Acrylamide Intake in Senior Center Canteens: A Total Exposure Assessment Applying the Duplicate Diet Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Food Samples
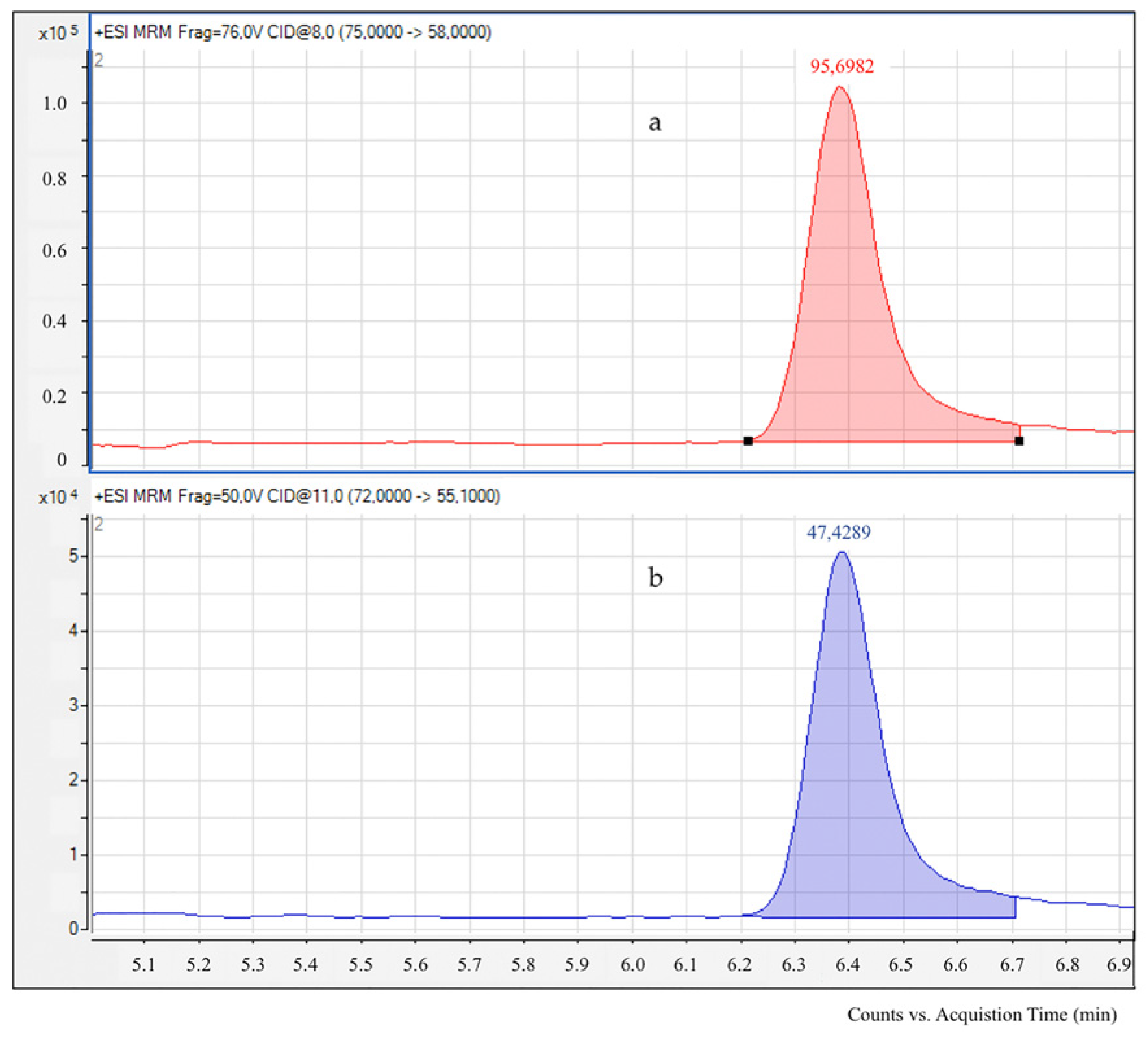
2.2. Acrylamide Determination by LC-ESI-MS/MS
2.3. Estimation of Acrylamide Exposure
2.4. Statistical Analysis
3. Results and Discussion
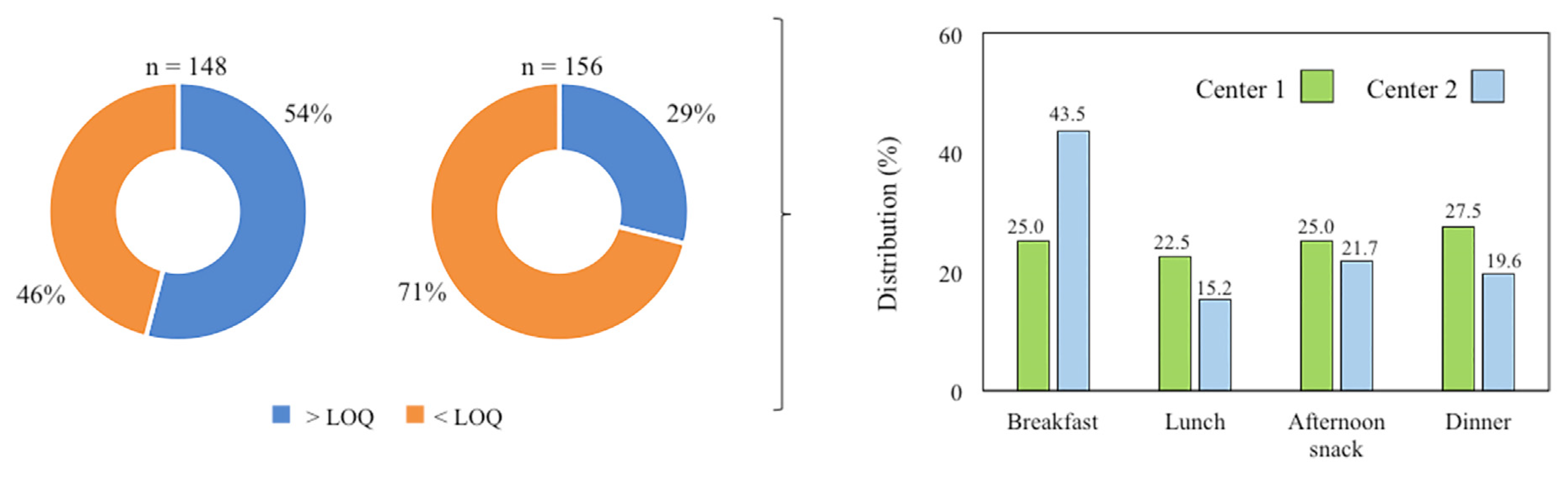
3.1. Analysis of Menus Offered in Senior Center Canteens
3.2. Acrylamide Content in Foods and Meals Provided in Senior Center Canteens
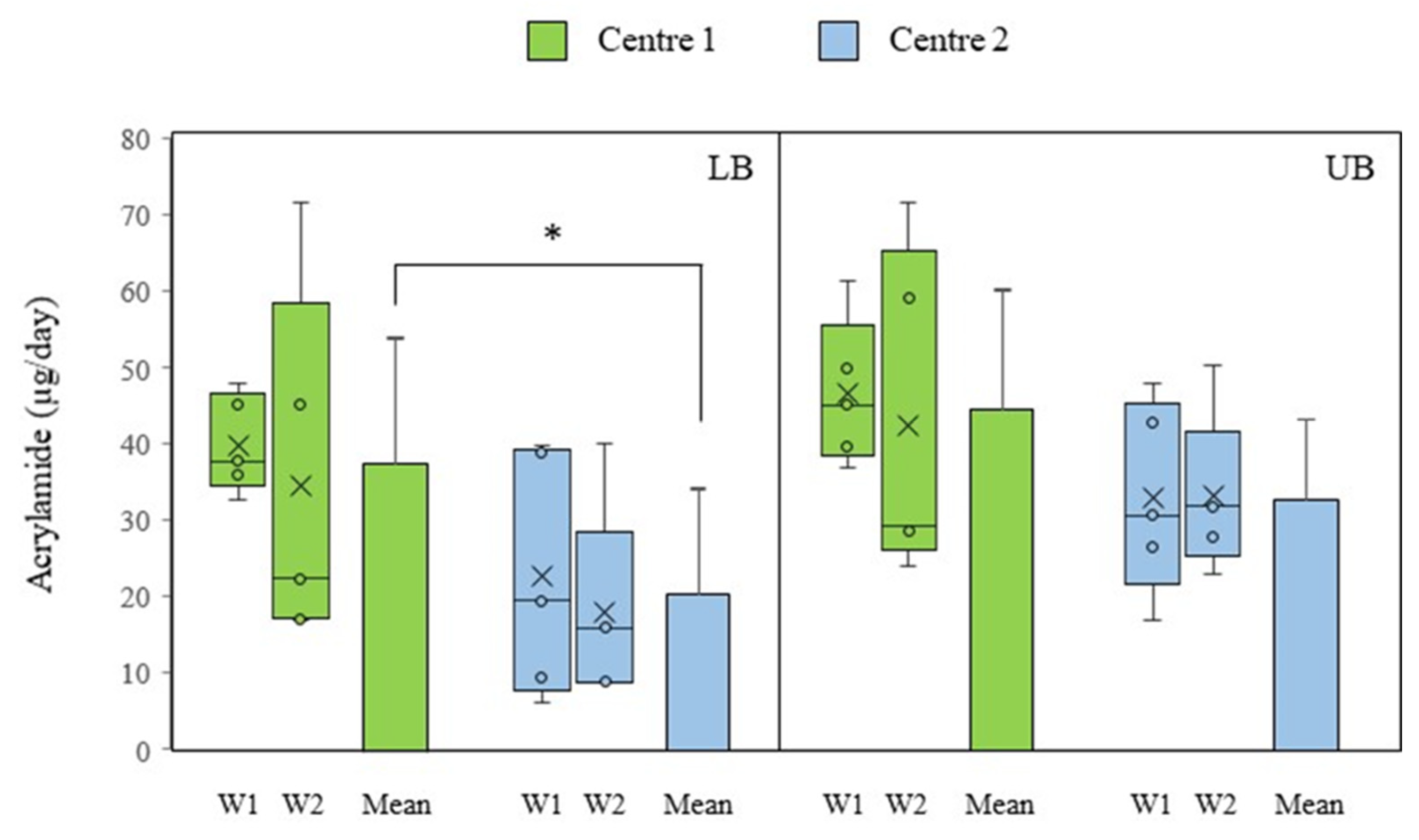
3.3. Acrylamide Intake in Senior Center Canteens
3.4. Dietary Exposure to Acrylamide in Senior Center Canteens and Risk Assessment
3.5. Limitations and Uncertainties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Rodrigo, C.; Gianzo, M.; Bárbara, G.H.; Aranceta-Bartrina, J. Prevalencia de obesidad y obesidad abdominal en la población española de 65 y más años de edad: Estudio ENPE. Med. Clín. 2022, 158, 49–57. [Google Scholar] [CrossRef]
- Schweighart, R.; O’Sullivan, J.L.; Klemmt, M.; Teti, A.; Neuderth, S. Wishes and needs of nursing home residents: A scoping review. Healthcare 2022, 10, 854. [Google Scholar] [CrossRef]
- Stokke, R.; Wibe, T.; Sogstad, M. Forming nursing home practices that support quality of care for residents. A qualitative observational study. J. Multidiscip. Healthc. 2023, 16, 2667–2680. [Google Scholar] [PubMed]
- Sahin, G.A.; Caferoglu, Z. The food service quality and its effects on nutritional status in nursing home residents. Clin. Nutr. ESPEN 2022, 47, 233–239. [Google Scholar]
- Food Safety Authority of Ireland. Monitoring & Surveillance Series, Chemical. Report on a Total Diet Study Carried Out by the Food Safety Authority of Ireland in the Period 2012–2014. Dublin. 2016. Available online: https://www.fsai.ie/getmedia/b96c171b-dce0-419b-a32e-43c3bce8e65a/tds-2014-final.pdf?ext=.pdf (accessed on 15 October 2024).
- AESAN (Agencia Española de Seguridad Alimentaria y Nutrición). Orientaciones para la Elaboración de Menús Saludables y Medioambientalmente Sostenibles en Centros Residenciales y Centros de día para Personas Mayores. 2023. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/nutricion/ORIENTACIONES_MAYORES.pdf (accessed on 25 November 2024).
- Lavriša, Ž.; Pravst, I.; Krušič, S.; Hren, N.; Gregorič, N.; Hren, I.; Koroušić Seljak, B.; Hristov, H. Nutrition among nursing home residents: Results from the NutriCare study. Front. Nutr. 2024, 11, 1423658. [Google Scholar]
- Abdelhay, S.M.M.; Ragab, H.M.; Hussein, Y.H.H. Assessment of Nutritional status and Malnutrition Problems among Elderly: An Egyptian Experience. Zagazig Univ. Med. J. 2024, 30, 1738–1749. [Google Scholar]
- Harris, J.A.; Castle, N.G. Obesity and nursing home care in the united states: A systematic review. Gerontologist 2019, 59, e196–e206. [Google Scholar]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Cieślik, I.; Cieślik, E.; Topolska, K.; Wiczkowski, W. Dietary acrylamide exposure from traditional food products in Lesser Poland and associated risk assessment. Ann. Agric. Environ. Med. 2020, 27, 225–230. [Google Scholar]
- Almeida Costa, S.S.; Correia, D.M.; Carvalho, C.; Vilela, S.; Severo, M.; Lopes, C.; Torres, D. Risk characterization of dietary acrylamide exposure and associated factors in the Portuguese population. Food Addit. Contam. 2022, 39, 888–900. [Google Scholar]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A.; Khaneghah, A.M. Acrylamide content of collected food products from Tehran’s market: A risk assessment study. Environ. Sci. Poll. Res. Int. 2020, 27, 30558–30570. [Google Scholar]
- González, N.; Marquès, M.; Calderón, J.; Collantes, R.; Corraliza, L.; Timoner, I.; Bosch, J.; Castell, V.; Domingo, J.L.; Nadal, M. Occurrence and dietary intake of food processing contaminants (FPCs) in Catalonia, Spain. J. Food Comp. Anal. 2022, 106, 104272. [Google Scholar]
- Kawahara, J.; Zheng, Y.; Terui, M.; Shinohara, A.; Uyama, K.; Yoneyama, M.; Nakajima, D.; Shibata, Y.; Adachi, S. Dietary exposure to acrylamide in a group of Japanese adults based on 24-hour duplicate diet samples. Food Add. Contam. Part A 2019, 36, 15–25. [Google Scholar]
- EFSA (European Food Safety Authority). The Food Classification and Description System FoodEx 2 (Draft-revision 1). EFSA Support. Publ. 2011, 215, 1–438. [Google Scholar]
- EFSA (European Food Safety Authority). The Food Classification and Description System FoodEx 2 (Revision 2). EFSA Support. Publ. 2015, 12, 1–90. [Google Scholar]
- González-Mulero, L.; Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Acrylamide exposure from common culinary preparations in Spain, in household, catering and industrial settings. Foods 2021, 10, 2008. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Altissimi, M.S.; Mercuri, M.L.; Haouet, N. Estimation of acrylamide exposure in Italian schoolchildren consuming a canteen menu: Health concern in three age groups. Int. J. Food Sci. Nutr. 2020, 71, 122–131. [Google Scholar]
- BOE (Boletín Oficial del Estado). 17/2011 Law, of July 5th, on Food Safety and Nutrition. Boletín Oficial del Estado 160. 6 July 2011. Available online: https://www.boe.es/boe/dias/2011/07/06/pdfs/BOE-A-2011-11604.pdf (accessed on 20 January 2023).
- AESAN (Agencia Española de Seguridad Alimentaria y Nutrición). Recomendaciones Dietéticas Saludables y Sostenibles Complementadas con Recomendaciones de Actividad Física Para la Población Española. 2022. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/nutricion/RECOMENDACIONES_DIETETICAS.pdf (accessed on 25 November 2024).
- EC (European Commission). Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, L304, 24–44. Available online: http://data.europa.eu/eli/reg/2017/2158/oj (accessed on 10 October 2022).
- Barón Cortés, W.R.; Vásquez Mejía, S.M.; Suárez Mahecha, H. Consumption study and margin of exposure of acrylamide in food consumed by the Bogotá population in Colombia. J. Food Comp. Anal. 2021, 100, 103934. [Google Scholar]
- Jeong, H.; Hwang, S.; Kwon, H. Survey for acrylamide in processed foods from Korean market and individual exposure estimation using a non-parametric probabilistic model. Food Add. Contam. Part A 2020, 37, 916–930. [Google Scholar]
- González-Mulero, L.; Delgado-Andrade, C.; Morales, F.J.; Mesías, M. Assessment of dietary acrylamide exposure in children attending school canteens using the duplicate diet method. Food Chem. Toxicol. 2023, 181, 114066. [Google Scholar] [PubMed]
- González-Mulero, L.; Delgado-Andrade, C.; Morales, F.J.; Mesías, M. Dietary exposure to acrylamide in Spanish university canteens by the duplicate diet method. Foods 2024, 12, 4263. [Google Scholar]
- Perestrelo, S.; Schwerbel, K.; Hessel-Pras, S.; Schäfer, B.; Kaminski, M.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: Acrylamide in foods from the German market with highest levels in vegetable crisps. Food Chem. X 2024, 22, 101403. [Google Scholar]
- Kuek, S.L.; Tarmizi, A.H.A.; Razak, R.A.A.; Jinap, A.; Norliza, S.; Sanny, M. Contribution of lipid towards acrylamide formation during intermittent frying of French fries. Food Contr. 2020, 118, 107430. [Google Scholar]
- Lee, S.; Kim, H.J. Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. Int. J. Environ. Res. Pub. Health 2020, 17, 7619. [Google Scholar] [CrossRef]
- Pogurschi, E.N.; Zugravu, C.A.; Ranga, I.N.; Trifunschi, S.; Munteanu, M.F.; Popa, D.C.; Tudorache, M.; Custura, I. Determination of Acrylamide in Selected Foods from the Romanian Market. Foods 2021, 10, 2110. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, J.N.; Razis, A.F.A.; Jambari, N.N.; Chai, L.C.; You, L.; Sanny, M. Dietary exposure to acrylamide among the Malaysian adult population. Food Chem. Toxicol. 2024, 185, 114502. [Google Scholar]
- Wong, W.W.K.; Chung, S.W.C.; Lam, C.-H.; Ho, Y.Y.; Xiao, Y. Dietary exposure of Hong Kong adults to acrylamide: Results of the first Hong Kong Total Diet Study. Food Add. Contam. Part A 2014, 31, 799–805. [Google Scholar]
- Food Safety Commission of Japan. Acrylamide in foods generated through heating. Food Saf. 2016, 4, 74–88. [Google Scholar]
- Zhu, F.; Wang, Y.; Liu, H.; Lyu, B.; Chen, D.; Li, J.; Ji, W.; Zhao, Y.; Wu, Y. Exposure to Acrylamide in the Sixth Total Diet Study—China, 2016–2019. China CDC Wkly. 2022, 4, 161–164. [Google Scholar] [CrossRef]
- Mojska, H.; Gieleciśska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Ishihara, J.; Yamamoto, J.; Hosoda, T.; Kotemori, A.; Takachi, R.; Nakamura, K.; Tanaka, J.; Yamaji, T.; Shimazu, T.; et al. Variations in the estimated intake of acrylamide from food in the Japanese population. Nutr. J. 2020, 19, 17. [Google Scholar] [CrossRef]
- Liu, Z.; Tse, L.A.; Ho, S.C.; Wu, S.; Chen, B.; Chan, D.; Wong, S.Y. Dietary acrylamide exposure was associated with increased cancer mortality in Chinese elderly men and women: A 11-year prospective study of Mr. and Ms. OS Hong Kong. J. Cancer Res. Clin. Oncol. 2017, 143, 2317–2326. [Google Scholar]
- Tran, N.; Barraj, L.; Murphy, M.; Bi, X. Dietary acrylamide exposure and hemoglobin adducts—National Health and Nutrition Examination Survey (2003–04). Food Chem. Toxicol. 2010, 48, 3098–3108. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Rosato, V.; Bracci, P.M.; Li, D.; Neale, R.E.; Lucenteforte, E.; Serraino, D.; Anderson, K.E.; Fontham, E.; Holly, E.A.; et al. Dietary acrylamide and the risk of pancreatic cancer in the International Pancreatic Cancer Case–Control Consortium (PanC4). Ann. Oncol. 2017, 28, 408–414. [Google Scholar]
- Lujan-Barroso, L.; González, C.A.; Slimani, N.; Obón-Santacana, M.; Ferrari, P.; Freisling, H.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Racine, A.; et al. Dietary intake of acrylamide and esophageal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Causes Control 2014, 25, 639–646. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.F.; Zhu, F.; Ma, Y.J.; Li, X.W.; Miao, H.; Wu, Y. Dietary exposure of acrylamide from the fifth Chinese Total Diet Study. Food Chem. Toxicol. 2016, 87, 97–102. [Google Scholar] [CrossRef]
- Mititelu, M.; Udeanu, D.I.; Docea, A.O.; Tsatsakis, A.; Calina, D.; Arsene, A.L.; Nedelescu, M.; Neacsu, S.M.; Velescu, B.S.; Ghica, M. New method for risk assessment in environmental health: The paradigm of heavy metals in honey. Environ. Res. 2023, 236, 115194. [Google Scholar]
- Health Canada. 2012. Health Canada’s Revised Exposure Assessment of Acrylamide in Food. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/securit/chem-chim/food-aliment/acrylamide/rev-eval-exposure-exposition-eng.pdf (accessed on 7 October 2024).
- Geraets, L.; te Biesebeek, J.D.; van Donkersgoed, G.; Koopman, N.; Boon, P.E. The Intake of Acrylamide, Nitrate and Ochratoxin A in People Aged 7 to 69 Living in The Netherlands; RIVM Letter Report: 2014-0002; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2014. Available online: https://www.rivm.nl/bibliotheek/rapporten/2014-0002.pdf (accessed on 20 July 2024).
- Sirot, V.; Hommet, F.; Tard, A.; Leblanc, J.C. Dietary acrylamide exposure of French population: Results of the second French Total Study. Food Chem. Toxicol. 2012, 50, 889–894. [Google Scholar]
- Liao, K.W.; Chang, F.C.; Chang, C.H.; Huang, Y.F.; Pan, W.H.; Chen, M.L. Associating acrylamide internal exposure with dietary pattern and health risk in the general population of Taiwan. Food Chem. 2022, 374, 131653. [Google Scholar] [CrossRef] [PubMed]
- Vryonidis, E.; Törnqvist, M.; Myhre, O.; Dirven, H.; Husøy, T. Dietary intake of acrylamide in the Norwegian EuroMix biomonitoring study: Comparing probabilistic dietary estimates with haemoglobin adduct measurements. Food Chem. Toxicol. 2023, 180, 114031. [Google Scholar] [PubMed]
| (A) Menus from Senior Center 1, Week 1 | ||||
| Breakfast | Lunch | Afternoon Snack | Dinner | |
| Monday | Milk Instant decaffeinated coffee Muffin | Stewed beans Breaded dab with French fries Orange Bread | Milk Instant cocoa Biscuits | Cauliflower gratin Breaded pork fillet with ham and cheese Yogurt Whole wheat bread |
| Tuesday | Milk Instant decaffeinated coffee Toasted bread with oil/butter | Soup Typical chickpea stew made with pork and beef meat and vegetables Banana Bread | Milk Instant cocoa Biscuits | Carrot puree Breaded eggs with bechamel sauce and sautéed vegetables Yogurt Whole wheat bread |
| Wednesday | Milk Instant decaffeinated coffee Muffin | Breadcrumbs with pork meat and vegetables Salmon stew with vegetables Apple Bread | Milk Instant cocoa Biscuits | Pork and beef meat and vegetable soup Breaded chicken with French fries Yogurt Whole wheat bread |
| Thursday | Milk Instant decaffeinated coffee Muffin | Noodles with pork meat and vegetables Breaded tilapia with salad Apple Bread | Milk Instant cocoa Biscuits | Vegetable puree Spanish omelet with roasted red peppers Yogurt Whole wheat bread |
| Friday | Milk Instant decaffeinated coffee Toasted bread with oil/butter | Rice with pork meat and vegetables Chicken and garlic stew with French fries Apple Bread | Milk Instant cocoa Biscuits | Sautéed green beans Assorted cold cuts Yogurt Whole wheat bread |
| (B) Menus from Senior Center 1, Week 2 | ||||
| Breakfast | Lunch | Afternoon Snack | Dinner | |
| Monday | Milk Instant decaffeinated coffee Muffin | Stewed lentils Breaded ham with bechamel sauce and salad Pear Bread | Milk Instant cocoa Biscuits | Fish soup Spanish omelet with roasted red peppers Yogurt Whole wheat bread |
| Tuesday | Milk Instant decaffeinated coffee Muffin | Rice with chicken Stewed pork with tomato and vegetables Banana Bread | Milk Instant cocoa Biscuits | Sautéed vegetables Breaded tilapia with French fries Yogurt Whole wheat bread |
| Wednesday | Milk Instant decaffeinated coffee Toasted bread with oil/butter | Soup Typical chickpea stew made with pork and beef meat and vegetables Pear Bread | Milk Instant cocoa Biscuits | Breaded squid Tomato salad Yogurt Whole wheat bread |
| Thursday | Milk Instant decaffeinated coffee Muffin | Macaroni with pork meat and tomato Fried eggs with French fries Apple Bread | Milk Instant cocoa Biscuits | Assorted cold cuts Yogurt Whole wheat bread |
| Friday | Milk Instant decaffeinated coffee Muffin | Stewed beans Breaded hake with sautéed vegetables Banana Bread | Milk Instant cocoa Biscuits | Mackerel with roasted red peppers Yogurt Whole wheat bread |
| (C) Menus from Senior Center 2, Week 1 | ||||
| Breakfast | Lunch | Afternoon Snack | Dinner | |
| Monday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Mixed salad Rice with pork meat, fish and vegetables Profiteroles Bread | Milk Instant cocoa Bakery products | Tomato soup Tuna omelet Mandarin oranges Bread |
| Tuesday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Stewed beans Tuna patties with salad Pear Bread | Milk Instant cocoa Bakery products | Zucchini puree Fried anchovies with roasted pepper salad Yogurt Bread |
| Wednesday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Roasted vegetables Potato stew with beef meat Yogurt Bread | Milk Instant cocoa Bakery products | Rice soup Fresh cheese with quince jam Mandarin orange Bread |
| Thursday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Fideuá with seafood Breaded pork fillet with ham and cheese Banana Bread | Milk Instant cocoa Bakery products | Mushroom cream Breaded hake with oven-baked potatoes Roasted apple Bread |
| Friday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Seafood soup Fried eggs with sautéed vegetables Custard Bread | Milk Instant cocoa Bakery products | Sautéed red cabbage Zucchini omelet Yogurt Bread |
| (D) Menus from Senior Center 2, Week 2 | ||||
| Breakfast | Lunch | Afternoon Snack | Dinner | |
| Monday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Stewed lentils Ham croquettes with salad Pear Bread | Milk Instant cocoa Bakery products | Vegetable soup Breaded cod Apple pie Bread |
| Tuesday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Stuffed eggplants with pork meat Potato stew with fish and seafood Yogurt Bread | Milk Instant cocoa Bakery products | Vegetable puree Roasted ham with oven-baked potatoes Mandarin orange Bread |
| Wednesday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Roasted red pepper salad Macaroni with tuna Banana Bread | Milk Instant cocoa Bakery products | Pork and beef meat and vegetable soup Vegetable omelet French toast Bread |
| Thursday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Soup Typical chickpea stew made with pork and beef meat and vegetables Custard Bread | Milk Instant cocoa Bakery products | Scrambled eggs with mushrooms Baked mako shark with oven-baked potatoes Mandarin orange Bread |
| Friday | Fruit juice Milk Instant decaffeinated coffee Mix of cereals and dairy products | Mixed salad Rice with pork meat, fish and vegetables Mandarin orange Bread | Milk Instant cocoa Bakery products | Tomato soup Stewed chicken with vegetables and French fries Yogurt Bread |
| (A) Senior Center 1 | ||||||
| Meal | Foods | n | FoodEx2 Code | Acrylamide Content (µg/kg) | Edible Serving (g) | Acrylamide Contribution (µg/Serving) |
| Breakfast and afternoon snack | Instant decaffeinated coffee | 10 | A03GR | 2831 ± 181 | 2 | 5.7 ± 0.4 |
| Instant cocoa | 10 | A03HG | 261 ± 30 | 14 | 3.6 ± 0.4 | |
| Muffins | 7 | A00BC | 21 ± 5 | 31 | 0.9 ± 0.1 | |
| Toasted bread | 3 | A006D | 34 ± 7 | 23 | 0.8 ± 0.2 | |
| Biscuits | 10 | A009X | 122 ± 120 | 21 | 2.7 ± 2.7 | |
| Lunches and dinners | Stewed beans | 2 | A03VT | 33 ± 25 | 413 | 11.6 ± 5.4 |
| Stewed lentils | 1 | A00QD#F28. A07GM | 27 | 282 | 7.6 | |
| Carrot puree | 1 | A03XY#F04. A05JG | 31 | 41 | 12.5 | |
| Pork and beef meat and vegetable soup | 1 | A041V | 22 | 432 | 9.4 | |
| Fish soup | 1 | A041X | 40 | 353 | 14.2 | |
| Typical chickpea stew made with pork and beef meat and vegetables | 2 | A03VK#F04. A00SL$F28. A07GM | 17 ± 4 | 339 | 5.8 ± 0.1 | |
| Breadcrumbs with pork meat and vegetables | 1 | A007A#F04. A022Y$F04. A00HC$F04. A05CN$F04. A05CP$F28. A07GT | 101 | 156 | 15.8 | |
| Breaded ham with bechamel sauce | 1 | A022T#F04. A043X$F28. A07HK | 33 | 117 | 3.8 | |
| Breaded pork fillet with ham and cheese | 1 | A03XD | 24 | 86 | 2.1 | |
| Breaded eggs with bechamel sauce | 1 | A032B#F04. A043X$F28. A07HK | 23 | 105 | 2.4 | |
| Breaded chicken | 1 | A01SP#F28. A07HK$F28. A07GS | 26 | 102 | 2.6 | |
| Spanish omelet | 2 | A03YN#F28. A00ZT | 161 ± 6 | 134 | 21.6 ± 0.1 | |
| Roasted red peppers | 2 | A05CN#F28. A07GX | 160 ± 10 | 42 | 6.7 ± 0.3 | |
| French fries | 5 | A0BYV | 213 ± 158 | 69 | 13.9 ± 11.9 | |
| Whole wheat bread | 10 | A005E | 29 ± 7 | 49 | 1.4 ± 0.5 | |
| Bread | 10 | A004Y | 28 ± 0.6 | 48 | 1.3 ± 0.1 | |
| (B) Senior center 2 | ||||||
| Meal | Foods | n | FoodEx2 code | Acrylamide Content (µg/kg) | Edible Serving (g) | Acrylamide Contribution (µg/Serving) |
| Breakfast and afternoon snack | Instant decaffeinated coffee | 10 | A03GR | 192 ± 4 | 2 | 0.4 ± 0.1 |
| Instant cocoa | 10 | A03HG | 20 ± 2 | 10 | 0.2 ± 0.1 | |
| Mix of cereals and dairy products | 10 | A06DX | 45 ± 2 | 124 | 5.5 ± 0.2 | |
| Lunches and dinners | Stuffed eggplants with pork meat | 1 | A03YF#F04. A00JD$F04. A049S$F04. A16FE | 36 | 290 | 10.5 |
| Stewed beans | 1 | A03VT | 23 | 405 | 9.2 | |
| Seafood soup | 1 | A03XK | 29 | 555 | 16.1 | |
| Tomato soup | 2 | A041N | 26 ± 15 | 486 | 14.0 ± 12.3 | |
| Tuna patties | 1 | A0FBT#F02. A00CC04. A00ZD | 41 | 104 | 4.2 | |
| Breaded pork fillet with ham and cheese | 1 | A03XD | 32 | 101 | 3.2 | |
| Vegetable omelet | 1 | A03YQ | 65 | 150 | 9.8 | |
| Zucchini omelet | 1 | A03YN#F04. A00JR | 55 | 180 | 9.9 | |
| Rice with pork meat, fish and vegetables | 2 | A041D | 25 ± 5 | 335 | 8.4 ± 2.5 | |
| Sautéed vegetables | 1 | A03YD | 25 | 309 | 7.7 | |
| Oven-baked potatoes | 3 | A011R | 20 ± 9 | 106 | 2.0 ± 0.6 | |
| French fries | 1 | A0BYV | 304 | 90 | 27.3 | |
| Apple pie | 1 | A00BA#F04. A01DJ | 30 | 93 | 2.8 | |
| (A) Senior Center 1 | |||||
| Meal | Foods | n | FoodEx2 Code | Edible Serving (g) | Acrylamide Contribution (µg/Serving) |
| Lunches and dinners | Noodles with pork meat and vegetables | 1 | A041V#F04. A007D | 527 | 7.9 |
| Rice with pork meat and vegetables | 1 | A041J | 305 | 4.6 | |
| Rice with chicken | 1 | A041H | 362 | 5.4 | |
| Macaroni with pork meat and tomato | 1 | A040Q#F04. A049S$F04. A16FE | 368 | 5.5 | |
| Sautéed vegetables | 1 | A03YD | 322 | 4.8 | |
| Vegetable puree | 1 | A03XY | 367 | 5.5 | |
| Cauliflower gratin | 1 | A03YF#F04. A00FR | 216 | 3.2 | |
| Sautéed green beans | 1 | A00PG#F28. A07GT | 186 | 2.8 | |
| Breaded dab | 1 | A02AS#F28. A07HK$F28. A07GS | 67 | 1.0 | |
| Salmon stew with vegetables | 1 | A028P#F04. A044E | 137 | 2.0 | |
| Breaded tilapia | 2 | A028A#F28. A07HK$F28. A07GS | 68 | 1.0 ± 0.1 | |
| Chicken and garlic stew | 1 | A01SP#F04. A045A$F28. A07GM | 114 | 1.7 | |
| Stewed pork with tomato and vegetables | 1 | A03VK | 169 | 2.5 | |
| Fried eggs | 1 | A032C | 45 | 0.7 | |
| Breaded hake | 1 | A02CB #F28. A07HK | 84 | 1.3 | |
| Sautéed vegetables | 2 | A03YD | 119 | 1.8 ± 0.3 | |
| Breaded squid | 1 | A02JJ#F28. A07HL$F28. A07GV | 77 | 1.2 | |
| Mackerel with roasted red peppers | 1 | A02CR#F04. A05CNA05CN$F28. A07GX | 99 | 1.5 | |
| (B) Senior center 2 | |||||
| Meal | Foods | n | FoodEx2 code | Edible Serving (g) | Acrylamide Contribution (µg/Serving) |
| Lunches and dinners | Roasted vegetables | 1 | A03YF | 169 | 2.5 |
| Roasted red pepper salad | 1 | A042D#F04. A05CN | 150 | 2.3 | |
| Potato stew with beef meat | 1 | A03VJ | 360 | 5.4 | |
| Fideuá with seafood | 1 | A040Q#F04. A0EYX | 229 | 3.4 | |
| Sautéed red cabbage | 1 | A00GA#F28. A07GT | 179 | 2.7 | |
| Stewed lentils | 1 | A00QD#F04. A025C$F28. A07GM | 512 | 7.7 | |
| Potato stew with fish and seafood | 1 | A03XQ#F04. A0EYX | 417 | 6.3 | |
| Roasted pepper salad | 1 | A05CN#F28. A07GX | 161 | 2.4 | |
| Zucchini pure | 1 | A03XY#F04. A00JR | 429 | 6.4 | |
| Mushroom cream | 1 | A03XY#F04. A00TQ | 451 | 6.8 | |
| Vegetable soup | 1 | A041S | 306 | 4.6 | |
| Vegetable puree | 1 | A03XY | 392 | 5.9 | |
| Pork and beef meat and vegetable soup | 1 | A041V | 359 | 5.4 | |
| Roasted ham | 1 | A023K | 62 | 0.9 | |
| Fried eggs | 1 | A032C | 122 | 1.8 | |
| Ham croquettes | 1 | A022TA022T#F04. A043X$F28. A07HL$F28. A07GS | 124 | 1.9 | |
| Macaroni with tuna | 1 | A007S#F04. A0FBT | 285 | 4.3 | |
| Typical chickpea stew made with pork and beef meat and vegetables | 1 | A03VK#F04. A00SL$F28. A07GM | 303 | 4.5 | |
| Tuna omelet | 1 | A03YN#$F04. A0FBT | 85 | 1.3 | |
| Fried anchovies | 1 | A02DD#F28. A07GS | 41 | 0.6 | |
| Quince jam | 1 | A01DR#F28. A07JQ | 66 | 1.0 | |
| Breaded hake | 1 | A02CB#F28. A07HK$F28. A07GS | 91 | 1.4 | |
| Breaded cod | 1 | A02BV#F28. A07HK$F28. A07GS | 185 | 2.8 | |
| Scrambled eggs with mushrooms | 1 | A03YN#$F04. A00TQ | 261 | 3.9 | |
| Baked mako shark | 1 | A052E#F28. A07GX | 145 | 2.2 | |
| Stewed chicken with vegetables | 1 | A03VK#F04. A01SP | 213 | 3.2 | |
| Roasted apple | 1 | A01DJ#F28. A07GX | 114 | 1.7 | |
| Profiteroles | 1 | A00AK | 58 | 0.9 | |
| French toast | 1 | A0DRD#F04. A02LT$F04. A 0BY6$F04. A019V$F28. A07GV | 132 | 2.0 | |
| Custard | 1 | A02PX | 120 | 1.8 ± 0.1 | |
| Bread | 20 | A004Y | 45 | 0.7 ± 0.1 | |
| Afternoon snack | Bakery products | 10 | A009T | 35 | 0.5 ± 0.1 |
| Lower Exposure Scenario | |||||
| Coffee (%) | Cereal-Based Products (%) | Potato-Based Dishes (%) | Cocoa (%) | Other Foods/Meals (%) | |
| Senior center 1 | |||||
| Week 1 | 11.8–17.3 | 8.0–20.7 | 0–53.5 | 7.6–11.1 | 0–58.0 |
| Week 2 | 7.9–33.4 | 8.9–45.7 | 0–70.6 | 5.1–21.5 | 0–46.2 |
| Senior center 2 | |||||
| Week 1 | 1.0–6.3 | 13.9–90.4 | 0 | 0.5–3.3 | 0–84.6 |
| Week 2 | 1.0–4.4 | 13.8–63.3 | 0–68.3 | 0.5–2.3 | 0–61.6 |
| Both centers | 1.0–33.4 | 8.0–90.4 | 0–70.6 | 0.5–21.5 | 0–84.6 |
| Upper Exposure Scenario | |||||
| Coffee (%) | Cereal-Based Products (%) | Potato-Based Dishes (%) | Cocoa (%) | Other Foods/Meals (%) | |
| Senior center 1 | |||||
| Week 1 | 9.2–15.3 | 7.7–19.3 | 0–42.7 | 5.9–9.9 | 20.1–59.7 |
| Week 2 | 7.9–23.7 | 6.8–32.0 | 0–54.1 | 5.1–15.3 | 21.7–46.2 |
| Senior center 2 | |||||
| Week 1 | 0.9–2.3 | 15.4–43.7 | 0–31.9 | 0.4–1.2 | 20.8–83.4 |
| Week 2 | 0.8–1.7 | 14.7–32.1 | 0–54.2 | 0.4–0.9 | 30.0–71.2 |
| Both centers | 0.8–23.7 | 6.8–43.7 | 0–54.2 | 0.4–15.3 | 20.1–83.4 |
| Women | Men | |||
|---|---|---|---|---|
| LB | UB | LB | UB | |
| Senior center 1 | ||||
| Exposure range (µg/kg bw/day) | 0.52 ± 0.23 a | 0.62 ± 0.22 a | 0.47 ± 0.21 a | 0.55 ± 0.19 a |
| MOE for neurotoxic effects | 820 | 691 | 923 | 307 |
| MOE for neoplastic effects | 324 | 273 | 365 | 777 |
| Senior center 2 | ||||
| Mean exposure (µg/kg bw/day) | 0.28 ± 0.19 b | 0.46 ± 0.15 a | 0.25 ± 0.17 b | 0.41 ± 0.13 a |
| MOE for neurotoxic effects | 1519 | 934 | 1708 | 1051 |
| MOE for neoplastic effects | 600 | 369 | 675 | 415 |
| p value | 0.021 | 0.069 | 0.021 | 0.069 |
| Both centers | ||||
| Mean exposure (µg/kg bw/day) | 0.40 ± 0.24 | 0.54 ± 0.20 | 0.36 ± 0.21 | 0.48 ± 0.18 |
| MOE for neurotoxic effects | 1065 | 795 | 1198 | 894 |
| MOE for neoplastic effects | 421 | 314 | 474 | 353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesias, M.; González-Mulero, L.; Morales, F.J.; Delgado-Andrade, C. Acrylamide Intake in Senior Center Canteens: A Total Exposure Assessment Applying the Duplicate Diet Approach. Foods 2025, 14, 1073. https://doi.org/10.3390/foods14061073
Mesias M, González-Mulero L, Morales FJ, Delgado-Andrade C. Acrylamide Intake in Senior Center Canteens: A Total Exposure Assessment Applying the Duplicate Diet Approach. Foods. 2025; 14(6):1073. https://doi.org/10.3390/foods14061073
Chicago/Turabian StyleMesias, Marta, Lucía González-Mulero, Francisco J. Morales, and Cristina Delgado-Andrade. 2025. "Acrylamide Intake in Senior Center Canteens: A Total Exposure Assessment Applying the Duplicate Diet Approach" Foods 14, no. 6: 1073. https://doi.org/10.3390/foods14061073
APA StyleMesias, M., González-Mulero, L., Morales, F. J., & Delgado-Andrade, C. (2025). Acrylamide Intake in Senior Center Canteens: A Total Exposure Assessment Applying the Duplicate Diet Approach. Foods, 14(6), 1073. https://doi.org/10.3390/foods14061073







