugar Formation in Scalding and Fermentation Processes. Foods 2024, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Klupsaite, D.; Starkute, V.; Zokaityte, E.; Cernauskas, D.; Mockus, E.; Kentra, E.; Sliazaite, R.; Abramaviciute, G.; Sakaite, P.; Komarova, V.; et al. The Contribution of Scalded and Scalded-Fermented Rye Wholemeal Flour to Quality Parameters and Acrylamide Formation in Semi-Wheat-Rye Bread. Foods 2023, 12, 937. [Google Scholar] [CrossRef]
- Christina Müller, D.; Nguyen, H.; Li, Q.; Schönlechner, R.; Miescher Schwenninger, S.; Wismer, W.; Gänzle, M. Enzymatic and Microbial Conversions to Achieve Sugar Reduction in Bread. Food Res. Int. 2021, 143, 110296. [Google Scholar] [CrossRef]
- Murniece, R.; Reidzane, S.; Galoburda, R.; Radenkovs, V.; Klava, D. The Impact of Fermented Scald on Rye and Hull-Less Barley Dough and Bread Structure Formation. Foods 2023, 12, 4475. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J.; Stankevicius, M.; Maruska, A. Thermophilic Lactic Acid Bacteria Affect the Characteristics of Sourdough and Whole-Grain Wheat Bread. Food Biosci. 2020, 38, 100791. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli—Do They Matter for Microbial Ecology and Bread Quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial Ecology and Process Technology of Sourdough Fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar] [CrossRef]
- Pontonio, E.; Arora, K.; Dingeo, C.; Carafa, I.; Celano, G.; Scarpino, V.; Genot, B.; Gobbetti, M.; Di Cagno, R. Commercial Organic Versus Conventional Whole Rye and Wheat Flours for Making Sourdough Bread: Safety, Nutritional, and Sensory Implications. Front. Microbiol. 2021, 12, 674413. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Impact of Sourdough Fermentation on Nutrient Transformations in Cereal-Based Foods: Mechanisms, Practical Applications, and Health Implications. Grain Oil Sci. Technol. 2024, 7, 124–132. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and Cereal Fermentation in a Nutritional Perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; di Palo, D.M.; Lorusso, M.P.; de Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough Fermented Breads Are More Digestible than Those Started with Baker’s Yeast Alone: An in Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Harasym, J. Enhancing Antioxidant Activity and Modulating FODMAPs Levels in Inoculated Sourdough Rye Bread. LWT 2024, 197, 115905. [Google Scholar] [CrossRef]
- Bohn, T.; Davidsson, L.; Walczyk, T.; Hurrell, R.F. Phytic Acid Added to White-Wheat Bread Inhibits Fractional Apparent Magnesium Absorption in Humans. Am. J. Clin. Nutr. 2004, 79, 418–423. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food Fermentations for Improved Digestibility of Plant Foods—An Essential Ex Situ Digestion Step in Agricultural Societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Weaver, C.M.; Kannan, S. Phytate and Mineral Bioavailability. In Food Phytates; Reddy, N.R., Sathe, S.K., Eds.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781420014419. [Google Scholar]
- Kumar, A.; Dash, G.K.; Sahoo, S.K.; Lal, M.K.; Sahoo, U.; Sah, R.P.; Ngangkham, U.; Kumar, S.; Baig, M.J.; Sharma, S.; et al. Phytic Acid: A Reservoir of Phosphorus in Seeds Plays a Dynamic Role in Plant and Animal Metabolism. Phytochem. Rev. 2023, 22, 1281–1304. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Damstrup, M.L.; Dal Thomsen, A.; Rasmussen, S.K.; Hansen, Å. Phytase Activity and Degradation of Phytic Acid during Rye Bread Making. Eur. Food Res. Technol. 2007, 225, 173–181. [Google Scholar] [CrossRef]
- Mikulski, D.; Klosowski, G. Phytic Acid Concentration in Selected Raw Materials and Analysis of Its Hydrolysis Rate with the Use of Microbial Phytases during the Mashing Process. J. Inst. Brew. 2015, 121, 213–218. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional Applications of Lactic Acid Bacteria: Enhancing Safety, Quality, and Nutritional Value in Foods and Fermented Beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Nuobariene, L.; Cizeikiene, D.; Gradzeviciute, E.; Hansen, Å.S.; Rasmussen, S.K.; Juodeikiene, G.; Vogensen, F.K. Phytase-Active Lactic Acid Bacteria from Sourdoughs: Isolation and Identification. LWT 2015, 63, 766–772. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.A.; Chanliaud, E.; Rémésy, C. Moderate Decrease of PH by Sourdough Fermentation Is Sufficient to Reduce Phytate Content of Whole Wheat Flour through Endogenous Phytase Activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Abrahmsohn, O.; David, G.J.P.; Staudacher, H.; Irving, P.; Lomer, M.C.E.; Ellis, P.R. Fructan Content of Commonly Consumed Wheat, Rye and Gluten-Free Breads. Int. J. Food Sci. Nutr. 2011, 62, 498–503. [Google Scholar] [CrossRef]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and Immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Loponen, J.; Gänzle, M.G. Use of Sourdough in Low FODMAP Baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Pejcz, E.; Spychaj, R.; Gil, Z. Technological Methods for Reducing the Content of Fructan in Rye Bread. Eur. Food Res. Technol. 2020, 246, 1839–1846. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Molognoni, L.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; Dea Lindner, J. De Use of Sourdough Fermentation to Reducing FODMAPs in Breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; De Marco, I.; Neves Oliveira dos Santos, N.; Costa Nunes, C.; Leite Cartabiano, C.E.; Molognoni, L.; Pereira, G.V.d.M.; Daguer, H.; De Dea Lindner, J. Reducing FODMAPs and Improving Bread Quality Using Type II Sourdough with Selected Starter Cultures. Int. J. Food Sci. Nutr. 2021, 72, 912–922. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Ellilä, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP Content by Bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. [Google Scholar] [CrossRef]
- Reidzane, S.; Kruma, Z.; Kazantseva, J.; Traksmaa, A.; Klava, D. Determination of Technological Parameters and Characterization of Microbiota of the Spontaneous Sourdough Fermentation of Hull-Less Barley. Foods 2021, 10, 2253. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F. Culture-Dependent Estimation of Lactic Acid Bacteria and Yeasts. In Basic Methods and Protocols on Sourdough; Gobbetti, M., Rizzello, C.G., Eds.; Springer: New York, NY, USA, 2024; pp. 17–27. ISBN 978-1-0716-3706-7. [Google Scholar]
- AACC 02-31.01; Titratable Acidity. AACC: St. Paul, MN, USA, 2000.
- AACC 02-52:1999; Hydrogen-Ion Activity (PH)—Electrometric Method. Approved Methods of the American Association of Cereal Chemists: St. Paul, MN, USA, 2000.
- Radenkovs, V.; Krasnova, I.; Cinkmanis, I.; Juhnevica-Radenkova, K.; Rubauskis, E.; Seglina, D. Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent. Horticulturae 2024, 10, 1362. [Google Scholar] [CrossRef]
- Mantzourani, I.; Daoutidou, M.; Terpou, A.; Plessas, S. Novel Formulations of Sourdough Bread Based on Supplements Containing Chokeberry Juice Fermented by Potentially probiotic L. Paracasei SP5. Foods 2024, 13, 4031. [Google Scholar] [CrossRef]
- AACC 32-32.01; Measurement of Total Fructan in Foods by an Enzymatic/Spectrophotometric Method. AACC: St. Paul, MN, USA, 2000.
- ISO 8589:2007; Sensory Analysis-General Guidance for Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- ISO 4121; Sensory Analysis-Guidelines for the Use of Quantitative Response Scales. ISO: Geneva, Switzerland, 2003.
- Kozlinskis, E.; Skudra, L.; Rakcejeva, T.; Kunkulberga, D. Changes in the Chemical and Microbiological Properties of Spontaneous Rye Sourdough during Fermentation. LLU Raksti 2010, 25, 67–77. [Google Scholar]
- Kulp, K.; Lorenz, K. Handbook of Dough Fermentations; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0824742648. [Google Scholar]
- Li, H.F.; Dai, F.J.; Zhang, L.L.; Li, Z.J. Characterization of Scalded Dough and Its Impact on the Growth of Mixed Yeasts Originating from Jiaozi. Food Biosci. 2022, 49, 101920. [Google Scholar] [CrossRef]
- Khakimov, B.; Jespersen, B.M.; Engelsen, S.B. Comprehensive and Comparative Metabolomic Profiling of Wheat, Barley, Oat and Rye Using Gas Chromatography-Mass Spectrometry and Advanced Chemometrics. Foods 2014, 3, 569–585. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Fernandes, Â.; Pinela, J.; Dias, M.I.; Pereira, C.; Pires, T.C.S.P.; Carocho, M.; Vasallo, E.F.; Ferreira, I.C.F.R.; Barros, L. Valorization of Cereal By-Products from the Milling Industry as a Source of Nutrients and Bioactive Compounds to Boost Resource-Use Efficiency. Agronomy 2021, 11, 972. [Google Scholar] [CrossRef]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces Marxianus, Lactobacillus Delbrueckii Ssp. Bulgaricus and L. Helveticus for Sourdough Bread Making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- Pérez-Alvarado, O.; Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; Vinderola, G.; García-Cayuela, T. Role of Lactic Acid Bacteria and Yeasts in Sourdough Fermentation during Breadmaking: Evaluation of Postbiotic-like Components and Health Benefits. Front. Microbiol. 2022, 13, 969460. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e0049421. [Google Scholar] [CrossRef] [PubMed]
- Ravyts, F.; De Vuyst, L. Prevalence and Impact of Single-Strain Starter Cultures of Lactic Acid Bacteria on Metabolite Formation in Sourdough. Food Microbiol. 2011, 28, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Sugar Reduction in Bakery Products: Current Strategies and Sourdough Technology as a Potential Novel Approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef]
- Haros, M.; Bielecka1, M.; Honke, J.; Sanz, Y. Phytate-Degrading Activity in Lactic Acid Bacteria. Pol. J. Food Nutr. Sci. 2008, 58, 33–40. [Google Scholar]
- Reale, A.; Mannina, L.; Tremonte, P.; Sobolev, A.P.; Succi, M.; Sorrentino, E.; Coppola, R. Phytate Degradation by Lactic Acid Bacteria and Yeasts during the Wholemeal Dough Fermentation: A 31P NMR Study. J. Agric. Food Chem. 2004, 52, 6300–6305. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Guy, G.; Messager, A.; Demigne, C.; Remesy, C. Prolonged Fermentation of Whole Wheat Sourdough Reduces Phytate Level and Increases Soluble Magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Afzal, M.; Pfannstiel, J.; Bertsche, U.; Melzer, T.; Ruf, A.; Heger, C.; Pfaff, T.; Schollenberger, M.; Rodehutscord, M. Mineral and Phytic Acid Content as Well as Phytase Activity in Flours and Breads Made from Different Wheat Species. Int. J. Mol. Sci. 2023, 24, 2770. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Arici, M. Effect of the Fermentation Temperature on the Degradation of Phytic Acid in Whole-Wheat Sourdough Bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Brearley, C.A.; Bruggraber, S.F.A.; Perfecto, A.; Shewry, P.; Fairweather-Tait, S. Assessment of Iron Bioavailability from Different Bread Making Processes Using an In Vitro Intestinal Cell Model. Food Chem. 2017, 228, 91–98. [Google Scholar] [CrossRef]
- Karppinen, S.; Myllymäki, O.; Forssell, P.; Poutanen, K. Fructan Content of Rye and Rye Products. Cereal Chem. 2003, 80, 168–171. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and Molecular-Weight Distribution of Dietary Fiber Components in Whole-Grain Rye Flour and Bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Loponen, J.; Gänzle, M.G. Characterization of the Extracellular Fructanase FruA in Lactobacillus Crispatus and Its Contribution to Fructan Hydrolysis in Breadmaking. J. Agric. Food Chem. 2020, 68, 8637–8647. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, M.; Yebra, M.J.; Monedero, V. Complex Oligosaccharide Utilization Pathways in Lactobacillus. Curr. Issues Mol. Biol. 2020, 40, 49–80. [Google Scholar] [CrossRef]
- Acín Albiac, M.; Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. How Fructophilic Lactic Acid Bacteria May Reduce the FODMAPs Content in Wheat-Derived Baked Goods: A Proof of Concept. Microb. Cell Fact. 2020, 19, 182. [Google Scholar] [CrossRef]
- Koj, K.; Pejcz, E. Rye Dietary Fiber Components upon the Influence of Fermentation Inoculated with Probiotic Microorganisms. Molecules 2023, 28, 1910. [Google Scholar] [CrossRef]
- Takagi, R.; Tsujikawa, Y.; Nomoto, R.; Osawa, R. Comparison of the Growth of Lactobacillus Delbrueckii, L. Paracasei and L. Plantarum on Inulin in Co-Culture Systems. Biosci. Microbiota, Food Health 2014, 33, 139–146. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces Cerevisiae and Kluyveromyces Marxianus Cocultures Allow Reduction of Fermentable Oligo-, Di-, and Monosaccharides and Polyols Levels in Whole Wheat Bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP Contents of Common Wheat and Rye Breads and the Effects of Processing on the Final Contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]



| Parameter | Sourdough Scald | Sourdough Dough |
|---|---|---|
| pH | 3.5 ± 0.0 | 3.7 ± 0.0 |
| TTA, mL 0.1 N NaOH | 16.7 ± 0.2 | 17.7 ± 0.6 |
| LAB, log10 CFU g−1 | 8.9 ± 0.1 | 8.6 ± 0.1 |
| Yeasts, log10 CFU g−1 | - | 7.1 ± 0.3 |
| Ingredients, kg | Scald | Dough |
|---|---|---|
| Rye wholemeal flour | 6.00 | 8.00 |
| Rye diastatic malt | 0.06 | - |
| Water (92 °C) | 9.00 | - |
| Sourdough Scald | 0.12 | - |
| Sourdough Dough | - | 0.40 |
| Salt | - | 0.11 |
| Scald | - | 15.18 |
| Total | 15.18 | 23.69 |
| Organic Acids, g 100 g−1 dw | Scald Samples | ||||
|---|---|---|---|---|---|
| RS0 | RS12 | RS24 | RS36 | RS48 | |
| Lactic acid | 0.27 ± 0.01 c | 2.83 ± 0.07 b | 3.74 ± 0.16 a | 3.85 ± 0.03 a | 3.72 ± 0.32 a |
| Acetic acid | 0.03 ± 0.01 b | 0.12 ± 0.02 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Propionic acid | ND | ND | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Butyric acid | 0.09 ± 0.02 b | 0.18 ± 0.01 a | 0.01 ± 0.00 c | 0.01 ± 0.00 c | 0.01 ± 0.00 c |
| Succinic acid | 0.07 ± 0.01 b | 0.16 ± 0.01 a | 0.14 ± 0.00 a | 0.14 ± 0.02 a | 0.13 ± 0.01 a |
| Oxalic acid | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.02 a |
| Quinic acid | 0.55 ± 0.03 a | 0.76 ± 0.07 a | 0.72 ± 0.16 a | 0.78 ± 0.13 a | 0.74 ± 0.21 a |
| Tartaric acid | 0.21 ± 0.02 a | 0.25 ± 0.03 a | 0.24 ± 0.06 a | 0.28 ± 0.06 a | 0.25 ± 0.06 a |
| Malic acid | 0.20 ± 0.01 a | 0.12 ± 0.01 b | 0.11 ± 0.00 b | 0.10 ± 0.01 b | 0.11 ± 0.01 b |
| TOTAL | 1.45 ± 0.04 b | 4.46 ± 0.16 a | 5.01 ± 0.40 a | 5.22 ± 0.20 a | 5.02 ± 0.02 a |
| Organic Acids, g 100 g−1 dw | Bread Samples | ||||
|---|---|---|---|---|---|
| RB0 | RB12 | RB24 | RB36 | RB48 | |
| Lactic acid | 0.81 ± 0.0 6 c | 1.54 ± 0.04 b | 1.91 ± 0.13 ab | 2.06 ± 0.12 a | 2.00 ± 0.13 ab |
| Acetic acid | 0.23 ± 0.03 a | 0.17 ± 0.03 a | 0.19 ± 0.01 a | 0.16 ± 0.02 a | 0.21 ± 0.03 a |
| Propionic acid | 0.08 ± 0.02 a | 0.01 ± 0.00 a | 0.07 ± 0.03 a | 0.06 ± 0.02 a | 0.04 ± 0.03 a |
| Butyric acid | 0.41 ± 0.05 a | 0.29 ± 0.01 a | 0.36 ± 0.03 a | 0.30 ± 0.01 a | 0.38 ± 0.03 a |
| Succinic acid | 0.03 ± 0.01 b | 0.13 ± 0.01 a | 0.16 ± 0.00 a | 0.15 ± 0.00 a | 0.17 ± 0.03 a |
| Oxalic acid | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a |
| Quinic acid | 0.84 ± 0.08 a | 0.77 ± 0.23 a | 0.82 ± 0.16 a | 0.86 ± 0.11 a | 0.84 ± 0.17 a |
| Tartaric acid | 0.24 ± 0.05 a | 0.19 ± 0.05 a | 0.20 ± 0.04 a | 0.20 ± 0.01 a | 0.21 ± 0.03 a |
| Malic acid | 0.27 ± 0.02 b | 0.30 ± 0.03 ab | 0.34 ± 0.03 ab | 0.36 ± 0.02 a | 0.35 ± 0.01 ab |
| TOTAL | 2.94 ± 0.10 b | 3.43 ± 0.33 ab | 4.08 ± 0.31 ab | 4.17 ± 0.20 a | 4.23 ± 0.40 a |
| Sugars, g 100 g−1 dw | Scald Samples | ||||
|---|---|---|---|---|---|
| RS0 | RS12 | RS24 | RS36 | RS48 | |
| Fructose | 0.40 ± 0.01 | ND | ND | ND | ND |
| Glucose | 0.99 ± 0.05 ab | 0.72 ± 0.08 b | 0.87 ± 0.15 ab | 0.88 ± 0.01 ab | 1.08 ± 0.01 a |
| Sucrose | 1.01 ± 0.09 a | 0.50 ± 0.03 b | 0.31 ± 0.04 bc | 0.36 ± 0.02 bc | 0.25 ± 0.01 c |
| Maltose | 25.33 ± 0.62 b | 27.56 ± 0.12 a | 26.6 ± 0.15 ab | 27.31 ± 1.06 ab | 26.99 ± 0.03 ab |
| Unknown | 3.05 ± 0.13 b | 4.47 ± 0.40 a | 4.86 ± 0.21 a | 4.89 ± 0.32 a | 4.21 ± 0.34 a |
| TOTAL | 30.78 ± 0.09 b | 33.25 ± 0.63 a | 32.64 ± 0.54 ab | 33.44 ± 1.42 ab | 33.53 ± 0.39 ab |
| Sugars, g 100 g−1 dw | Bread Samples | ||||
|---|---|---|---|---|---|
| RB0 | RB12 | RB24 | RB36 | RB48 | |
| Fructose | 0.37 ± 0.04 b | 0.63 ± 0.01 a | 0.89 ± 0.01 a | 0.57 ± 0.02 a | 0.67 ± 0.01 a |
| Glucose | 0.49 ± 0.06 ab | 0.45 ± 0.03 a | 0.46 ± 0.01 ab | 0.56 ± 0.01 ab | 0.43 ± 0.03 b |
| Sucrose | 0.50 ± 0.05 a | 0.32 ± 0.06 b | 0.31 ± 0.05 bc | 0.37 ± 0.06 bc | 0.30 ± 0.03 c |
| Maltose | 13.32 ± 0.14 a | 12.62 ± 0.15 b | 12.20 ± 0.18 ab | 12.11 ± 0.12 ab | 12.12 ± 0.27 ab |
| Unknown | 1.33 ± 0.01 b | 2.13 ± 0.10 a | 2.03 ± 0.22 a | 2.27 ± 0.05 a | 2.23 ± 0.10 a |
| TOTAL | 16.58 ± 0.42 a | 16.82 ± 0.37 a | 16.56 ± 0.47 a | 16.54 ± 0.26 a | 16.41 ± 0.45 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murniece, R.; Reidzane, S.; Radenkovs, V.; Straumite, E.; Keke, A.; Kobrin, E.-G.; Klava, D. Scald Fermentation Time as a Factor Determining the Nutritional and Sensory Quality of Rye Bread. Foods 2025, 14, 979. https://doi.org/10.3390/foods14060979
Murniece R, Reidzane S, Radenkovs V, Straumite E, Keke A, Kobrin E-G, Klava D. Scald Fermentation Time as a Factor Determining the Nutritional and Sensory Quality of Rye Bread. Foods. 2025; 14(6):979. https://doi.org/10.3390/foods14060979
Chicago/Turabian StyleMurniece, Ruta, Sanita Reidzane, Vitalijs Radenkovs, Evita Straumite, Anete Keke, Eeva-Gerda Kobrin, and Dace Klava. 2025. "Scald Fermentation Time as a Factor Determining the Nutritional and Sensory Quality of Rye Bread" Foods 14, no. 6: 979. https://doi.org/10.3390/foods14060979
APA StyleMurniece, R., Reidzane, S., Radenkovs, V., Straumite, E., Keke, A., Kobrin, E.-G., & Klava, D. (2025). Scald Fermentation Time as a Factor Determining the Nutritional and Sensory Quality of Rye Bread. Foods, 14(6), 979. https://doi.org/10.3390/foods14060979









