Metabolomic Analysis of Different Parts of Black Wax Gourd (Cucurbita pepo)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. UPLC-MS/MS Acquisition Analysis
2.2.1. UPLC Conditions
2.2.2. ESI-Q TRAP-MS/MS
2.3. Qualitative and Quantitative Result Analysis
2.4. Statistical Analyses of Metabolite Data
3. Results
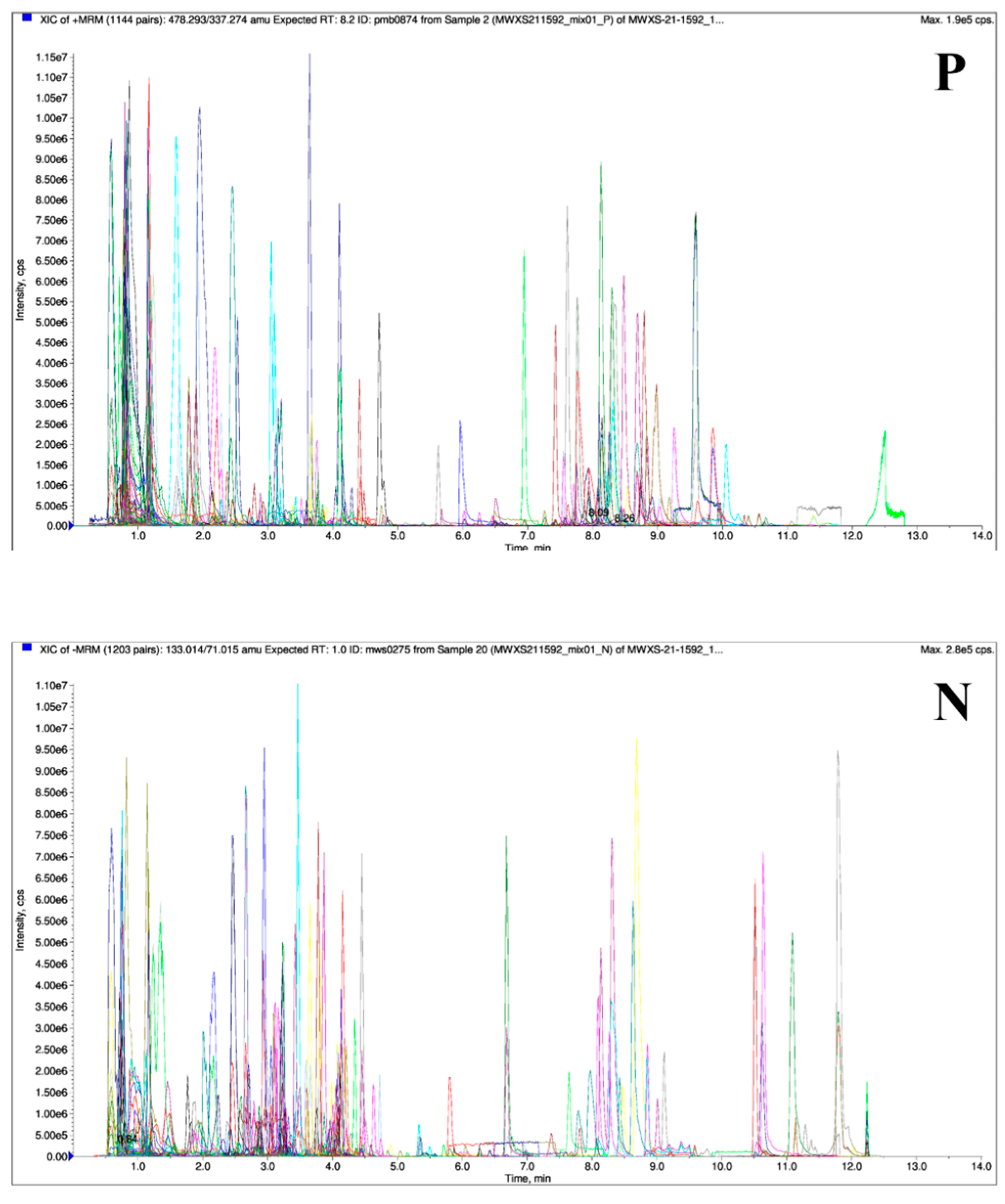
3.1. UPLC-MS/MS Analysis of Different Parts of Wax Gourds
3.1.1. Quality Control, Statistics, and Metabolite Identification of Different Parts of Wax Gourd Samples
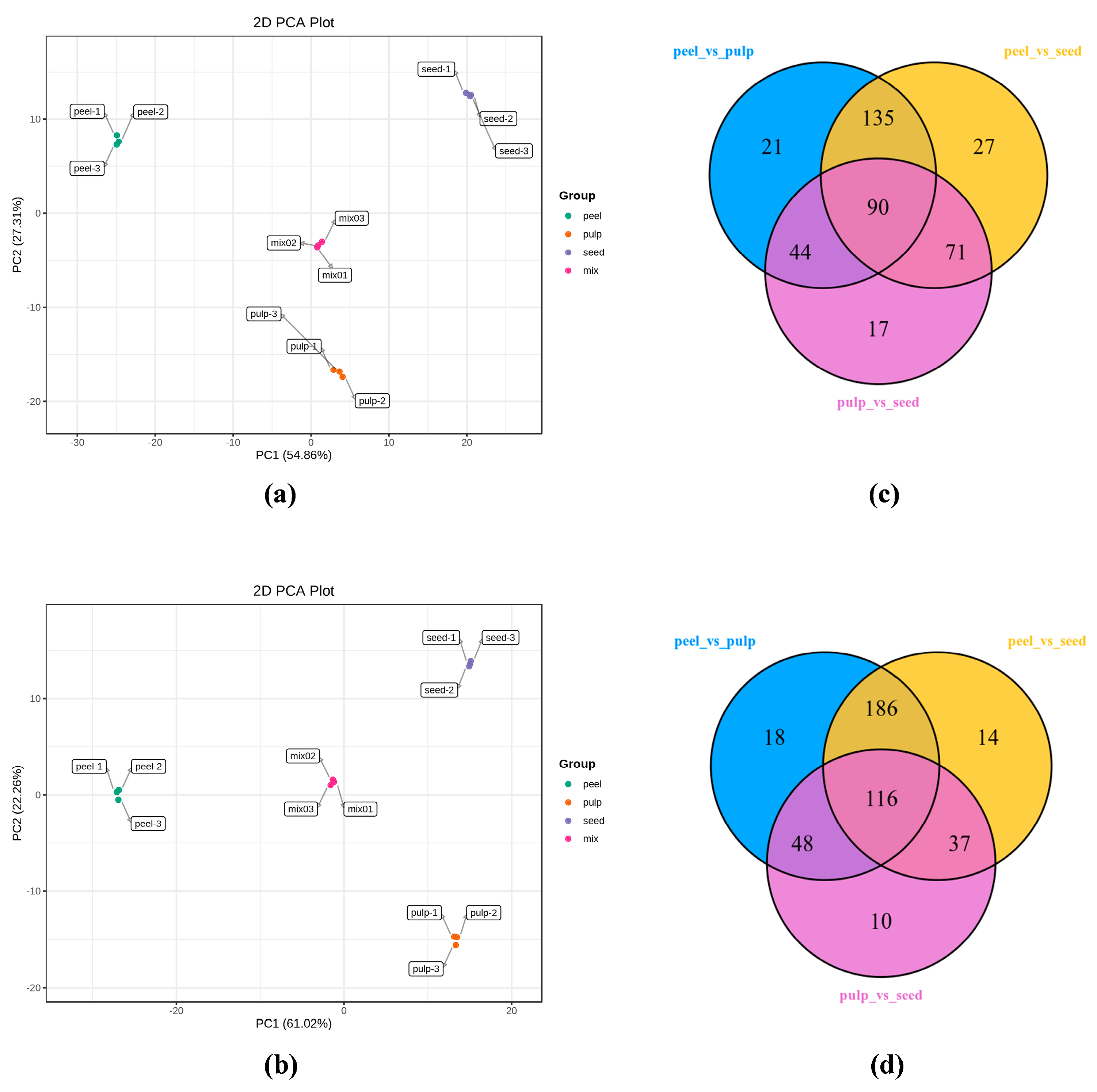
3.1.2. Screening for Differential Metabolites
3.1.3. Analysis of Common Differential Metabolites
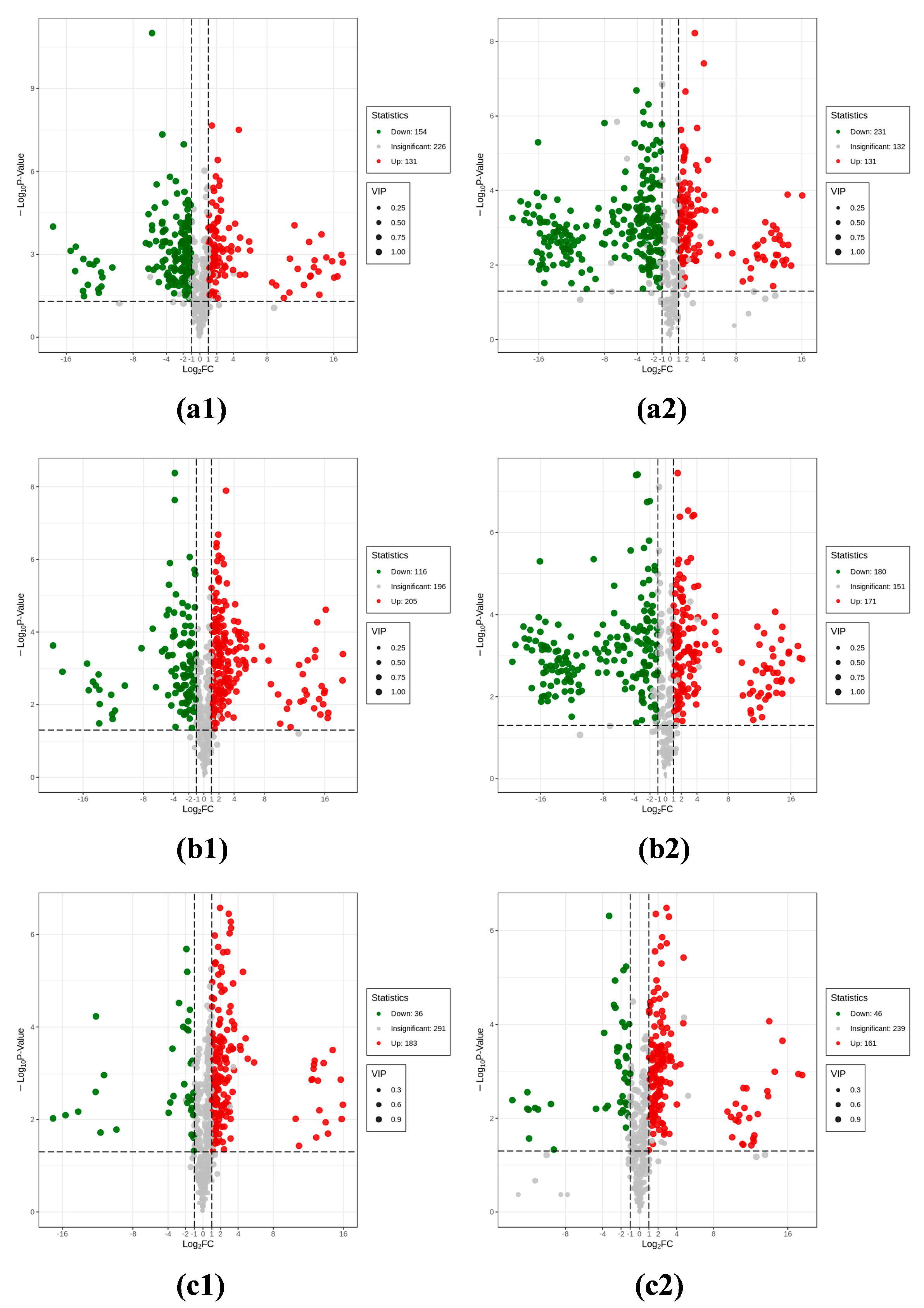
3.1.4. Analysis of Differential Metabolites
3.1.5. Analysis of Unique Differential Metabolites in Peel vs. Pulp
3.1.6. Analysis of Unique Differential Metabolites in Peel vs. Seeds
3.1.7. Analysis of Unique Differential Metabolites in Pulp vs. Seeds
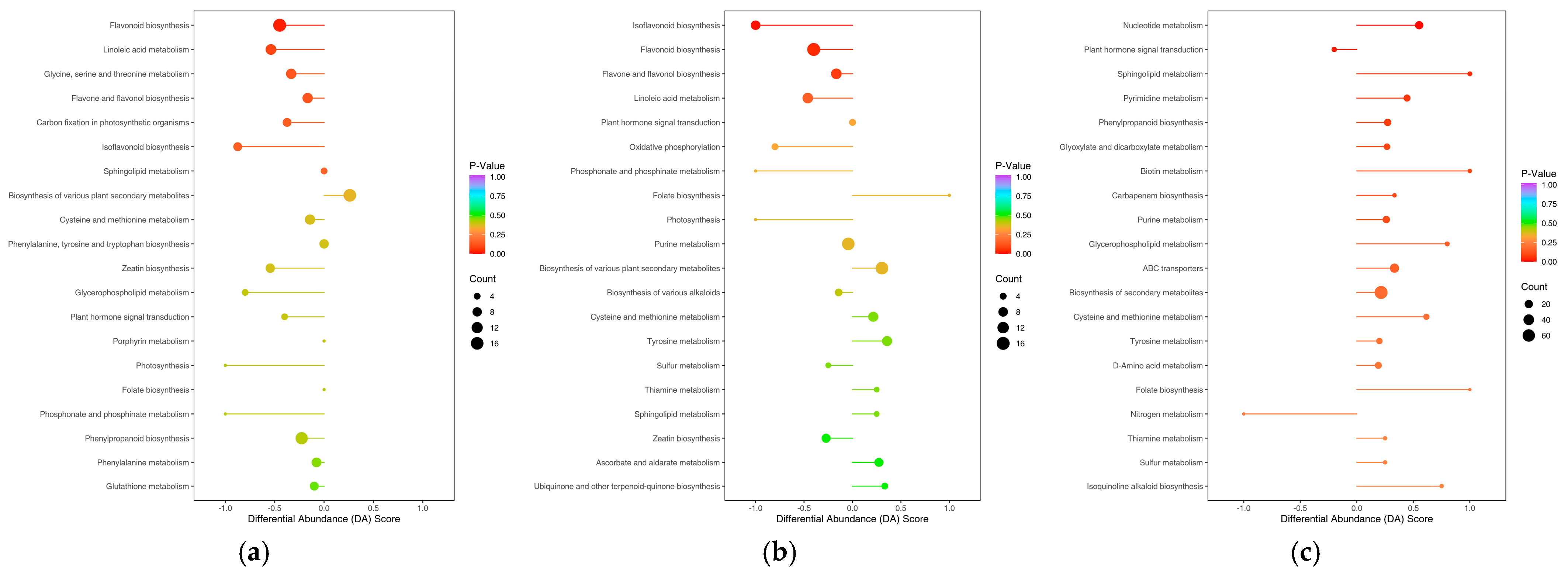
3.2. Differential Metabolite KEGG Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Chen, Z.; He, L.; Feng, Q.; Zhang, H.; Du, G.; Shi, C.; Wang, S. Comparative Chloroplast Genome Analysis of Wax Gourd (Benincasa Hispida) with Three Benincaseae Species, Revealing Evolutionary Dynamic Patterns and Phylogenetic Implications. Genes 2022, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Rosskopf, E.N.; Jeffries, K.A.; Zhao, W.; Plotto, A. Soil Amendment and Storage Effect the Quality of Winter Melons (Benincasa Hispida (Thunb) Cogn.) and Their Juice. Foods 2023, 12, 209. [Google Scholar] [CrossRef]
- Parvathi, M.S.; Antony, P.D.; Kutty, M.S. Multiple Stressors in Vegetable Production: Insights for Trait-Based Crop Improvement in Cucurbits. Front. Plant Sci. 2022, 13, 861637. [Google Scholar] [CrossRef]
- Mondal, I.H.; Rangan, L.; Uppaluri, R.V.S. Process-Product Characteristics of Tray-Dried Benincasa Hispida. J. Food Process. Preserv. 2020, 44, e14697. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.A.; Manthey, J.; Dorado, C.; Rivera, T.; Bai, J.; Hernández, F. Effect of Preprocessing Storage Temperature and Time on the Physicochemical Properties of Winter Melon Juice. J. Food Qual. 2022, 2022, 3237639. [Google Scholar] [CrossRef]
- Kubo, K.; Pritchard, B.; Phyo, A.S. How Chinese Demand for Fresh Fruit and Vegetables Is Creating New Landscapes of Rural Development and Vulnerability in Southeast Asia: Insights from the Myanmar Melon Frontier. Geoforum 2021, 122, 32–40. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Hasan, N.; Begum, K.; Hoque, S.M.Z. Degradation Kinetics of Lycopene from Red Amaranth & Preparation of Winter Melon Jelly Using This Lycopene and Comparison with Commercial Jelly. Heliyon 2024, 10, e31135. [Google Scholar]
- Kaleem, M.M.; Nawaz, M.A.; Ding, X.; Wen, S.; Shireen, F.; Cheng, J.; Bie, Z. Comparative Analysis of Pumpkin Rootstocks Mediated Impact on Melon Sensory Fruit Quality through Integration of Non-Targeted Metabolomics and Sensory Evaluation. Plant Physiol. Biochem. 2022, 192, 320–330. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, M.B.P.P.; Costa, H.S. Melon (Cucumis melo L.) by-Products: Potential Food Ingredients for Novel Functional Foods? Trends Food Sci. Technol. 2020, 98, 181–189. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible Seeds from Cucurbitaceae Family as Potential Functional Foods: Immense Promises, Few Concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and Solutions of Extracting Value-Added Ingredients from Fruit and Vegetable by-Products: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Silva, J.A.L.; Pintado, M. Fruit and Vegetable by-Products’ Flours as Ingredients: A Review on Production Process, Health Benefits and Technological Functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Al-Nablsi, S.; El-Keblawy, A.; Ali, M.A.; Mosa, K.A.; Hamoda, A.M.; Shanableh, A.; Almehdi, A.M.; Soliman, S.S.M. Phenolic Contents and Antioxidant Activity of Citrullus Colocynthis Fruits, Growing in the Hot Arid Desert of the UAE, Influenced by the Fruit Parts, Accessions, and Seasons of Fruit Collection. Antioxidants 2022, 11, 656. [Google Scholar] [CrossRef]
- Romo-Tovar, J.; Cerda, R.B.; González-Chávez, M.L.C.; Rodríguez-Jasso, R.M.; Sepúlveda, S.A.L.; Salas, M.G.; Treviño, A.L. Importance of Certain Varieties of Cucurbits in Enhancing Health: A Review. Foods 2024, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.R.; Meghwal, M.; Prabhakar, P.K.; Giuffrè, A.M. A Comparative Study on the Nutritional, Antioxidant, Thermal, Morphological and Diffraction Properties of Selected Cucurbit Seeds. Agronomy 2022, 12, 2242. [Google Scholar] [CrossRef]
- Azpiazu, C.; Medina, P.; Adán, A.; Sánchez-Ramos, I.; Estal, P.D.; Fereres, A.; Vinuela, E. The Role of Annual Flowering Plant Strips on a Melon Crop in Central Spain. Influence on Pollinators and Crop. Insects 2020, 11, 66. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Yang, X.; Deng, X.; Dang, J.; Wang, Z.; Yusop, M.R.; Abdullah, S. Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita Moschata Duch.) and Winter Squash (Cucurbita Maxima Duch.). Horticulturae 2022, 8, 596. [Google Scholar] [CrossRef]
- Wu, W.; Wang, P.; Huang, X.; Su, L.; Lv, H.; Gou, J.; Cheng, Z.; Ma, L.; Yu, W.; Liu, Z. Fine Mapping and Functional Analysis of Major Regulatory Genes of Soluble Solids Content in Wax Gourd (Benincasa Hispida). Int. J. Mol. Sci. 2022, 23, 6999. [Google Scholar] [CrossRef]
- Xue, S.; Wan, X.; Lu, S.; Zhong, Y.; Xie, D. A Time-Course Transcriptome Analysis of Wax Gourd Fruit Development Reveals Predominant Genes Regulating Taste and Nutrition. Front. Plant Sci. 2022, 13, 971274. [Google Scholar] [CrossRef]
- Su, L.; Gou, J.; Lv, H.; Cheng, Z.; Ma, L.; Huang, X.; Wu, W.; Yu, W.; Wang, P.; Liu, Z. High-Density Genetic Map and Quantitative Trait Loci Map of Fruit-Related Traits in Wax Gourd (Benincasa Hispida). Euphytica 2022, 218, 117. [Google Scholar] [CrossRef]
- Yan, J.; Sun, P.; Liu, W.; Xie, D.; Wang, M.; Peng, Q.; Sun, Q.; Jiang, B. Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa Hispida). Agronomy 2022, 12, 2045. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, L.; Zhu, J.; Li, H.; Duan, H. Comparative Analysis of Tuberous Root Metabolites between Cultivated and Wild Varieties of Rehmannia Glutinosa by Widely Targeted Metabolomics. Sci. Rep. 2021, 11, 11460. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, Z.; Gao, L.; Zhang, N.; Chang, W. Isoimperatorin Alleviates Lipopolysaccharide-Induced Periodontitis by Downregulating Erk1/2 and Nf-Kappab Pathways. Open Life Sci. 2023, 18, 20220541. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Lv, L.L.; Chen, J.X.; Ying, H.F.; Ruan, M.; Zhu, W.H.; Xu, J.Y.; Zhang, C.Y.; Zhang, K.Y.; et al. Nobiletin Inhibits Breast Cancer Cell Migration and Invasion by Suppressing the Il-6-Induced Erk-Stat and Jnk-C-Jun Pathways. Phytomedicine 2023, 110, 154610. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Choi, Y.; Shim, J.; Kim, M. Genistin: A Novel Potent Anti-Adipogenic and Anti-Lipogenic Agent. Molecules 2020, 25, 2042. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, J.; Wang, Y.; Zhang, J.; Liu, D. Ethnopharmacology, Phytochemistry and Pharmacology of Benincasae Exocarpium: A Review. Chin. Herb. Med. 2023, 15, 15–26. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Lin, J.; Sun-Waterhouse, D.; Cui, C. The Therapeutic Potential of Diet on Immune-Related Diseases: Based on the Regulation on Tryptophan Metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 8793–8811. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cavanaugh, C.R.; Hornby, P.J. Emerging Effects of Tryptophan Pathway Metabolites and Intestinal Microbiota on Metabolism and Intestinal Function. Amino Acids 2022, 54, 57–70. [Google Scholar] [CrossRef]
- Mullen, N.J.; Singh, P.K. Nucleotide Metabolism: A Pan-Cancer Metabolic Dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, K.; Opielka, M.; Nazar, W.; Kowianski, P.; Smolenski, R.T. Neuroprotective Effects of Guanosine in Ischemic Stroke—Small Steps Towards Effective Therapy. Int. J. Mol. Sci. 2021, 22, 6898. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Metabolic Aspects of Adenosine Functions in the Brain. Front. Pharmacol. 2021, 12, 672182. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, C.L.; Li, P.; Li, H.R.; Liu, Q.; Deng, X.M.; Wang, J.F. Nicotinamide Mononucleotide Attenuates Lps-Induced Acute Lung Injury with Anti-Inflammatory, Anti-Oxidative and Anti-Apoptotic Effects. J. Surg. Res. 2023, 283, 9–18. [Google Scholar] [CrossRef]
- Jung, M.; Lee, K.M.; Im, Y.; Seok, S.H.; Chung, H.; Kim, D.Y.; Han, D.; Lee, C.H.; Hwang, E.H.; Park, S.Y.; et al. Nicotinamide (Niacin) Supplement Increases Lipid Metabolism and ROS-Induced Energy Disruption in Triple-Negative Breast Cancer: Potential for Drug Repositioning as an Anti-Tumor Agent. Mol. Oncol. 2022, 16, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Bao, W.; Gouife, M.; Xu, J.; Han, J.; Lu, C.; Ming, T.; Zhou, J.; Zhang, W.; Su, X. Exploring the Health Benefits of Traditionally Fermented Wax Gourd: Flavor Substances, Probiotics, and Impact on Gut Microbiota. Front. Sustain. Food Syst. 2024, 8, 1314537. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, G.; Varghese, T. Effect of Dietary Gamma-Aminobutyric Acid on Growth Performance, Haemato-Immunological Responses, Antioxidant Enzymes Activity, Ghrelin and Igf-I Expression of Labeo Rohita (Hamilton, 1822) Fingerlings. Comp. Clin. Pathol. 2022, 32, 53–65. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Alhasaniah, A.H. L-Carnitine: Nutrition, Pathology, and Health Benefits. Saudi J. Biol. Sci. 2023, 30, 103555. [Google Scholar] [CrossRef]
- Syed, Q.A.; Rashid, Z.; Ahmad, M.H.; Shukat, R.; Ishaq, A.; Muhammad, N.; Rahman, H.U.R. Nutritional and Therapeutic Properties of Fenugreek (Trigonella Foenum-Graecum): A Review. Int. J. Food Prop. 2020, 23, 1777–1791. [Google Scholar] [CrossRef]
| No. | Metabolite Category | Quantity (Relative Content %) | Number of Metabolites (Relative Content %) | |||
|---|---|---|---|---|---|---|
| Peel | Pulp | Seed | ||||
| Primary metabolites | 1 | Amino acids and derivatives | 126 (18.33 ± 0.12) | 117 (5.32 ± 0.08) | 121 (6.14 ± 0.03) | 124 (6.87 ± 0.03) |
| 2 | Nucleotides and derivatives | 69 (9.55 ± 0.15) | 65 (3.91 ± 0.08) | 68 (1.58 ± 0.01) | 69 (4.06 ± 0.22) | |
| 3 | Organic acids | 89 (9.89 ± 0.15) | 81 (2.80 ± 0.04) | 86 (3.17 ± 0.14) | 87 (3.92 ± 0.07) | |
| 4 | Lipids | 142 (17.90 ± 0.10) | 141 (4.79 ± 0.07) | 134 (4.84 ± 0.10) | 136 (8.27 ± 0.12) | |
| 5 | Sugars and alcohols | 73 (5.79 ± 0.07) | 65 (1.32 ± 0.02) | 67 (1.88 ± 0.03) | 69 (2.59 ± 0.10) | |
| 6 | Vitamins | 21 (2.06 ± 0.06) | 21 (0.53 ± 0.00) | 19 (0.62 ± 0.05) | 20 (0.91 ± 0.02) | |
| Total | 520 (63.52 ± 0.43) | 490 (18.67 ± 0.10) | 493 (18.23 ± 0.20) | 505 (26.62 ± 0.27) | ||
| Secondary metabolites | 7 | Flavonoids | 149 (7.29 ± 0.15) | 136 (3.38 ± 0.05) | 114 (1.86 ± 0.05) | 117 (2.05 ± 0.06) |
| 8 | Phenolic acids | 204 (19.86 ± 0.27) | 187 (7.59 ± 0.05) | 171 (5.19 ± 0.17) | 182 (7.08 ± 0.16) | |
| 9 | Alkaloids | 61 (7.00 ± 0.02) | 57 (2.01 ± 0.01) | 58 (2.26 ± 0.04) | 60 (2.73 ± 0.05) | |
| 10 | Lignans and coumarins | 61 (1.75 ± 0.04) | 53 (0.95 ± 0.05) | 54 (0.26 ± 0.01) | 55 (0.54 ± 0.01) | |
| 11 | Terpenoids | 25 (0.58 ± 0.04) | 21 (0.34 ± 0.01) | 13 (0.12 ± 0.04) | 15 (0.12 ± 0.01) | |
| Total | 500 (36.48 ± 0.48) | 454 (14.27 ± 0.10) | 410 (9.69 ± 0.25) | 429 (12.52 ± 0.25) | ||
| metabolites | 1020 (100) | 944 (32.94 ± 0.00) | 905 (27.92 ± 0.06) | 934 (39.14 ± 0.06) | ||
| No. | Category | Peel vs. Pulp | Peel vs. Seed | Pulp vs. Seed | |
|---|---|---|---|---|---|
| Total (Up/Down) | Total (Up/Down) | Total (Up/Down) | |||
| Primary metabolites | 1 | Amino acids and their derivatives | 62 (38/24) | 77 (61/16) | 60 (47/13) |
| 2 | Nucleotides and their derivatives | 41 (5/36) | 40 (18/22) | 41 (36/5) | |
| 3 | Organic acids | 56 (30/26) | 55 (36/19) | 37 (29/8) | |
| 4 | Lipids | 88 (41/47) | 103 (60/43) | 60 (54/6) | |
| 5 | Sugars and alcohols | 36 (17/19) | 35 (23/12) | 20 (15/5) | |
| 6 | Vitamins | 7 (2/ 5) | 13 (9/4) | 6 (5/1) | |
| Total | 290 (133/157) | 323 (207/116) | 224 (186/38) | ||
| Secondary metabolites | 1 | Flavonoids | 123 (45/78) | 121 (49/72) | 33 (25/8) |
| 2 | Phenolic acids | 148 (51/97) | 135 (73/62) | 103 (77/26) | |
| 3 | Alkaloids | 32 (13/19) | 27 (16/11) | 28 (24/4) | |
| 4 | Lignans and coumarins | 47 (22/25) | 46 (25/21) | 34 (27/7) | |
| 5 | Terpenoids | 18 (4/14) | 22 (6/16) | 11 (9/2) | |
| Total | 368 (135/233) | 351 (169/182) | 209 (162/47) | ||
| Total differential metabolites | 658 (268/390) | 674 (376/298) | 433 (348/85) | ||
| ID | m/z | Name of Fold Change (Pulp/Peel) | Chromatographic Peak Areas | VIP | Difference Multiple | p-Value | Type | ||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Peel | Pulp | ||||||
| Primary Differential Metabolites | |||||||||
| mws0230 | 120.07 | 74 | l-Threonine | 4,503,833 ± 152,026 | 1,546,467 ± 41,357 | 1.1 | 0.34 | 0 | Down |
| mws0425 | 129.02 | 85 | Citraconic acid | 86,139 ± 16,658 | 221,163 ± 21,215 | 1.06 | 2.57 | 0 | Up |
| Lmbn000198 | 133.01 | 71.01 | 3-Dehydro-l-threonic acid | 413,356 ± 16,314 | 902,200 ± 137,376 | 1.07 | 2.18 | 0.02 | Up |
| mws0275 | 133.01 | 71.01 | l-malic acid | 409,073 ± 30,929 | 853,487 ± 81,403 | 1.08 | 2.09 | 0.01 | Up |
| pme0040 | 136.06 | 119 | Adenine | 69,214,000 ± 2,743,185 | 29,226,333 ± 1,229,554 | 1.09 | 0.42 | 0 | Down |
| mws0208 | 145.05 | 101 | Adipic acid | 1,581,100 ± 42,670 | 730,963 ± 42,042 | 1.09 | 0.46 | 0 | Down |
| Lmbn001609 | 145.05 | 83.05 | 2-Acetyl-2-hydroxybutyric acid | 281,627 ± 32,358 | 139,250 ± 7700 | 1.07 | 0.49 | 0.01 | Down |
| mws0854 | 163 | 59.01 | Rhamnose | 213,700 ± 42,067 | 61,154 ± 7047 | 1.07 | 0.29 | 0.02 | Down |
| pme0021 | 166.09 | 120.08 | l-Phenylalanine | 899,753 ± 16,929 | 1,879,000 ± 35,172 | 1.1 | 2.09 | 0 | Up |
| pmc0274 | 167.1 | 121.1 | 6-Methylmercaptopurine | 3,258,100 ± 76,300 | 7,710,100 ± 278,967 | 1.1 | 2.37 | 0 | Up |
| MWSmce165 | 165.08 | 59.02 | l-Fucose | 1,901,633 ± 83,155 | 738,243 ± 101,290 | 1.08 | 0.39 | 0 | Down |
| Zmzn000079 | 199 | 78.96 | d-Erythrose-4-phosphate | 103,930 ± 16,989 | 28,742 ± 3662 | 1.08 | 0.28 | 0.01 | Down |
| Zmgn002106 | 206.08 | 58.03 | N-Acetyl-l-phenylalanine | 371,247 ± 69,554 | 165,817 ± 4643 | 1.05 | 0.45 | 0.04 | Down |
| pme2566 | 217.08 | 199 | 5-l-glutamyl-l-amino acid | 54,064 ± 3577 | 22,579 ± 5356 | 1.05 | 0.42 | 0 | Down |
| Lmcn009122 | 253.22 | 235.21 | (7Z)-hexadec-10-enoic acid | 32,369 ± 3598 | 15,783 ± 1431 | 1.07 | 0.49 | 0.01 | Down |
| MWSmce356 | 275.07 | 114.02 | l-tryptophan sulfate | 48,404 ± 6298 | 155,297 ± 13,533 | 1.08 | 3.21 | 0 | Up |
| pme3188 | 323.03 | 211 | Uridine 5′-monophosphate | 8,032,433 ± 276,343 | 2,456,367 ± 243,901 | 1.09 | 0.31 | 0 | Down |
| pme3311 | 339 | 97 | d-fructose-1,6-diphosphate | 92,208 ± 3048 | 25,099 ± 7497 | 1.05 | 0.27 | 0 | Down |
| Lmhp007836 | 448.25 | 307.23 | Lysophosphatidylethanolamine 16:3 | 5298 ± 1586 | 11,567 ± 1600 | 1 | 2.18 | 0.01 | Up |
| Lmhp008763 | 452.28 | 311.26 | Lysophosphatidylethanolamine 16:1 (2n isomer) | 3,094,700 ± 135,834 | 1,236,767 ± 131,562 | 1.09 | 0.4 | 0 | Down |
| MWS0155 | 462.07 | 96.97 | Adenosine-5′-monophosphate | 57,563 ± 3035 | 27,998 ± 5880 | 1.03 | 0.49 | 0 | Down |
| Secondary Differential Metabolites | |||||||||
| pmp001287 | 120.08 | 103.05 | N-benzylidene methylamine | 9,232,867 ± 243,854 | 20,316,333 ± 498,097 | 1.06 | 2.2 | 0 | Up |
| NK10264324 | 127.04 | 81.03 | 1,3,5-Benzenetriol | 172,853 ± 22,483 | 396,137 ± 43,430 | 1.03 | 2.29 | 0 | Up |
| pmb0501 | 131.13 | 114.1 | Butylguanidine | 84,127 ± 9112 | 29,414 ± 3450 | 1.04 | 0.35 | 0 | Down |
| mws1195 | 179.07 | 147.1 | Methyl p-coumarate | 316,540 ± 4518 | 156,163 ± 3780 | 1.06 | 0.49 | 0 | Down |
| Lmyp003951 | 227.09 | 181.09 | 3-Hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one | 728,393 ± 62,642 | 339,023 ± 20,476 | 1.04 | 0.47 | 0 | Down |
| Lmzn001925 | 153.02 | 109.03 | 3,4′-Dihydroxy-3′-methoxybenzenepentanoic acid | 32,558 ± 7055 | 13,457 ± 1146 | 1.01 | 0.41 | 0.04 | Down |
| mws1383 | 243.09 | 145 | 7,8-Dimethylxanthine | 51,294 ± 5875 | 124,537 ± 5641 | 1.04 | 2.43 | 0 | Up |
| MWSslk172 | 247.06 | 217.02 | Isorhoifolin | 2696 ± 1425 | 28,141 ± 2149 | 1.01 | 10.44 | 0 | Up |
| pmf0526 | 271.1 | 147.2 | Isorhoifolin | 2398 ± 595 | 7845 ± 1202 | 1.02 | 3.27 | 0.01 | Up |
| pmn001367 | 315.07 | 153.02 | Protocatechuic acid 4-O-glucoside | 12,788,000 ± 25,229 | 5,425,533 ± 280,837 | 1.05 | 0.42 | 0 | Down |
| Jmwn002172 | 331.1 | 153.02 | 3,4-Dihydroxyphenylethanol-β-d-glucopyranoside | 269,997 ± 20,971 | 24,212 ± 8396 | 1.04 | 0.09 | 0 | Down |
| pmb0235 | 323.09 | 177.4 | Feruloylcoumarin | 40,454 ± 5226 | 95,878 ± 8366 | 1.03 | 2.37 | 0 | Up |
| Lmmn001294 | 327.14 | 165.09 | 3,5-Dimethoxy-4-hydroxyphenol-1-O-glucoside | 3,362,700 ± 153,119 | 964,387 ± 101,376 | 1.05 | 0.29 | 0 | Down |
| pmn001519 | 183.03 | 124.02 | Methyl gallate | 31,494 ± 5903 | 94,635 ± 3909 | 1.04 | 3 | 0 | Up |
| pmp000384 | 419.13 | 257.08 | Iso-Glycitin | 41,081 ± 6680 | 8902 ± 2384 | 1.03 | 0.22 | 0.01 | Down |
| pmp001079 | 609.18 | 301 | Luteolin-7-O-neohesperidoside | 32,514 ± 4815 | 78,608 ± 5825 | 1.03 | 2.42 | 0 | Up |
| pmb3002 | 595.17 | 449.1 | Luteolin-7-O-rutinoside | 116,570 ± 12,194 | 373,607 ± 11,425 | 1.05 | 3.2 | 0 | Up |
| Zmxp003107 | 458.1 | 116.3 | Luteolin-7,3′-O-diglucoside | 73,390 ± 13,231 | 28,595 ± 3214 | 1.02 | 0.39 | 0.02 | Down |
| ID | m/z | Name of Fold Change (Peel/Seed) | Chromatographic Peak Areas | VIP | Difference Multiple | p-Value | Type | ||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Peel | Seed | ||||||
| Primary Differential Metabolites | |||||||||
| mws0889 | 135.03 | 75.01 | d-threonic acid | 3,411,633 ± 221,075 | 1,192,733 ± 103,148 | 1.07 | 0.35 | 0 | Down |
| Hmsp000364 | 144.1 | 98.09 | Cyclopentylglycine | 171,100 ± 20,154 | 353,580 ± 19,226 | 1.05 | 2.07 | 0 | Up |
| pme3351 | 144.1 | 126 | Allyl ester | 42,915 ± 9746 | 126,007 ± 16,787 | 1.04 | 2.94 | 0 | Up |
| mws0851 | 143.11 | 143.11 | Sodium valproate | 1,235,133 ± 38,875 | 59,2097 ± 13,556 | 1.07 | 0.48 | 0 | Down |
| mws0981 | 178.04 | 136.02 | Isoxanthopterin | 30,873 ± 5210 | 74,217 ± 4327 | 1.05 | 2.4 | 0 | Up |
| mws4170 | 179.06 | 59.01 | d-glucose | 9,070,467 ± 1,228,309 | 29,221,000 ± 1,467,887 | 1.07 | 3.22 | 0 | Up |
| pmf0139 | 179.06 | 59 | d-galactose | 6,565,200 ± 361,045 | 20,125,667 ± 666,863 | 1.07 | 3.07 | 0 | Up |
| pme2596 | 170.08 | 134.1 | 4-pyridoxine | 233,487 ± 7043 | 552,763 ± 3316 | 1.08 | 2.37 | 0 | Up |
| Zmzp000145 | 189.16 | 84.08 | Trimethyllysine | 1,996,867 ± 378,707 | 5,128,667 ± 224,260 | 1.05 | 2.57 | 0 | Up |
| Lmrj002244 | 195.11 | 70.06 | Cyclo(proline-proline) | 10,155 ± 1392 | 27,690 ± 7078 | 1.02 | 2.73 | 0.05 | Up |
| MWS1771 | 196.1 | 91.05 | l-tyrosine methyl ester | 145,483 ± 14,822 | 404,253 ± 17,053 | 1.07 | 2.78 | 0 | Up |
| pmb2640 | 199.17 | 199.17 | Lauric acid | 286,630 ± 15,541 | 125,020 ± 7052 | 1.07 | 0.44 | 0 | Down |
| mws1337 | 220.12 | 202.1 | d-pantothenic acid | 9,018,600 ± 310,680 | 18,228,000 ± 364,037 | 1.07 | 2.02 | 0 | Up |
| pme1228 | 221.09 | 204 | 5-hydroxy-l-tryptophan | 167,030 ± 20,955 | 69,374 ± 4561 | 1.06 | 0.42 | 0.01 | Down |
| pmn001689 | 293.21 | 235.17 | 9-hydroxy-12-oxo-15(Z)-octadecenoic acid | 9273 ± 169 | 22,291 ± 2785 | 1.06 | 2.4 | 0.01 | Up |
| Lmqn000351 | 325.11 | 59.01 | Rhamnose | 48,141 ± 11,530 | 111,822 ± 16,984 | 1.01 | 2.32 | 0.01 | Up |
| pmb0789 | 332.13 | 152.07 | Pyridoxal-5′-O-glucoside | 494,887 ± 95,367 | 1,263,000 ± 100,484 | 1.04 | 2.55 | 0 | Up |
| MWSslk074 | 343.12 | 59.01 | Lactitol | 4,802,767 ± 98,615 | 10,730,000 ± 226,316 | 1.08 | 2.23 | 0 | Up |
| pme1014 | 445.3 | 341.3 | Menatetrenone (vitamin K2) | 112,310 ± 3104 | 238,977 ± 14,500 | 1.07 | 2.13 | 0 | Up |
| pmd0160 | 496.34 | 184.07 | Lysophosphatidylethanolamine 16:0 (2n isomer) | 333,030 ± 18,370 | 890,643 ± 25,151 | 1.07 | 2.67 | 0 | Up |
| pmb0876 | 483.27 | 255.23 | Lysophosphatidylethanolamine 16:0 | 337,597 ± 30,415 | 878,040 ± 30,869 | 1.07 | 2.6 | 0 | Up |
| MWS5083 | 455.1 | 96.97 | Fumarate mononucleotide (FMN) | 237,953 ± 20,041 | 93,554 ± 26,151 | 1.01 | 0.39 | 0 | Down |
| pmb0883 | 482.32 | 341.31 | Lysophosphatidylethanolamine 18:0 | 408,780 ± 41,361 | 1,060,963 ± 218,639 | 1.04 | 2.6 | 0.03 | Up |
| pma1303 | 492.31 | 184.07 | Lysophosphatidylcholine 16:2 | 412,553 ± 7690 | 1,303,500 ± 12,217 | 1.08 | 3.16 | 0 | Up |
| pmb0863 | 454.29 | 313.27 | Lysophosphatidylcholine 16:0 (2n isomers) | 11,407,000 ± 304,242 | 36,728,000 ± 416,476 | 1.08 | 3.22 | 0 | Up |
| Lmhp010573 | 517.34 | 263.24 | 1-linoleoylglycerol-3-O-glucoside | 94,823 ± 13,666 | 16,682 ± 2030 | 1.07 | 0.18 | 0.01 | Down |
| pmp001281 | 522.36 | 184.07 | Lysophosphatidylcholine 18:1 | 9,784,400 ± 513,013 | 38,107,000 ± 637,846 | 1.07 | 5.71 | 0.01 | Up |
| Secondary Differential Metabolites | |||||||||
| ML10177402 | 152.03 | 108.05 | 4-aminosalicylic acid | 20,380 ± 5761 | 63,312 ± 8733 | 1 | 3.11 | 0 | Up |
| MWS1839 | 165.06 | 92.03 | Ethyl 4-hydroxybenzoate | 16,106 ± 1936 | 51,140 ± 4049 | 1.04 | 3.18 | 0 | Up |
| mws1071 | 217.05 | 201.4 | Bergamottin | 16,066 ± 2629 | 32,993 ± 2524 | 1.01 | 2.05 | 0 | Up |
| mws0063 | 271.06 | 153.02 | Genistein | 291,050 ± 3581 | 13,189 ± 1626 | 1.05 | 0.05 | 0 | Down |
| Lmyn003835 | 289.04 | 137.02 | 4-(3,4,5-trihydroxybenzyloxy)benzoic acid | 12,071 ± 2540 | 504,17 ± 9587 | 1.03 | 4.18 | 0.02 | Up |
| Lmmn003663 | 329.09 | 167.04 | Methyl 5-glucoxy-2-hydroxybenzoate | 158,563 ± 27,550 | ND | 1.05 | 0 | 0.01 | Down |
| Jmbp006554 | 389.12 | 359.07 | Ethyl rosmarinate | 102,944 ± 5198 | 210,320 ± 7153 | 1.05 | 2.04 | 0 | Up |
| Lmcp007265 | 389.12 | 359.08 | 3′-demethylnobiletin | 35,375 ± 587 | 114,287 ± 3024 | 1.05 | 3.23 | 0 | Up |
| MWSmce052 | 455.36 | 455.36 | 3-epibenzoic acid | 456,187 ± 40,667 | ND | 1.05 | 0 | 0 | Down |
| Zmhn003106 | 461.15 | 337.09 | 4-O-(6′-O-glucopyranosyl-imino)-4-hydroxybenzyl alcohol | ND | 248,107 ± 28,303 | 1.05 | 27,567.41 | 0 | Up |
| Zmpn008194 | 471.35 | 471.35 | Corosolic acid | 1,465,757 ± 596,114 | 10,446 ± 2121 | 1.05 | 0.01 | 0.05 | Down |
| Lmhn003799 | 501.1 | 307.05 | Feruloylferulic acid feruloyl-l-tartaric acid | ND | 55,070 ± 3954 | 1.05 | 6118 | 0 | Up |
| Wmzn002116 | 515.12 | 353.09 | 3,5-dicaffeoylquinic acid | 125,287 ± 5973 | ND | 1.05 | 0 | 0 | Down |
| Lmsn003628 | 547.17 | 223.1 | 6′-O-sinapoylsucrose | 289,620 ± 47,716 | ND | 1.05 | 0 | 0.01 | Down |
| ID | m/z | Name of Fold Change (Pulp/Seed) | Chromatographic Peak Areas | VIP | Difference Multiple | p-Value | Type | ||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Pulp | Seed | ||||||
| Primary Differential Metabolites | |||||||||
| pme3017 | 104.07 | 58.00 | 2-aminoisobutyric acid | 2,619,800 ± 204,823 | 7,807,867 ± 772,368 | 1.12 | 2.98 | 0 | Up |
| MWSmce706 | 129.07 | 56.05 | Cyclo(glycyl-l-alanyl) | 262,900 ± 32,361 | 102,312 ± 13,370 | 1.1 | 0.39 | 0.01 | Down |
| MWS2413 | 130.05 | 58.03 | N-acetyl-β-alanine | 25,521 ± 3942 | 70,898 ± 15,610 | 1.08 | 2.78 | 0.03 | Up |
| pme0193 | 147.08 | 84.00 | L-glutamine | 170,230 ± 239,687 | 52,551 ± 17,783 | 1.07 | 0.31 | 0 | Down |
| pme0183 | 152.06 | 135.00 | Isoguanine | 63,000 ± 5290 | 163,067 ± 21,330 | 1.11 | 2.59 | 0.01 | Up |
| pme0256 | 151.03 | 151.00 | Xanthine | 232,217 ± 58,144 | 704,923 ± 174,233 | 1.06 | 3.04 | 0.03 | Up |
| mws0254 | 156.08 | 110.00 | l-histidine | 5,254,033 ± 276,779 | 10,624,967 ± 769,176 | 1.11 | 2.02 | 0 | Up |
| mws0214 | 181.07 | 71.00 | d-sorbitol | 97,320 ± 5823 | 201,470 ± 2260 | 1.12 | 2.07 | 0 | Up |
| mws0237 | 187.10 | 125.00 | Azelaic acid | 6,140,633 ± 240,694 | 12,480,667 ± 456,462 | 1.12 | 2.03 | 0 | Up |
| pme2060 | 204.25 | 136.00 | N6-isopentenyladenine | 38,353 ± 7567 | 17,936 ± 4052 | 1.01 | 0.47 | 0.02 | Down |
| pme0170 | 217.13 | 158.00 | N-acetyl-l-arginine | 116,436 ± 18,342 | 57,584 ± 6193 | 1.07 | 0.49 | 0.02 | Down |
| pme2890 | 269.06 | 136.00 | l-cysteine | 5680 ± 1368 | 42,967 ± 3715 | 1.11 | 7.56 | 0 | Up |
| Lmbn009444 | 297.24 | 183.14 | Ricinoleic acid | 81,700 ± 5000 | 37,971 ± 1328 | 1.12 | 0.46 | 0 | Down |
| pmf0297 | 297.32 | 183.10 | n-docosanol | 78,398 ± 6882 | 35,779 ± 730 | 1.12 | 0.46 | 0.01 | Down |
| Hmqp006023 | 326.27 | 309.27 | Ethyl 9-hydroxy-10,12-octadecadienoate | 17,511 ± 3640 | 46,363 ± 1003 | 1.09 | 2.65 | 0 | Up |
| MWSmce203 | 341.12 | 73.03 | d-(+)-cellobiose | 19,646 ± 3737 | 50,640 ± 14,435 | 1.04 | 2.58 | 0.06 | Up |
| MWS0442 | 527.16 | 365.10 | Maltotriose | 130,434 ± 35,925 | 56,861 ± 5581 | 1.03 | 0.44 | 0.07 | Down |
| Secondary Differential Metabolites | |||||||||
| pmb0484 | 104.10 | 60.10 | Choline | 519,1300 ± 20,2733 | 15,198,333 ± 362,467 | 1.14 | 2.93 | 0 | Up |
| mws1382 | 165.06 | 122.04 | 4′-hydroxy-3′-methoxyacetophenone | 91,188 ± 10,580 | 33,927 ± 4393 | 1.12 | 0.37 | 0.01 | Down |
| pmn001492 | 187.10 | 123.10 | Zelandine | 394,527 ± 27,333 | 824,017 ± 19,184 | 1.13 | 2.09 | 0 | Up |
| pmn001380 | 187.10 | 169.09 | Eucommiol | 630,580 ± 20,976 | 1,292,967 ± 50,931 | 1.14 | 2.05 | 0 | Up |
| Lmhn001477 | 311.04 | 179.04 | Caffeoyl tartaric acid | 17,671 ± 2004 | 40,448 ± 2249 | 1.12 | 2.29 | 0 | Up |
| mws0983 | 326.31 | 62.06 | Oleoyl monoethanolamine | 135,163 ± 30,152 | 340,493 ± 7545 | 1.09 | 2.52 | 0 | Up |
| pmb0108 | 375.20 | 137.10 | Feruloyl syringic acid | 73,136 ± 10,371 | 1566,00 ± 21,570 | 1.09 | 2.14 | 0.01 | Up |
| pmn001710 | 521.13 | 359.08 | Rosmarinic acid 3′-O-glucoside | 169,020 ± 35,578 | 755,923 ± 108,774 | 1.12 | 4.47 | 0.01 | Up |
| Lmjp003822 | 531.15 | 177.06 | 3,5-O-bis-caffeoylquinic acid methyl ester | 32,926 ± 3284 | 115,763 ± 8785 | 1.13 | 3.52 | 0 | Up |
| mab0299 | 677.35 | 659.35 | Dihydroisocoumarin I-glucoside | 166,360 ± 21194 | 48,042 ± 6209 | 1.12 | 0.29 | 0.01 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, H.; Xu, Y.; Yang, J.; Yu, Y.; Wen, J.; Xie, D.; Zhong, Y.; Wu, J.; Fu, M. Metabolomic Analysis of Different Parts of Black Wax Gourd (Cucurbita pepo). Foods 2025, 14, 1046. https://doi.org/10.3390/foods14061046
Li J, Liu H, Xu Y, Yang J, Yu Y, Wen J, Xie D, Zhong Y, Wu J, Fu M. Metabolomic Analysis of Different Parts of Black Wax Gourd (Cucurbita pepo). Foods. 2025; 14(6):1046. https://doi.org/10.3390/foods14061046
Chicago/Turabian StyleLi, Jun, Haocheng Liu, Yujuan Xu, Jiguo Yang, Yuanshan Yu, Jing Wen, Dasen Xie, Yujuan Zhong, Jijun Wu, and Manqin Fu. 2025. "Metabolomic Analysis of Different Parts of Black Wax Gourd (Cucurbita pepo)" Foods 14, no. 6: 1046. https://doi.org/10.3390/foods14061046
APA StyleLi, J., Liu, H., Xu, Y., Yang, J., Yu, Y., Wen, J., Xie, D., Zhong, Y., Wu, J., & Fu, M. (2025). Metabolomic Analysis of Different Parts of Black Wax Gourd (Cucurbita pepo). Foods, 14(6), 1046. https://doi.org/10.3390/foods14061046






