Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. UPLC-IM-QTOF MS Conditions
2.3. Sample Preparation
3. Results and Discussion
3.1. Database Construction for Hazardous Substances with Regulatory Differences
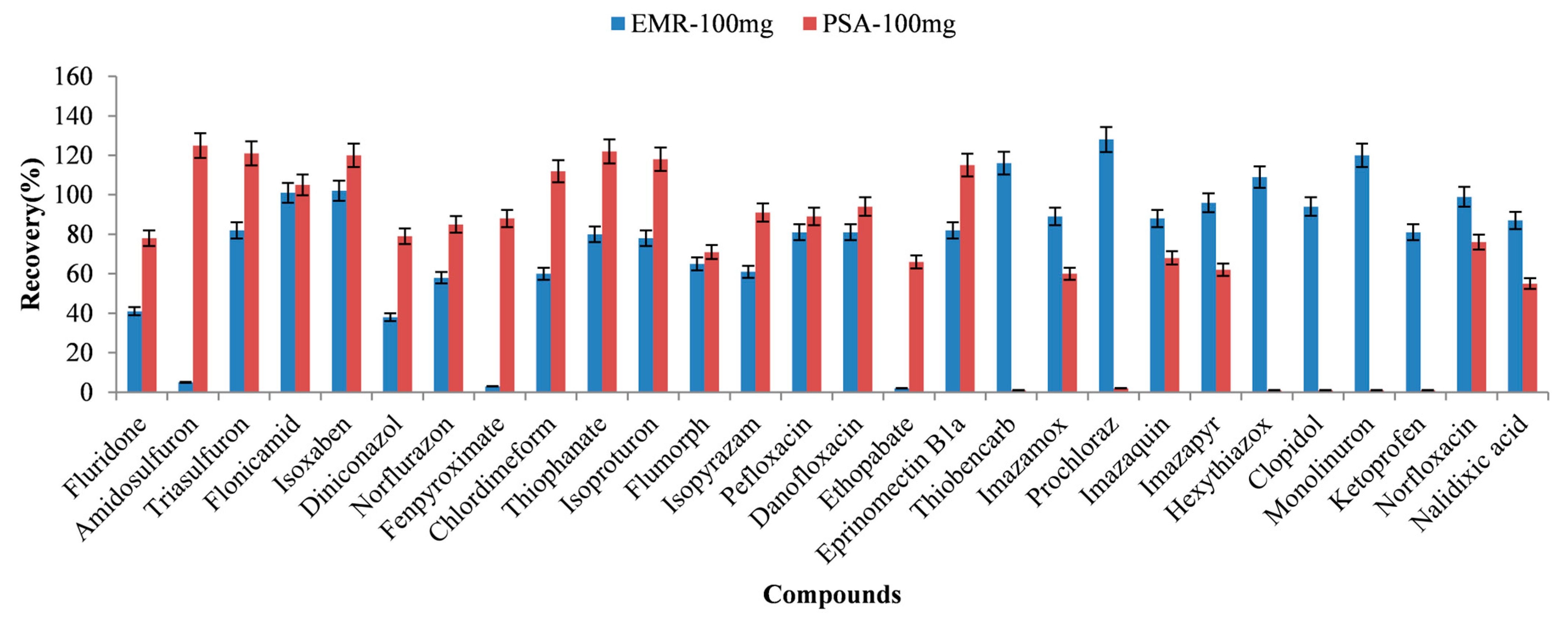
3.2. Optimization of Sample Preparation
3.2.1. Extraction Solvent
3.2.2. Volume of Extraction Solvent
3.2.3. Purified Sorbents
3.2.4. The Amount of Purified Sorbents
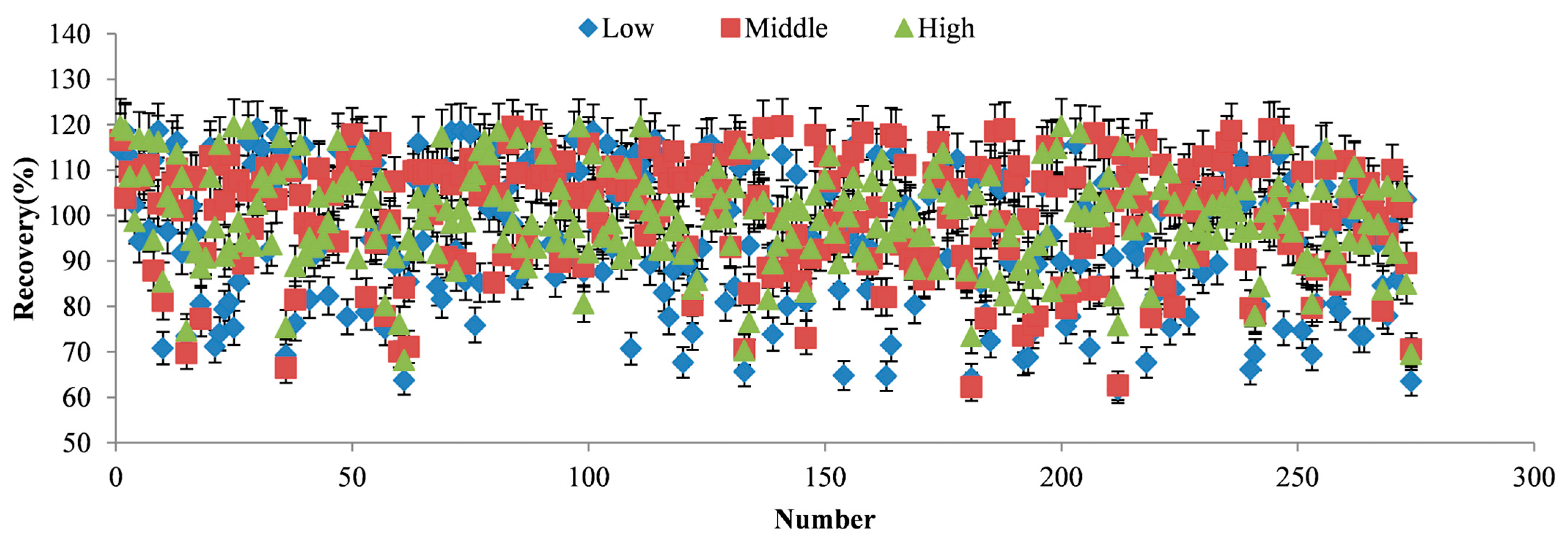
3.3. Method Validation
3.4. Detection of the Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Li, R.; Li, M.; Wang, Y.; Wang, J.; Yuan, R. Evaluation of the effects of Chinese Dietary Guidelines on nutrition, environment, and cost. Chin. J. Popul. Resour. Environ. 2024, 22, 515–525. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Zhu, X. The evolution process, characteristics and adjustment of Chinese dietary guidelines: A global perspective. Resour. Conserv. Recycl. 2023, 193, 106964. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Residues of pesticides and veterinary drugs in diets of dairy cattle from conventional and organic farms in Austria. Environ. Pollut. 2023, 316, 120626. [Google Scholar] [CrossRef] [PubMed]
- Calahorrano-Moreno, M.B.; Ordoñez-Bailon, J.J.; Baquerizo-Crespo, R.J.; Dueñas-Rivadeneira, A.A.; Montenegro, M.C.B.; Rodríguez-Díaz, J.M. Contaminants in the cow’s milk we consume? Pasteurization and other technologies in the elimination of contaminants. F1000 Res. 2022, 11, 91. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Boussadia, M.I.; Gueroui, Y.; Habila, S.; Bousbia, A.; Symeon, G.K. Pesticide residues levels in raw cow’s milk and health risk assessment across the globe: A systematic review. Environ. Adv. 2022, 9, 100266. [Google Scholar] [CrossRef]
- Rahmani, J.; Alipour, S.; Miri, A.; Fakhri, Y.; Riahi, S.-M.; Keramati, H.; Moradi, M.; Amanidaz, N.; Pouya, R.H.; Bahmani, Z.; et al. The prevalence of aflatoxin M1 in milk of Middle East region: A systematic review, meta-analysis and probabilistic health risk assessment. Food Chem. Toxicol. 2018, 118, 653–666. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2022 European Union report on pesticide residues in food. EFSA J. 2024, 22, e8753. [Google Scholar]
- Shingal, A.; Ehrich, M. The EU’s pesticides MRLs harmonization: Effect on trade, prices and quality. Food Policy 2024, 125, 102634. [Google Scholar] [CrossRef]
- Traoré, O.Z.; Tamini, L.D. African trade of mangoes to OECD countries: Disentangling the effects of compliance with maximum residue limits on production, export supply and import demand. Eur. Rev. Agric. Econ. 2022, 49, 383–432. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade impact of maximum residue limits in fresh fruits and vegetables. Food Policy 2022, 106, 102203. [Google Scholar] [CrossRef]
- Fiankor, D.D.D.; Dalheimer, B.; Mack, G. Pesticide regulatory heterogeneity, foreign sourcing, and global agricultural value chains. Am. J. Agric. Econ. 2024, 107, 611–634. [Google Scholar] [CrossRef]
- Xiong, B.; Beghin, J.C. Stringent Maximum Residue Limits, Protectionism, and Competitiveness: The Cases of the US and Canada. In Nontariff Measures with Market Imperfections: Trade and Welfare Implications; International Agricultural Trade Research Consortium, Emerald Press: Davis, CA, USA, 2012. [Google Scholar]
- Okunola, A.; Dennis, E.; Beghin, A.J. Are Veterinary Drug Maximum Residue Limits Protectionist? International Evidence. Agricultural Economics Staff Paper, Research in Agricultural & Applied Economics. 2024. Available online: https://ageconsearch.umn.edu/record/342915/?v=pdf (accessed on 2 January 2025).
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Shabeer, T.A.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Mahdavi, V.; Gordan, H.; Rezadoost, H.; Conti, G.O.; Khaneghah, A.M. Determination of multi-class pesticides residues of cow and human milk samples from Iran using UHPLC-MS/MS and GC-ECD: A probabilistic health risk assessment. Environ. Res. 2022, 208, 112730. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Chen, J.; Pang, X.; Yang, L.; Fan, Y.; Yang, Q.; Chen, A. High-sensitive detection of organophosphate and carbamate pesticide residues in milk based on bioluminescence method. Food Control 2025, 168, 110933. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Yu, Z.-N.; Ho, H.; Wang, J.; Wang, Y.-T.; Fan, R.-B.; Han, R.-W. Analysis of Veterinary Drug Residues in Pasteurized Milk Samples in Chinese Milk Bars. J. Food Prot. 2020, 83, 204–210. [Google Scholar] [CrossRef]
- Shi, R.; Yu, Z.; Wu, W.; Ho, H.; Wang, J.; Wang, Y.; Han, R. A Survey of 61 Veterinary Drug Residues in Commercial Liquid Milk Products in China. J. Food Prot. 2020, 83, 1227–1233. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, W.; Xu, X.; Han, Y.; Jie, M.; Xu, G.; Bai, Y. Development of a modified quick, easy, cheap, effective, rugged, and safe method based on melamine sponge for multi-residue analysis of veterinary drugs in milks by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1651, 462333. [Google Scholar] [CrossRef]
- Melekhin, A.; Tolmacheva, V.; Goncharov, N.; Apyari, V.; Dmitrienko, S.; Shubina, E.; Grudev, A. Multi-class, multi-residue determination of 132 veterinary drugs in milk by magnetic solid-phase extraction based on magnetic hypercrosslinked polystyrene prior to their determination by high-performance liquid chromatography—Tandem mass spectrometry. Food Chem. 2022, 387, 132866. [Google Scholar] [CrossRef]
- Khaled, O.; Ryad, L.; Nagi, M.; Eissa, F. Multiclass method for detecting 41 antibiotic residues in bovine liver, muscle, and milk using LC-Q-Orbitrap-HRMS. J. Food Compos. Anal. 2024, 132, 106299. [Google Scholar] [CrossRef]
- Goessens, T.; Mouchtaris-Michailidis, T.; Tesfamariam, K.; Truong, N.; Vertriest, F.; Bader, Y.; De Saeger, S.; Lachat, C.; De Boevre, M. Dietary mycotoxin exposure and human health risks: A protocol for a systematic review. Environ. Int. 2024, 184, 108456. [Google Scholar] [CrossRef]
- Yadav, K.; Moovendaran, K.; Dhenadhayalan, N.; Lee, S.-F.; Leung, M.-K.; Sankar, R. From food toxins to biomarkers: Multiplexed detection of aflatoxin B1 and aflatoxin M1 in milk and human serum using PEGylated ternary transition metal sulfides. Sens. Actuators Rep. 2023, 5, 100156. [Google Scholar] [CrossRef]
- Kenjeric, L.; Sulyok, M.; Malachova, A.; Greer, B.; Kolawole, O.; Quinn, B.; Elliott, C.T.; Krska, R. Extention and interlaboratory comparison of an LC-MS/MS multi-class method for the determination of 15 different classes of veterinary drug residues in milk and poultry feed. Food Chem. 2024, 449, 138834. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lan, H.; Wu, Z.; Pan, D.; Wu, Y. Two-dimensional metal-organic framework nanosheets coated solid-phase microextraction Arrow coupled with UPLC-Q-ToF-MS for the determination of three veterinary residues in milk and pork. J. Chromatogr. A 2024, 1736, 465373. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Wang, Z.; Chen, J.; Xie, J.W. Development and validation of a QuEChERS-HPLC-DAD method using polymer-functionalized melamine sponges for the analysis of antipsychotic drugs in milk. Food Chem. 2024, 444, 138553. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, M.; Hou, F.; He, Y.; Zhu, Y.; Dai, H.; Huang, M.; Yang, Y.; Wu, L. Multi-residue analysis of pesticides and veterinary drugs by ultra-performance liquid chromatography-tandem mass spectrometry using a modified QuEChERS method. Microchem. J. 2025, 208, 112384. [Google Scholar] [CrossRef]
- GB 2763-2019; National Food Safety Standard Maximum Residue Limits for Pesticides in Food of China. Standards Press of China: Beijing, China, 2019.
- Belova, L.; Caballero-Casero, N.; van Nuijs, A.L.; Covaci, A. Ion mobility-high resolution mass spectrometry (IM-HRMS) for the analysis of Contaminants of Emerging Concern (CECs): Database compilation and application to urine samples. Anal. Chem. 2021, 16, 6428–6436. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, M.; Chen, X.; Yin, Y.; Xiong, X.; Wang, R.; Zhu, Z.-J. Ion mobility collision cross-section atlas for known and unknown metabolite annotation in untargeted metabolomics. Nat. Commun. 2020, 11, 4334. [Google Scholar] [CrossRef]
- Jia, T.; Ouyang, S.; Chen, W.; Zhang, T.; Lu, M.; Zhou, X.; Lei, H.; Wei, X. Develop an external standard method for high-flux determination of veterinary drug residues liquid milk without solid phase extraction. Food Chem. 2024, 433, 137269. [Google Scholar] [CrossRef]
- Acceptance Criteria for Confirmation of Identity of Chemical Residues using Exact Mass Data for the FDA Foods and Veterinary Medicine Program; FDA: Silver Spring, MD, USA, 2015.
- GB/T 23210-2008; Determination of 511 Pesticides and Related Chemicals Residues in Milk and Milk Power-GC-MS Method. Standards Press of China: Beijing, China, 2008.
- GB/T 23211-2008; Determination of 493 Pesticides and Related Chemicals Residues in Milk and Milk Power-LC-MS-MS method. Standards Press of China: Beijing, China, 2008.
- GB 29696-2013; Determination of Avermectins Residues in Milk by High Performance Liquid Chromatographic Method. Standards Press of China: Beijing, China, 2013.
- GB 23200.49; Determination of Difenoconazole Residue in Foods Gas Chromatography-Mass Spectrometry. Standards Press of China: Beijing, China, 2016.
- GB 23200.56; Determination of Quinoxyfen Residue in Foods. Standards Press of China: Beijing, China, 2016.
- GB 29692-2013; Determination of Quinolones Residues in Milk by High Performance Liquid Chromatographic Method. Standards Press of China: Beijing, China, 2013.
- GB 5009.209-2016; Determination of Zearalenone in Foods. Standards Press of China: Beijing, China, 2016.
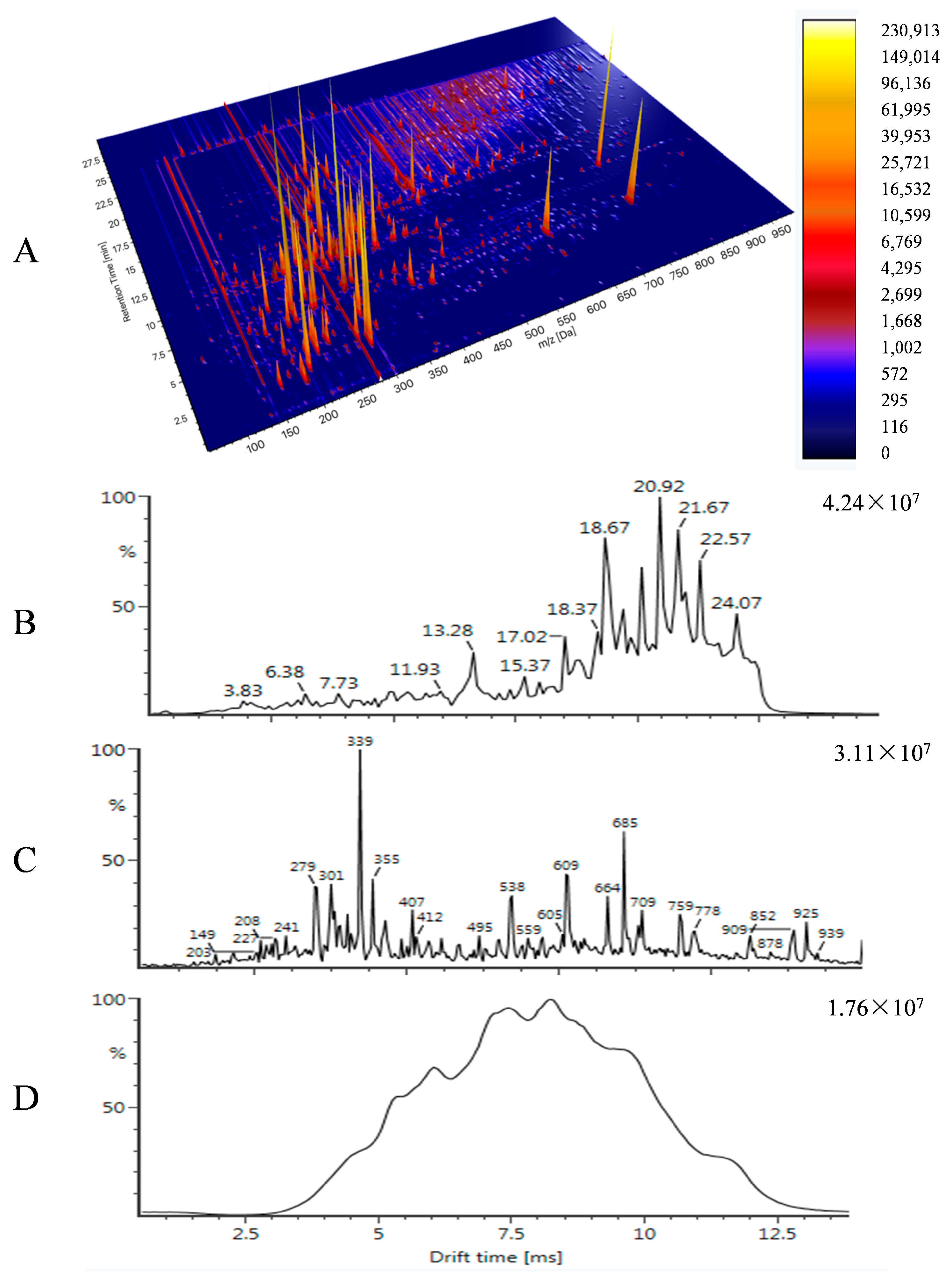



| Time (min) | %A | %B |
|---|---|---|
| 0 | 2 | 98 |
| 0.5 | 2 | 98 |
| 20 | 99 | 1 |
| 24 | 99 | 1 |
| 25 | 2 | 98 |
| 30 | 2 | 98 |
| Compound | LOQ in This Method (μg/kg) | LOQ in National Standard in China (μg/kg) | Reference Method |
|---|---|---|---|
| Ametryn | 1 | 12.5 | GB/T 23210-2008 Determination of 511 pesticides and related chemicals residues in milk and milk power-GC-MS method [33] |
| Anilofos | 1 | 8.3 | |
| Atrazine | 1 | 4.2 | |
| Benalaxyl | 1 | 4.2 | |
| Buprofezin | 1 | 8.3 | |
| Carfentrazone-ethyl | 1 | 8.3 | |
| Chlordimeform | 1 | 16.8 | |
| Cloquintocet-mexyl | 1 | 4.2 | |
| Cyanazine | 1 | 12.5 | |
| Cyproconazole | 1 | 4.2 | |
| Diazinon | 1 | 4.2 | |
| Dimethachlor | 1 | 12.5 | |
| Diniconazol | 1 | 12.5 | |
| Edifenphos | 1 | 8.3 | |
| Etoxazole | 1 | 25 | |
| Fenamidone | 1 | 16.8 | |
| Fenamiphos | 1 | 12.5 | |
| Fenarimol | 1 | 8.3 | |
| Fenpropimorph | 1 | 4.2 | |
| Fenpyroximate | 1 | 33.3 | |
| Fluazifop-butyl | 1 | 4.2 | |
| Flusilazole | 1 | 12.5 | |
| Halosulfuron methyl | 1 | 333.2 | |
| Hexazinone | 1 | 12.5 | |
| Metribuzin | 1 | 12.5 | |
| Myclobutanil | 1 | 4.2 | |
| Napropamide | 1 | 12.5 | |
| Norflurazon | 1 | 4.2 | |
| Pirimicarb | 1 | 8.3 | |
| Pirimiphos-ethyl | 1 | 8.3 | |
| Pirimiphos-methyl | 1 | 4.2 | |
| Propyzamide | 1 | 8.3 | |
| Spiroxamine | 1 | 8.3 | |
| Acrinathrin | 5 | 8.3 | |
| Boscalid | 5 | 10 | |
| Butamifos | 5 | 4.2 | |
| Ciprofloxacin | 5 | 10 | |
| Clodinafop-propargyl | 5 | 8.3 | |
| Enrofloxacin | 5 | 10 | |
| Fenhexamid | 5 | 83.3 | |
| Fluquinconazole | 5 | 4.2 | |
| Acrinathrin | 5 | 8.3 | |
| Boscalid | 5 | 10 | |
| Butamifos | 5 | 4.2 | |
| Ciprofloxacin | 5 | 10 | |
| Clodinafop-propargyl | 5 | 8.3 | |
| Enrofloxacin | 5 | 10 | |
| Fenhexamid | 5 | 83.3 | |
| Fluquinconazole | 5 | 4.2 | |
| Profenofos | 5 | 10 | |
| Pyraflufen-ethyl | 5 | 8.3 | |
| Pyridaben | 5 | 4.2 | |
| Thiabendazole | 5 | 30 | |
| Tricyclazole | 5 | 25 | |
| Zoxamide | 5 | 8.3 | |
| Tribenuron-methyl | 10 | 12 | |
| Diuron | 1 | 1.2 | GB/T 23211-2008 Determination of 493 pesticides and related chemicals residues in milk and milk power-LC-MS-MS method [34] |
| Furathiocarb | 1 | 1.5 | |
| Isoprothiolane | 1 | 1.35 | |
| Pyrazophos | 1 | 1.2 | |
| Omethoate | 5 | 7 | |
| Sulfentrazone | 5 | 22.4 | |
| Cyhalofop | 10 | 25 | |
| Triallate | 10 | 15 | |
| Doramectin | 1 | 2 | GB 29696-2013 Determination of Avermectins residues in milk by High Performance Liquid Chromatographic method [35] |
| Difenoconazole | 1 | 5 | GB 23200.49 Determination of difenoconazole residue in foods Gas chromatography-mass spectrometry [36] |
| Quinoxyfen | 1 | 3 | GB 23200.56 Determination of quinoxyfen residue in foods [37] |
| Sarafloxacin | 5 | 10 | GB 29692-2013 Determination of quinolones residues in milk by High Performance Liquid Chromatographic method [38] |
| Zearalenone | 1 | 4 | GB 5009.209-2016 Determination of zearalenone in foods [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Zhang, J.; Shao, B.; Yin, J.; Yang, Y.; Yang, Y. Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Foods 2025, 14, 967. https://doi.org/10.3390/foods14060967
Guo Q, Zhang J, Shao B, Yin J, Yang Y, Yang Y. Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Foods. 2025; 14(6):967. https://doi.org/10.3390/foods14060967
Chicago/Turabian StyleGuo, Qiaozhen, Jing Zhang, Bing Shao, Jie Yin, Yunjia Yang, and Yi Yang. 2025. "Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry" Foods 14, no. 6: 967. https://doi.org/10.3390/foods14060967
APA StyleGuo, Q., Zhang, J., Shao, B., Yin, J., Yang, Y., & Yang, Y. (2025). Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Foods, 14(6), 967. https://doi.org/10.3390/foods14060967






