Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges
Abstract
1. Introduction
2. Transmission Pathways of Viruses in the Seafood Supply Chain
2.1. Viruses in Seafood
2.1.1. Norovirus
2.1.2. Hepatitis A Virus (HAV)
2.1.3. Hepatitis E Virus (HEV)
2.1.4. Human Adenovirus (HAdV)
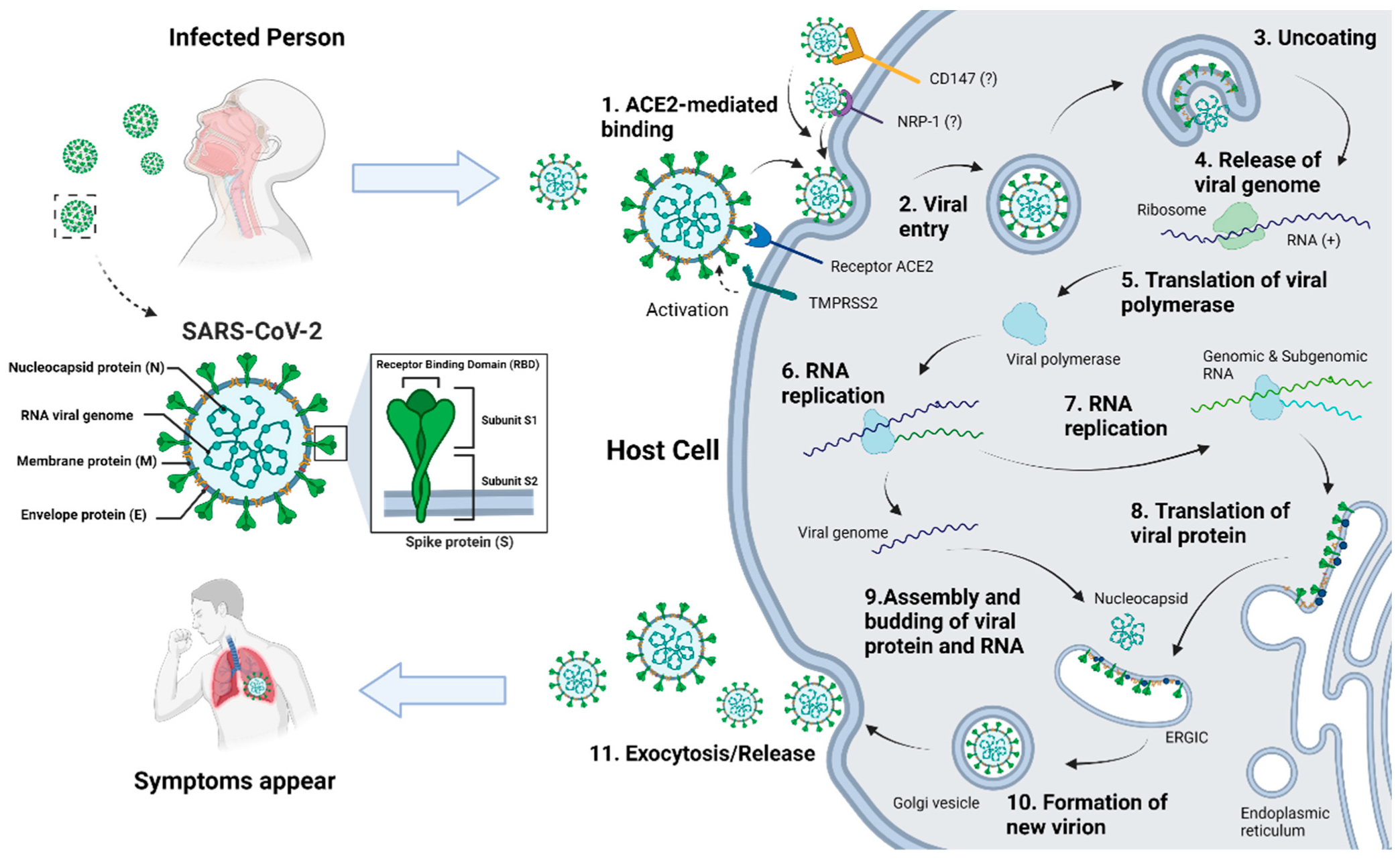
2.1.5. Coronavirus (CoV)
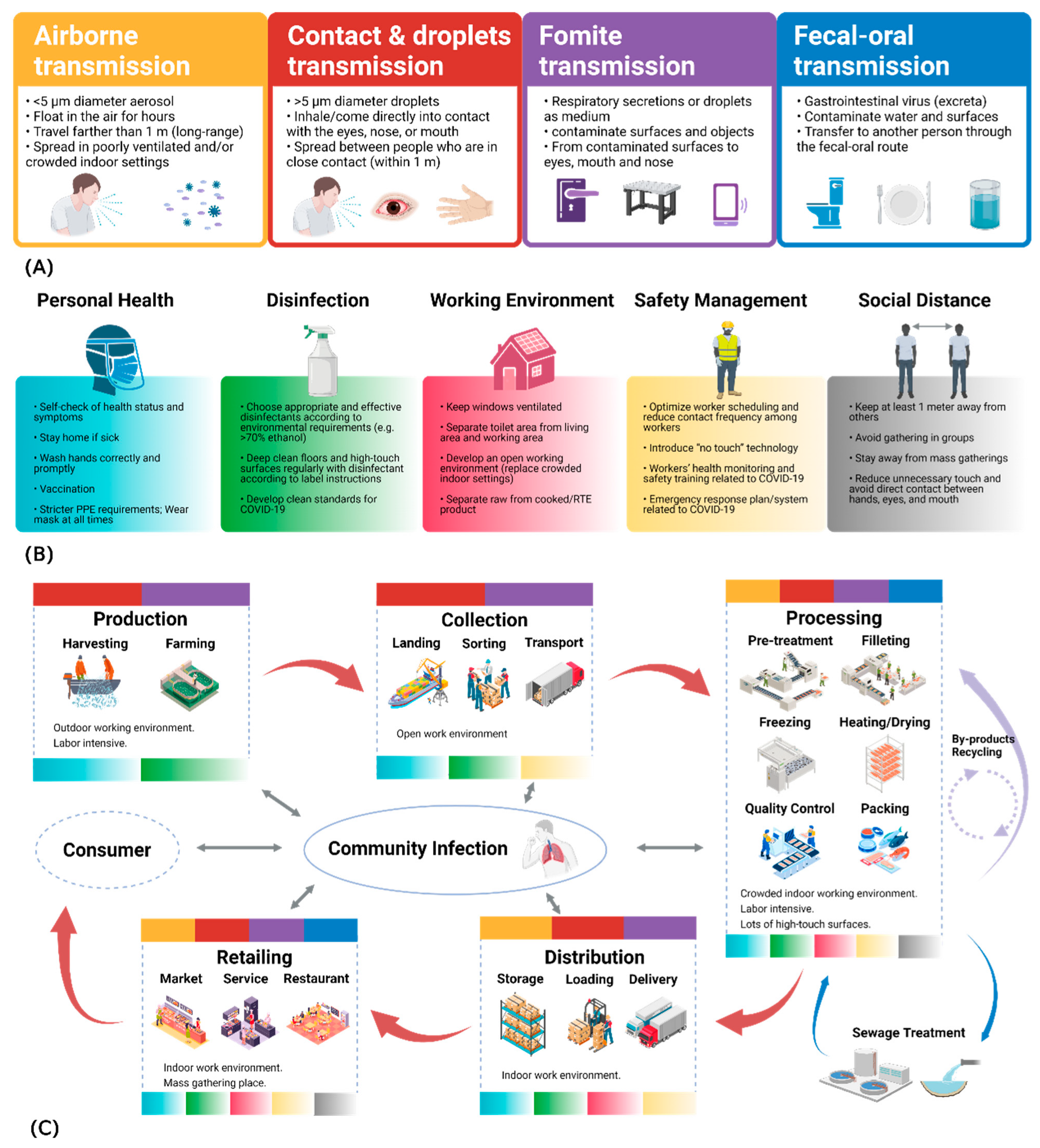
2.2. Transmission Routes
2.2.1. Respiratory Transmission
2.2.2. Contact Transmission
2.2.3. Fecal–Oral Transmission and Foodborne Transmission
2.2.4. Zoonotic Transmission
3. Industry Practices and New Technologies for Potential Virus Prevention
3.1. Prevention and Control Measures in the Seafood Industry
- Train employees on skills to prevent the spread of virus, strengthen food safety education, and ensure employees’ mental health [138].
- Install and provide additional handwashing stations at high-traffic and fixed locations, equipped with warm water and hand sanitizer [79].
- Establish health screening procedures, such as using quick, non-contact thermometers to check the temperature of every worker or visitor entering the workplace [139].
- Restrict visitors and personnel movement to minimize unnecessary contact.
- Provide workers with adequate and clean personal protective equipment (PPE).
- Introduce labor-saving technologies, such as artificial intelligence [140].
- Maintain good manufacturing practices (GMP) and good hygiene practices (GHP) [139].
- Increase water treatment and purification systems at aquaculture farms to filter out viruses and reduce the viral content within seafood products, thus lowering the viral load at the source.
- Regularly monitor the viral concentration in wastewater produced at each stage of the process and take preemptive measures to investigate and address any abnormalities as soon as they are detected.
- It is recommended that employees be vaccinated against the relevant viruses.
- Strengthen the traceability system, so that once a virus is detected, it is possible to promptly determine which stage of the process is problematic and take timely measures to address it.
- Improve the production line by incorporating commercially viable non-thermal treatments, such as ultraviolet (UV) light, to reduce the quantity of viruses on packaging and products.
- Adhere to food safety management system (FSMS) protocols established by authorities in accordance with Hazard Analysis and Critical Control Points (HACCP) principles [141,142]. Maintaining social distance above 1 m effectively reduces the probability of airborne virus transmission [143]. However, maintaining social distancing throughout the entire supply chain or within food facilities is complex [124]. For example, most sanitation measures in capture fisheries production complicate fishing activities [121]. Moreover, strengthening port or border restrictions may lead to fishermen remaining at sea for extended periods (due to being unable to land), making physical distancing on fishing vessels less important [28]. Therefore, managers involved in the seafood supply chain should assess operational modifications in implementing physical distancing measures in crowded public facilities such as factories, restaurants, and markets, and adopt optimal strategies to minimize non-work-related interactions [124].
3.2. Policy Implications in the Seafood Industry
3.3. Recent Viral Protection Technologies for the Seafood Industry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. In Brief to The State of World Fisheries and Aquaculture 2024. In Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- FAO. In Brief to The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Tokur, B.; Korkmaz, K. Seafood associated human pathogenic non-enveloped viruses. Ege J. Fish. Aquat. Sci. 2021, 38, 253–262. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Lekshmi, M.; Das, O.; Kumar, S.; Nayak, B.B. Occurrence of Human Enteric Adenoviruses in Fresh Tropical Seafood from Retail Markets and Landing Centers. J. Food Sci. 2019, 84, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Predmore, A.; Divers, E.; Lou, F. New interventions against human norovirus: Progress, opportunities, and challenges. Annu. Rev. Food Sci. Technol. 2012, 3, 331–352. [Google Scholar] [CrossRef]
- Butt, A.A.; Aldridge, K.E.; Sanders, C.V. Infections related to the ingestion of seafood Part I: Viral and bacterial infections. Lancet Infect. Dis. 2004, 4, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, R.; Frösner, G.G.; Hochstein-Mintzel, V.; Riedemann, S.; Reinhardt, G. Accumulation and persistence of hepatitis A virus in mussels. J. Med. Virol. 1992, 37, 174–179. [Google Scholar] [CrossRef]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef]
- Khora, S.S. Risk from Viral Pathogens in Seafood. In Diet, Microbiome and Health; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–481. [Google Scholar] [CrossRef]
- Krugman, S.; Giles, J.P.; Hammond, J. Hepatitis virus: Effect of heat on the infectivity and antigenicity of the MS-1 and MS-2 strains. J. Infect. Dis. 1970, 122, 432–436. Available online: https://www.jstor.org/stable/30108355 (accessed on 3 February 2025). [CrossRef]
- Dolin, R.; Blacklow, N.R.; Dupont, H.; Buscho, R.F.; Wyatt, R.G.; Kasel, J.A.; Hornick, R.; Chanock, R.M. Biological Properties of Norwalk Agent of Acute Infectious Nonbacterial Gastroenteritis. Exp. Biol. Med. 1972, 140, 578–583. [Google Scholar] [CrossRef]
- Polo, D.; Álvarez, C.; Longa, Á.; Romalde, J.L. Effectiveness of depuration for hepatitis A virus removal from mussels (Mytilus galloprovincialis). Int. J. Food Microbiol. 2014, 180, 24–29. [Google Scholar] [CrossRef]
- Kang, S.; Park, S.Y.; Ha, S.-D. Application of gamma irradiation for the reduction of norovirus in traditional Korean half-dried seafood products during storage. LWT 2016, 65, 739–745. [Google Scholar] [CrossRef]
- Huang, Y.M.; Chang, W.C.; Hsu, C.L. Inactivation of norovirus by atmospheric pressure plasma jet on salmon sashimi. Food Res. Int. 2021, 141, 110108. [Google Scholar] [CrossRef]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-CoV-2. Turk. J. Med Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef]
- Hashim, Z.H.; Azra, M.N.; Noor, M.I.M.; Kasan, N.A.; Tan, S.H. Impact of COVID-19 on marine fisheries supply chains: Case study of Malaysia. Adv. Food Secur. Sustain. 2021, 6, 169–210. [Google Scholar] [CrossRef]
- Dyal, J.W.; Grant, M.P.; Broadwater, K.; Bjork, A.; Waltenburg, M.A.; Gibbins, J.D.; Hale, C.; Silver, M.; Fischer, M.; Steinberg, J.; et al. COVID-19 Among Workers in Meat and Poultry Processing Facilities—19 States. Morb. Mortal. Wkly. Rep. 2020, 69, 557–561. [Google Scholar] [CrossRef]
- Porter, K.A.; Ramaswamy, M.; Koloski, T.; Castrodale, L.; McLaughlin, J. COVID-19 Among Workers in the Seafood Processing Industry: Implications for Prevention Measures—Alaska, March–October 2020. Morbidity Mortal. Wkly. Rep. 2021, 70, 622–626. [Google Scholar] [CrossRef]
- Lu, L.C.; Quintela, I.; Lin, C.H.; Lin, T.C.; Lin, C.H.; Wu VC, H.; Lin, C.S. A review of epidemic investigation on cold-chain food-mediated SARS-CoV-2 transmission and food safety consideration during COVID-19 pandemic. J. Food Saf. 2021, 41, e12932. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Ren, L.; Wu, S.; Ma, W.; Yang, J.; Di, L.; Li, J.; Xiao, Y.; Kang, L.; Du, S.; et al. Cold-chain food contamination as the possible origin of COVID-19 resurgence in Beijing. Natl. Sci. Rev. 2020, 7, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Louten, J. Virus Structure and Classification. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–29. [Google Scholar] [CrossRef]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Godoy, M.G.; Kibenge, M.J.T.; Kibenge, F.S.B. SARS-CoV-2 transmission via aquatic food animal species or their products: A review. Aquaculture 2021, 536, 736460. [Google Scholar] [CrossRef]
- Glass, R.I.; Noel, J.; Mitchell, D.; Herrmann, J.E.; Blacklow, N.R.; Pickering, L.K.; Dennehy, P.; Ruiz-Palacios, G.; De Guerrero, M.L.; Monroe, S.S. The Changing Epidemiology of Astrovirus-Associated Gastroenteritis: A Review; Springer: Vienna, Austria, 1996; pp. 287–300. [Google Scholar] [CrossRef]
- Woods, J.W.; Calci, K.R.; Marchant-Tambone, J.G.; Burkhardt, W. Detection molecular characterization of norovirus from oysters implicated in outbreaks in the, U.S. Food Microbiol. 2016, 59, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rowan, N.J. Current decontamination challenges and potentially complementary solutions to safeguard the vulnerable seafood industry from recalcitrant human norovirus in live shellfish: Quo Vadis? Sci. Total Environ. 2023, 874, 162380. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F. Reflections about the impact of the SARS-CoV-2/COVID-19 pandemic on mental health. Rev. Bras. Psiquiatr. 2020, 42, 329. [Google Scholar] [CrossRef]
- Brucker, R.; Bui, T.; Kwan-Gett, T.; Stewart, L.; Hall, A.J.; Kinzer, M.H. Norovirus Infections Associated with Frozen Raw Oysters—Washington, 2011. MMWR: Morbidity & Mortality Weekly Report; 2012; 61. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6106a3.htm (accessed on 3 February 2025).
- Cook, N.; Knight, A.; Richards, G.P. Persistence and Elimination of Human Norovirus in Food and on Food Contact Surfaces: A Critical Review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef]
- Leduc, A.; Leclerc, M.; Challant, J.; Loutreul, J.; Robin, M.; Maul, A.; Majou, D.; Boudaud, N.; Gantzer, C. F-Specific RNA Bacteriophages Model the Behavior of Human Noroviruses during Purification of Oysters: The Main Mechanism Is Probably Inactivation Rather than Release. Appl. Environ. Microbiol. 2020, 86, e00526-20. [Google Scholar] [CrossRef]
- Calci, K.R.; Meade, G.K.; Tezloff, R.C.; Kingsley, D.H. High-Pressure Inactivation of Hepatitis A Virus within Oysters. Appl. Environ. Microbiol. 2005, 71, 339–343. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escámez, P.S.F.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Guidance on the requirements for the development of microbiological criteria. EFSA J. 2017, 15, e05052. [Google Scholar] [CrossRef]
- de Abreu Corrêa, A.; Carratala, A.; Barardi CR, M.; Calvo, M.; Girones, R.; Bofill-Mas, S. Comparative inactivation of murine norovirus, human adenovirus, and human JC polyomavirus by chlorine in seawater. Appl. Environ. Microbiol. 2012, 78, 6450–6457. [Google Scholar] [CrossRef]
- Younger, A.D.; Neish, A.; Walker, D.I.; Jenkins, K.L.; Lowther, J.A.; Stapleton, T.A.; Alves, M.T. Strategies to reduce norovirus (NoV) contamination from oysters under depuration conditions. Food Chem. Toxicol. 2020, 143, 111509. [Google Scholar] [CrossRef]
- D’Souza, D.H.; Jaykus, L.A. Zirconium hydroxide effectively immobilizes and concentrates human enteric viruses. Lett. Appl. Microbiol. 2002, 35, 414–418. [Google Scholar] [CrossRef]
- Viral, H.; Liver, D.; Hollinger, F.B.; Lemon, S.M.; Margolis, H. Viral Hepatitis and Liver Disease: Proceedings of the 1990 International Symposium on Viral Hepatitis and Liver Disease: Contemporary Issues and Future Prospects; Williams & Wilkins: Philadelphia, PA, USA, 1991; Available online: https://cir.nii.ac.jp/crid/1130282272009208448 (accessed on 3 February 2025).
- Halliday, M.L.; Kang, L.Y.; Zhou, T.K.; Hu, M.D.; Pan, Q.C.; Fu, T.Y.; Huang, Y.S.; Hu, S.L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J. Infect. Dis. 1991, 164, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Bellou, M.; Kokkinos, P.; Vantarakis, A. Shellfish-borne viral outbreaks: A systematic review. Food Environ. Virol. 2013, 5, 13–23. [Google Scholar] [CrossRef]
- Pinto, R.M.; Costafreda, M.I.; Bosch, A. Risk assessment in shellfish-borne outbreaks of hepatitis, A. Appl. Environ. Microbiol. 2009, 75, 7350–7355. [Google Scholar] [CrossRef] [PubMed]
- Myrmel, M.; Berg, E.M.M.; Rimstad, E.; Grinde, B. Detection of enteric viruses in shellfish from the Norwegian coast. Appl. Environ. Microbiol. 2004, 70, 2678–2684. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Hepatitis A Vaccine Administration|Hepatitis A|CDC. 2024. Available online: https://www.cdc.gov/hepatitis-a/hcp/vaccine-administration/index.html (accessed on 3 February 2025).
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Pham, B.; Duval, B.; de Serres, G.; Gilca, V.; Vrbova, L.; Anonychuk, A.; Krahn, M.; Moher, D. A review of interventions triggered by hepatitis A infected food-handlers in Canada. BMC Health Serv. Res. 2006, 6, 157. [Google Scholar] [CrossRef]
- di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Park, S.Y.; Kim, A.N.; Oh, M.H.; Ha, S.-D. Survival of hepatitis A virus on various food-contact surfaces during 28 days of storage at room temperature. Food Res. Int. 2014, 64, 849–854. [Google Scholar] [CrossRef]
- Cromeans, T.L.; Favorov, M.O.; Nainan, O.V.; Margolis, H.S. (Eds.) Hepatitis A and E viruses. In Foodborne Disease Handbook, 2nd ed.; Taylor & Francis Group: Oxfordshire, UK, 2018; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781351072083-2/hepatitis-viruses-theresa-cromeans-michael-favorov-omana-nainan-harold-margolis (accessed on 3 February 2025).
- Smith, D.B.; Simmonds, P. Classification and Genomic Diversity of Enterically Transmitted Hepatitis Viruses. Cold Spring Harb. Perspect. Med. 2018, 8, a031880. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D. Hepatitis E Outbreak on Cruise Ship. Emerg. Infect. Dis. 2009, 15, 1738. [Google Scholar] [CrossRef]
- van der Poel, W.H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Vergara, A. Hepatitis E Virus in the Food of Animal Origin: A Review. Foodborne Pathog. Dis. 2021, 18, 368–377. [Google Scholar] [CrossRef]
- Wei, M.; Wang, J.; Wang, Y.; Liu, L.; Xu, X.; Wang, J. Development and Application of a Multiplex Reverse Transcription–Droplet Digital PCR Assay for Simultaneous Detection of Hepatitis A Virus and Hepatitis E Virus in Bivalve Shellfish. Foods 2024, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Record. Wkly. Epidemiol. Rec. 2014, 89, 321–336. Available online: https://iris.who.int/handle/10665/242244 (accessed on 3 February 2025).
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Katayama, H.; Oguma, K.; Ohgaki, S. Quantitative analysis of human enteric adenoviruses in aquatic environments. J. Appl. Microbiol. 2007, 103, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Lees, D. Viruses and bivalve shellfish. Int. J. Food Microbiol. 2000, 59, 81–116. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chen, J.-S.; Hsu, G.-J.; Chen, H.-P.; Chao, H.-C.; Huang, S.-W.; Tsai, I.-S.; Hsu, B.-M. Surveillance of adenovirus and norovirus contaminants in the water and shellfish of major oyster breeding farms and fishing ports in Taiwan. Pathogens 2022, 11, 316. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Fieldhouse, J.K.; Seto, D.; Gray, G.C. Are adenoviruses zoonotic? A systematic review of the evidence. Emerg. Microbes Infect. 2019, 8, 1679–1687. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Ahmed, N.I.; Shaheen MN, F.; Mohamed EC, B.; Loutfy, S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health 2019, 17, 287–294. [Google Scholar] [CrossRef]
- Kajon, A.E.; Lu, X.; Erdman, D.D.; Louie, J.; Schnurr, D.; George, K.; Koopmans, M.P.; Allibhai, T.; Metzgar, D. Molecular Epidemiology and Brief History of Emerging Adenovirus 14—Associated Respiratory Disease in the United States. J. Infect. Dis. 2010, 202, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tong, Y.G.; Wo, Y.; Wang, H.Y.; Liu, E.M.; Gray, G.C.; Liu, W.; Cao, W.C. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in Chin, 2009–2012. Influ. Other Respir. Viruses 2014, 8, 302–308. [Google Scholar] [CrossRef]
- Radin, J.M.; Hawksworth, A.W.; Blair, P.J.; Faix, D.J.; Raman, R.; Russell, K.L.; Gray, G.C. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 962–968. [Google Scholar] [CrossRef]
- Girones, R.; Carratalà, A.; Calgua, B.; Calvo, M.; Rodriguez-Manzano, J.; Emerson, S. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J. Water Health 2014, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Salahshoori, I.; Mobaraki-Asl, N.; Seyfaee, A.; Nasirabad, N.M.; Dehghan, Z.; Faraji, M.; Ganjkhani, M.; Babapoor, A.; Shadmehr, S.Z.; Hamrang, A. Overview of COVID-19 disease: Virology, epidemiology, prevention diagnosis, treatment, and vaccines. Biologics 2021, 1, 2–40. [Google Scholar] [CrossRef]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef]
- Lebeau, G.; Vagner, D.; Frumence, É.; Ah-Pine, F.; Guillot, X.; Nobécourt, E.; Raffray, L.; Gasque, P. Deciphering SARS-CoV-2 Virologic and Immunologic Features. Int. J. Mol. Sci. 2020, 21, 5932. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis ML, C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Fernández-Ríos, A.; Campos, C.; Laso, J.; Margallo, M.; Aldaco, R. The fishing and seafood sector in the time of COVID-19: Considerations for local and global opportunities and responses. Curr. Opin. Environ. Sci. Health 2021, 23, 100286. [Google Scholar] [CrossRef]
- O’Brien, B.; Goodridge, L.; Ronholm, J.; Nasheri, N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021, 95, 103709. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, H.; Yan, N.; Huang, J.; Zhao, L.; Xu, S.; Wu, J.; Jiang, S.; Pan, C.; Liao, M. Long-term Survival of SARS-CoV-2 on Salmon as a Source for International Transmission. J. Infect. Dis. 2021, 223, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- García-Aljaro, C.; Ballesté, E.; Muniesa, M.; Jofre, J. Determination of crAssphage in water samples and applicability for tracking human faecal pollution. Microb. Biotechnol. 2017, 10, 1775–1780. [Google Scholar] [CrossRef]
- McLeod, C.; Polo, D.; Le Saux, J.C.; Le Guyader, F.S. Depuration and Relaying: A Review on Potential Removal of Norovirus from Oysters. Compr. Rev. Food Sci. Food Saf. 2017, 16, 692–706. [Google Scholar] [CrossRef]
- Ganesh, A.; Lin, J. Waterborne human pathogenic viruses of public health concern. Int. J. Environ. Health Res. 2013, 23, 544–564. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Jalava, K. First respiratory transmitted food borne outbreak? Int. J. Hyg. Environ. Heal. 2020, 226, 113490. [Google Scholar] [CrossRef]
- Nakat, Z.; Bou-Mitri, C. COVID-19 and the food industry: Readiness assessment. Food Control 2021, 121, 107661. [Google Scholar] [CrossRef]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA 2020, 324, 441–442. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an Alternative to Reduce the Spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chang, L.-Y.; Li, C.-S. Detection of airborne viruses in a pediatrics department measured using real-time qPCR coupled to an air-sampling filter method. J. Environ. Health 2010, 73, 22–28. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20103375872 (accessed on 3 February 2025). [PubMed]
- Centers for Disease Control and Prevention (CDC). How Norovirus Spreads|Norovirus|CDC. 2024. Available online: https://www.cdc.gov/norovirus/causes/index.html (accessed on 3 February 2025).
- Bhangar, S.; Mullen, N.A.; Hering, S.V.; Kreisberg, N.M.; Nazaroff, W.W. Ultrafine particle concentrations and exposures in seven residences in northern California. Indoor Air 2011, 21, 132–144. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Leclerc, Q.J.; Fuller, N.M.; Knight, L.E.; Funk, S.; Knight, G.M. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Kraay, A.N.M.; Brouwer, A.F.; Lin, N.; Collender, P.A.; Remais, J.V.; Eisenberg, J.N.S. Modeling environmentally mediated rotavirus transmission: The role of temperature and hydrologic factors. Proc. Natl. Acad. Sci. USA 2018, 115, E2782–E2790. [Google Scholar] [CrossRef]
- Castaño, N.; Cordts, S.C.; Kurosu Jalil, M.; Zhang, K.S.; Koppaka, S.; Bick, A.D.; Paul, R.; Tang SK, Y. Fomite Transmission, Physicochemical Origin of Virus-Surface Interactions, and Disinfection Strategies for Enveloped Viruses with Applications to SARS-CoV-2. ACS Omega 2021, 6, 6509–6527. [Google Scholar] [CrossRef]
- Clay, S.; Maherchandani, S.; Malik, Y.S.; Goyal, S.M. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am. J. Infect. Control 2006, 34, 41–43. [Google Scholar] [CrossRef]
- Mbithi, J.N.; Springthorpe, V.S.; Sattar, S.A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl. Environ. Microbiol. 1990, 56, 3601–3604. [Google Scholar] [CrossRef] [PubMed]
- Abad, F.X.; Pinto, R.M.; Bosch, A. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 1994, 60, 3704–3710. [Google Scholar] [CrossRef]
- Mehraeen, E.; Salehi, M.A.; Behnezhad, F.; Moghaddam, H.R.; SeyedAlinaghi, S. Transmission Modes of COVID-19: A Systematic Review. Infect. Disord. Drug Targets 2021, 21, 27–34. [Google Scholar] [CrossRef]
- Pastorino, B.; Touret, F.; Gilles, M.; de Lamballerie, X.; Charrel, R.N. Prolonged Infectivity of SARS-CoV-2 in Fomites. Emerg. Infect. Dis. 2020, 26, 2256–2257. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Chen, B.; Edirisinghe, M. Surface interactions and viability of coronaviruses. J. R. Soc. Interface 2021, 18, 20200798. [Google Scholar] [CrossRef]
- Finger, J.A.F.F.; Lima, E.M.F.; Coelho, K.S.; Behrens, J.H.; Landgraf, M.; Franco, B.D.G.M.; Pinto, U.M. Adherence to food hygiene and personal protection recommendations for prevention of COVID-19. Trends Food Sci. Technol. 2021, 112, 847–852. [Google Scholar] [CrossRef]
- Kratzel, A.; Kratzel, A.; Todt, D.; V’kovski, P.; Steiner, S.; Steiner, S.; Gultom, M.; Gultom, M.; Thao TT, N.; Thao TT, N.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Pan, Y.; Zhao, Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020, 525, 135–140. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Shin-Ya, M.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Marin, E.; Zhu, W.; et al. Mechanisms of instantaneous inactivation of SARS-CoV-2 by silicon nitride bioceramic. Mater. Today Bio. 2021, 12, 100144. [Google Scholar] [CrossRef]
- Criscuolo, C.; Gal, P.; Leidecker, T.; Losma, F.; Nicoletti, G.; Criscuolo, C.; Gal, P.; Leidecker, T.; Losma, F.; Nicoletti, G. The Role of Telework for Productivity During and Post-COVID-19: Results from an OECD Survey Among Managers and Workers; OECD Publishing: Paris, France, 2021; Volume 31. [Google Scholar] [CrossRef]
- Eastaugh, J.; Shepherd, S. Infectious and Toxic Syndromes From Fish and Shellfish Consumption: A Review. Arch. Intern. Med. 1989, 149, 1735–1740. [Google Scholar] [CrossRef]
- Anelich LE, C.M.; Lues, R.; Farber, J.M.; Parreira, V.R. SARS-CoV-2 and Risk to Food Safety. Front. Nutr. 2020, 7, 580551. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-H.; Ni, W.; Wu, Q.; Li, W.-J.; Li, G.-J.; Wang, W.-D.; Tong, J.-N.; Song, X.-F.; Wong, G.W.-K.; Xing, Q.-S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020, 53, 473–480. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef]
- Hu, L.; Gao, J.; Yao, L.; Zeng, L.; Liu, Q.; Zhou, Q.; Zhang, H.; Lu, D.; Fu, J.; Liu, Q.S.; et al. Evidence of foodborne transmission of the coronavirus (COVID-19) through the animal products food supply chain. Environ. Sci. Technol. 2021, 55, 2713–2716. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; Koopmans, M.P. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021, 371, 172–177. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, C.; Yang, Q.; Xu, Y.; Xu, J.; Li, Y.; Yu, X.; Zhu, H.; Liu, J. Characteristics of respiratory virus infection during the outbreak of 2019 novel coronavirus in Beijing. Int. J. Infect. Dis. Off. Publ. Int. Soc. Infect. Dis. 2020, 96, 266–269. [Google Scholar] [CrossRef]
- Deardorff, T.L.; Overstreet, R.M. Seafood-Transmitted Zoonoses in the United States: The Fishes, the Dishes, and the Worms. In Microbiology of Marine Food Products; Springer: Berlin/Heidelberg, Germany, 1991; pp. 211–265. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, Z. Viral hepatitis A and E. In Molecular Medical Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 2311–2319. [Google Scholar] [CrossRef]
- Chen, D.; Sun, J.; Zhu, J.; Ding, X.; Lan, T.; Zhu, L.; Xiang, R.; Ding, P.; Wang, H.; Wang, X.; et al. Single-cell screening of SARS-CoV-2 target cells in pets, livestock, poultry and wildlife. BioRxiv 2020. [Google Scholar] [CrossRef]
- Zhou, P.; Shi, Z.L. SARS-CoV-2 spillover events. Science 2021, 371, 120–122. [Google Scholar] [CrossRef]
- Wei, C.; Shan, K.J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a mouse origin of the SARS-CoV-2 Omicron variant. J. Genet. Genom. 2021, 48, 1111–1121. [Google Scholar] [CrossRef]
- Wee, J.J.; Chen, J.; Wei, G.W. Preventing future zoonosis: SARS-CoV-2 mutations enhance human-animal cross-transmission. Comput. Biol. Med. 2024, 182, 109101. [Google Scholar] [CrossRef]
- Damas, J.; Hughes, G.M.; Keough, K.C.; Painter, C.A.; Persky, N.S.; Corbo, M.; Hiller, M.; Koepfli, K.P.; Pfenning, A.R.; Zhao, H.; et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 22311–22322. [Google Scholar] [CrossRef]
- Nabi, G.; Khan, S. Risk of COVID-19 pneumonia in aquatic mammals. Environ. Res. 2020, 188, 109732. [Google Scholar] [CrossRef]
- World Health Organization. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. 2020. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 4 February 2025).
- FAO. The Impact of COVID-19 on Fisheries and Aquaculture Food Systems, Possible Responses; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Griffin, J.; McAloon, C.; Byrne, A.; Madden, J.; Mc Evoy, D.; Collins, Á.; Hunt, K.; Barber, A.; Butler, F.; et al. Presymptomatic transmission of SARS-CoV-2 infection: A secondary analysis using published data. BMJ Open 2021, 11, e041240. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Issues Best Practices on Safe Food Handling and Employee Health in Retail Food Settings During COVID-19 Pandemic|FDA. 2020. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-issues-best-practices-safe-food-handling-and-employee-health-retail-food-settings-during-covid (accessed on 3 February 2025).
- Food and Drug Administration. Food Safety and the Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://www.fao.org/urban-food-actions/resources/resources-detail/en/c/1271363/ (accessed on 24 October 2024).
- van Damme, P.; Pintó, R.M.; Feng, Z.; Cui, F.; Gentile, A.; Shouval, D. Hepatitis A virus infection. Nat. Rev. Dis. Primers 2023, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Yeh, T.-Y.; Contreras, G.P. Full vaccination suppresses SARS-CoV-2 delta variant mutation frequency. MedRxiv 2021, 2021-08. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Shares Resources for the Food and Agriculture Sector About COVID-19 Vaccination|FDA. 2021. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-shares-resources-food-and-agriculture-sector-about-covid-19-vaccination (accessed on 3 February 2025).
- World Health Organization. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations During Severe Shortages. 2020. Available online: https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages (accessed on 4 February 2025).
- Centers for Disease Control and Prevention. Use Masks to Slow the Spread of COVID-19. 2020. Available online: https://www.cdc.gov/socialmedia/syndication/405380/405874.html (accessed on 24 October 2024).
- Rizou, M.; Galanakis, I.M.; Aldawoud TM, S.; Galanakis, C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Khaniki, G.J.; Salehi, A. Hygienic and safety requisites for consumption a safe food during the pandemic of the Covid-19 disease. J. Food Saf. Hyg. 2019, 5, 126–130. [Google Scholar] [CrossRef]
- Onofre, N.S.; Soler, C.; Merino-Torres, J.F.; Soriano, J.M. “Five Keys to Safer Food” and COVID-19. Nutrients 2021, 13, 4491. [Google Scholar] [CrossRef] [PubMed]
- Adelodun, B.; Ajibade, F.O.; Ibrahim, R.G.; Bakare, H.O.; Choi, K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: Any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total. Environ. 2020, 742, 140680. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, F.M.; Abdullah, A.F.; Aris, A.Z.; Umi, W.A.D. Impacts of COVID-19 on the Aquatic Environment and Implications on Aquatic Food Production. Sustainability 2021, 13, 11281. [Google Scholar] [CrossRef]
- Huss, H.H.; Reilly, A.; Embarek, K.B.P. Prevention and control of hazards in seafood. Food Control 2000, 11, 149–156. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Meat and Poultry Processing Workers and Employers: Interim guiance from CDC and the Occupational Safety and Health Administration (OSHA). 2020. Available online: https://stacks.cdc.gov/view/cdc/87280 (accessed on 24 October 2024).
- Jyoti; Bhattacharya, B. Impact of COVID-19 in Food Industries and potential innovations in Food Packaging to combat the pandemic—A review. Sci. Agropecu. 2021, 12, 133–140. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Vaishya, R.; Bahl, S.; Suman, R.; Vaish, A. Industry 4.0 technologies and their applications in fighting COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 419–422. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Seafood HACCP and the FDA Food Safety Modernization Act|FDA. 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-seafood-haccp-and-fda-food-safety-modernization-act (accessed on 3 February 2025).
- Olaimat, A.N.; Shahbaz, H.M.; Fatima, N.; Munir, S.; Holley, R.A. Food Safety During and After the Era of COVID-19 Pandemic. Front. Microbiol. 2020, 11, 1854. [Google Scholar] [CrossRef]
- Food and Agriculture Organization; World Health Organization. COVID-19 and Food Safety: Guidance for Food Businesses: Interim Guidance 2020. Available online: https://iris.who.int/handle/10665/331705 (accessed on 3 February 2025).
- Panisello, P.J.; Rooney, R.; Quantick, P.C.; Stanwell-Smith, R. Application of foodborne disease outbreak data in the development and maintenance of HACCP systems. Int. J. Food Microbiol. 2000, 59, 221–234. [Google Scholar] [CrossRef]
- Edmunds, K.L.; Hunter, P.R.; Few, R.; Bell, D.J. Hazard analysis of critical control points assessment as a tool to respond to emerging infectious disease outbreaks. PLoS ONE 2013, 8, e72279. [Google Scholar] [CrossRef]
- Munaò, G.; Cianti, L.; Benucci, P.; Bisori, A.; Iacopini, G.; Manuelli, D.; Rossi, F.; Scarlini, C.; Tentenni, L. Foodborne Viruses and Food Handlers Training: A Specific Project for Official Control. Ital. J. Food Saf. 2009, 1, 11–16. [Google Scholar]
- Ezzatpanah, H.; Gómez-López, V.M.; Koutchma, T.; Lavafpour, F.; Moerman, F.; Mohammadi, M.; Raheem, D. Risks and new challenges in the food chain: Viral contamination and decontamination from a global perspective, guidelines, and cleaning. Compr. Rev. Food Sci. Food Saf. 2022, 21, 868–903. [Google Scholar] [CrossRef]
- Casanova, L.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Coronavirus Survival on Healthcare Personal Protective Equipment. Infect. Control Hosp. Epidemiol. 2010, 31, 560–561. [Google Scholar] [CrossRef]
- Hersi, M.; Stevens, A.; Quach, P.; Hamel, C.; Thavorn, K.; Garritty, C.; Skidmore, B.; Vallenas, C.; Norris, S.L.; Egger, M.; et al. Effectiveness of personal protective equipment for healthcare workers caring for patients with filovirus disease: A rapid review. PLoS ONE 2015, 10, e0140290. [Google Scholar] [CrossRef]
- Konecka-Matyjek, E.; Turlejska, H.; Pelzner, U.; Szponar, L. Actual situation in the area of implementing quality assurance systems GMP, GHP and HACCP in Polish food production and processing plants. Food Control 2005, 16, 1–9. [Google Scholar] [CrossRef]
- Roy, P.K.; Roy, A.; Jeon, E.B.; DeWitt CA, M.; Park, J.W.; Park, S.Y. Comprehensive analysis of predominant pathogenic bacteria and viruses in seafood products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13410. [Google Scholar] [CrossRef]
- Zakrevskii, V.; Lopatin, S.; Yuvanen, E. Microbiological safety of marine products (seafood). Vestn. St. Petersburg Univ. Med. 2020, 15, 134–141. [Google Scholar] [CrossRef]
- Chintagari, S.; Hazard, N.; Edwards, G.; Jadeja, R.; Janes, M. Risks associated with fish and seafood. In Preharvest Food Safety; Wiley: Hoboken, NJ, USA, 2018; pp. 123–142. [Google Scholar] [CrossRef]
- Richards, G.P. Outbreaks of shellfish-associated enteric virus illness in the United States: Requisite for development of viral guidelines. J. Food Prot. 1985, 48, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Improvement strategies of food supply chain through novel food processing technologies during COVID-19 pandemic. Food Control 2021, 125, 108010. [Google Scholar] [CrossRef] [PubMed]
- Ham, S. Explaining Gender Gaps in the South Korean Labor Market During the COVID-19 Pandemic. Fem. Econ. 2021, 27, 133–151. [Google Scholar] [CrossRef]
- Denuwara, N.; Maijala, J.; Hakovirta, M. The impact of unmanned stores’ business models on sustainability. SN Bus. Econ. 2021, 1, 143. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Recent breakthroughs of antibacterial and antiviral protective polymeric materials during COVID-19 pandemic and after pandemic: Coating, packaging, and textile applications. Curr. Opin. Colloid Interface Sci. 2021, 55, 101480. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioff, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Agnihothram, S.; Mullis, L.; Townsend, T.A.; Watanabe, F.; Mustafa, T.; Biris, A.; Manjanatha, M.G.; Azevedo, M.P.; Food, U.S. Titanium dioxide nanoparticles evoke proinflammatory response during Murine norovirus infection despite having minimal effects on virus replication. Int. J. Nanotechnol. Med. Eng. 2016, 1, 63. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1592. [Google Scholar] [CrossRef]
- Abera, G.; Giorgis, W. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Lou, F.; Neetoo, H.; Chen, H.; Li, J. High hydrostatic pressure processing: A promising nonthermal technology to inactivate viruses in high-risk foods. Annu. Rev. Food Sci. Technol. 2015, 6, 389–409. [Google Scholar] [CrossRef]
- Murchie, L.W.; Kelly, A.L.; Wiley, M.; Adair, B.M.; Patterson, M. Inactivation of a calicivirus and enterovirus in shellfish by high pressure. Innov. Food Sci. Emerg. Technol. 2007, 8, 213–217. [Google Scholar] [CrossRef]
- Rupnik, A.; Doré, W.; Devilly, L.; Fahy, J.; Fitzpatrick, A.; Schmidt, W.; Hunt, K.; Butler, F.; Keaveney, S. Evaluation of Norovirus Reduction in Environmentally Contaminated Pacific Oysters During Laboratory Controlled and Commercial Depuration. Food Environ. Virol. 2021, 13, 229–240. [Google Scholar] [CrossRef]
- Mohamed, M.E.A.; Eissa, A.H.A.; Mohamed, M.E.A.; Eissa, A.H.A. Pulsed Electric Fields for Food Processing Technology. In Structure and Function of Food Engineering; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]

| Virus Common Name (Abbreviation and/Serotype) | Virus Family | Food Commodity | Causes of Diseases |
|---|---|---|---|
| Hepatitis A virus (HAV), | Picornaviridae | Bivalve molluscan shellfish (including oysters, clams, cockles, and mussels); fresh produce; prepared foods | Hepatitis |
| Norovirus (NoV), | Caliciviridae | Bivalve molluscan shellfish (including oysters, clams, cockles, and mussels); fresh produce; prepared foods | Gastroenteritis |
| Sapovirus | Caliciviridae | Salad; river water; oysters | Gastroenteritis |
| Enterovirus (e.g., poliovirus, Coxsackie A, B virus) | Picornaviridae | Oysters; contaminated water or food | Associated with a range of symptoms, including neurological symptoms |
| Hepatitis E virus (HEV) | Hepeviridae | Raw or undercooked meat of pig or wild boar or Sika deer; unpasteurized milk, shellfish, and ethnic foods; contaminated water | Hepatitis |
| Astrovirus | Astroviridae | Transmission is fecal–oral via food or water (<1% of astrovirus infections are considered food-borne) [25] | Gastroenteritis |
| Human parvovirus | Parvoviridae | Shellfish | Erythema infectiosum |
| Human adenovirus (HAdv) | Adenoviridae | Shellfish | Gastroenteritis |
| Hantavirus | Contamination of food or water with saliva or urine or through the dust of feces from infected wild rodents | Hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome | |
| Aichi virus | Picornaviridae | Oysters and seafood | Gastroenteritis |
| Environmental Conditions | Temperature | Viability Time |
|---|---|---|
| Aerosol | 21–23 °C | Up to 3–4 h |
| Plastic | 21–23 °C | Up to 72 h |
| Stainless steel | 21–23 °C | Up to 72 h |
| Copper | 21–23 °C | Up to 4 h |
| Cardboard | 21–23 °C | Up to 24 h |
| Glass | 21–23 °C | Up to 96 h |
| Cloth | 22 °C | Up to 24 h |
| Steel | 21–23 °C | Up to 48 h |
| Surgical mask | 22 °C | Up to 96 h |
| Disinfectants/Methods | Working Concentration or Condition | Treatment Time | Reduction of the Virus Titer | References |
|---|---|---|---|---|
| Heat treatment | >75 °C | 45 s to 5 min | N/A | [98] |
| Sodium hypochlorite | 0.05% (500 ppm) | 5 min | SARS-CoV-2 reduced by about 3 logs | [98] |
| Silicon nitride | 15 wt% aqueous suspensions | 1 min | Inactivate 99% of SARS-CoV-2 | [99] |
| Heat treatment | 70 °C | 5 min | SARS-CoV-2 reduced by about 7 logs | [73] |
| Ozone (O3) | 4 ppm (gas exposure) | 90 min | >98.2% viral titer reduction | [100] |
| Household bleach | 1:49 | 5 min | SARS-CoV-2 reduced by about 5.8 log (>99.9%) | [73] |
| Household bleach | 1:99 aq. | 5 min | SARS-CoV-2 reduced by about 5.8 log (>99.9%) | [73] |
| Ethanol | 70% aq. | 5 min | SARS-CoV-2 reduced by about 5.8 log (>99.9%) | [73] |
| Povidone-iodine | 7.5% aq. | 5 min | SARS-CoV-2 reduced by about 3.8 log (>99.9%) | [73] |
| Chloroxylenol | 0.05% aq. | 5 min | SARS-CoV-2 reduced by about 4.8 log (>99.9%) | [73] |
| Chlorhexidine (0.05%) | 0.05% aq. | 5 min | SARS-CoV-2 reduced by about 3.8 log (>99.9%) | [73] |
| Benzalkonium chloride | 0.1% aq. | 5 min | SARS-CoV-2 reduced by about 3.8 log (>99.9%) | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Chen, W.; Lin, Z.; Liu, L.; Zhang, M.; Yang, H.; Liu, Z.; Chen, L. Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges. Foods 2025, 14, 1071. https://doi.org/10.3390/foods14061071
Lin D, Chen W, Lin Z, Liu L, Zhang M, Yang H, Liu Z, Chen L. Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges. Foods. 2025; 14(6):1071. https://doi.org/10.3390/foods14061071
Chicago/Turabian StyleLin, Dingsong, Wendi Chen, Zejia Lin, Lingdai Liu, Molan Zhang, Hongshun Yang, Zifei Liu, and Lin Chen. 2025. "Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges" Foods 14, no. 6: 1071. https://doi.org/10.3390/foods14061071
APA StyleLin, D., Chen, W., Lin, Z., Liu, L., Zhang, M., Yang, H., Liu, Z., & Chen, L. (2025). Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges. Foods, 14(6), 1071. https://doi.org/10.3390/foods14061071








