Anti-Melanoma Activity and Potential Mechanism of Purified Potato Protease Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instruments
2.3. Recovery and Purification of Potato Protein
2.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. BCA Method for Detecting Protein Content
2.6. Solubility Determination of Potato Protein
2.7. Glycoprotein Staining
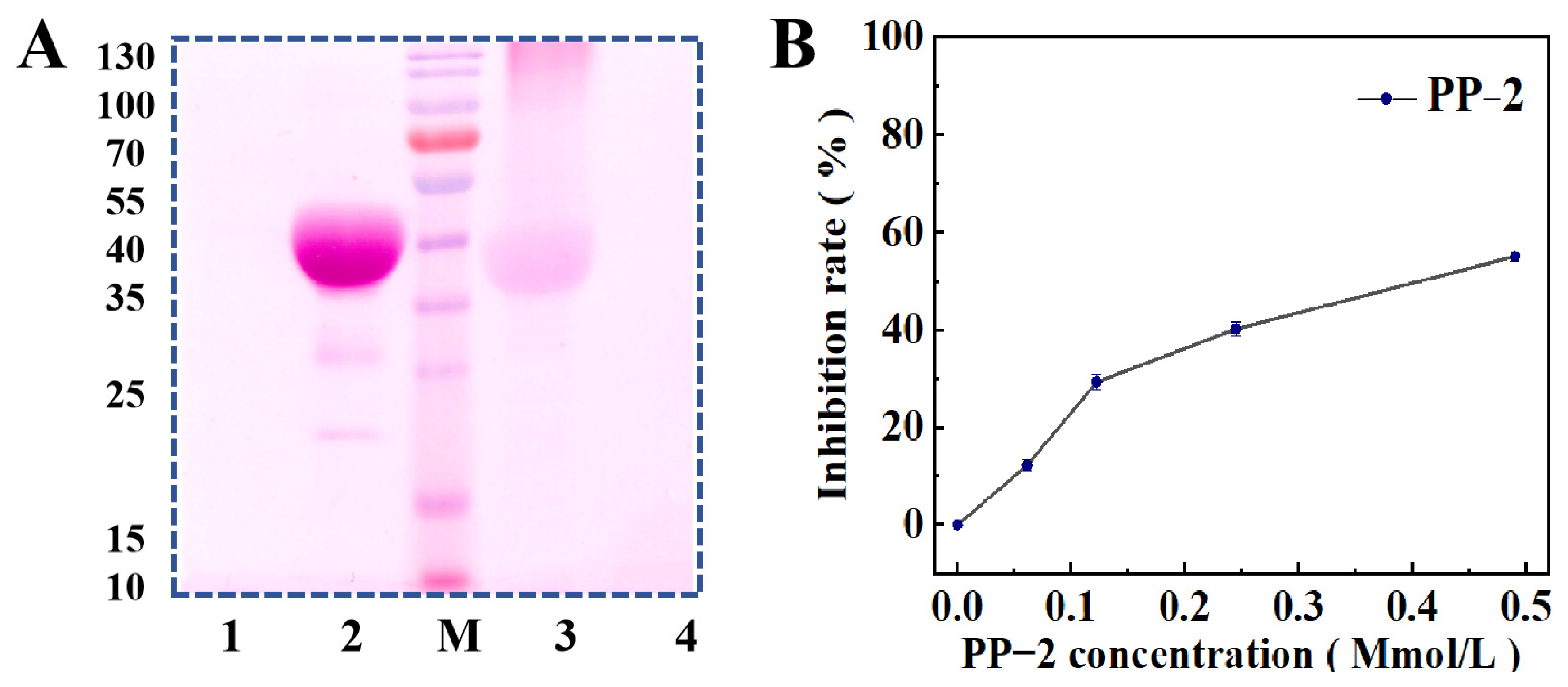
2.8. Pepsin Inhibition Activity Assay
2.9. Hydrolyzed Amino Acid Composition of Potato Protein
2.10. Scanning Electron Microscope (SEM) Analysis
2.11. Antioxidant Activity
2.11.1. Reducing Power Activity
2.11.2. Hydroxyl Radical Scavenging Activity
2.11.3. DPPH Radicals Scavenging Activity
2.12. Cell Culture
2.13. Cell Viability Assay
2.14. Melanin Content Assay
2.15. Cell Tyrosinase Activity Assay
2.16. Colony Formation Assay
2.17. Cell Wound Scratch Assay
2.18. Cell Cycle Analysis
2.19. Apoptosis Analysis
2.20. Western Blotting
2.21. Statistical Analysis
3. Results and Discussion
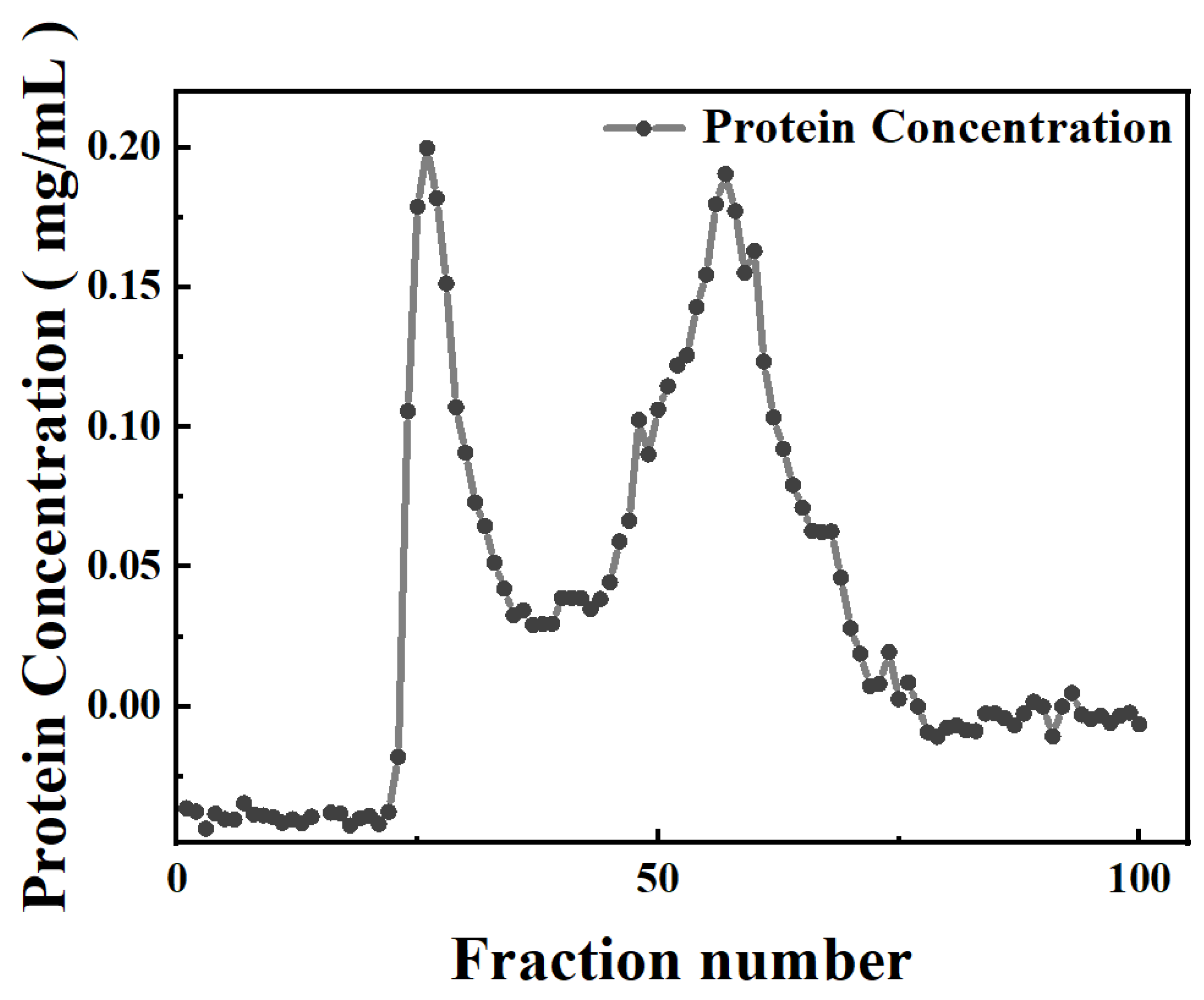
3.1. Purification of Protein Components from Potato Juice
3.2. Homogeneity and Molecular Weight
3.3. Solubility
3.4. Protein Type Determination
3.5. Amino Acid Composition
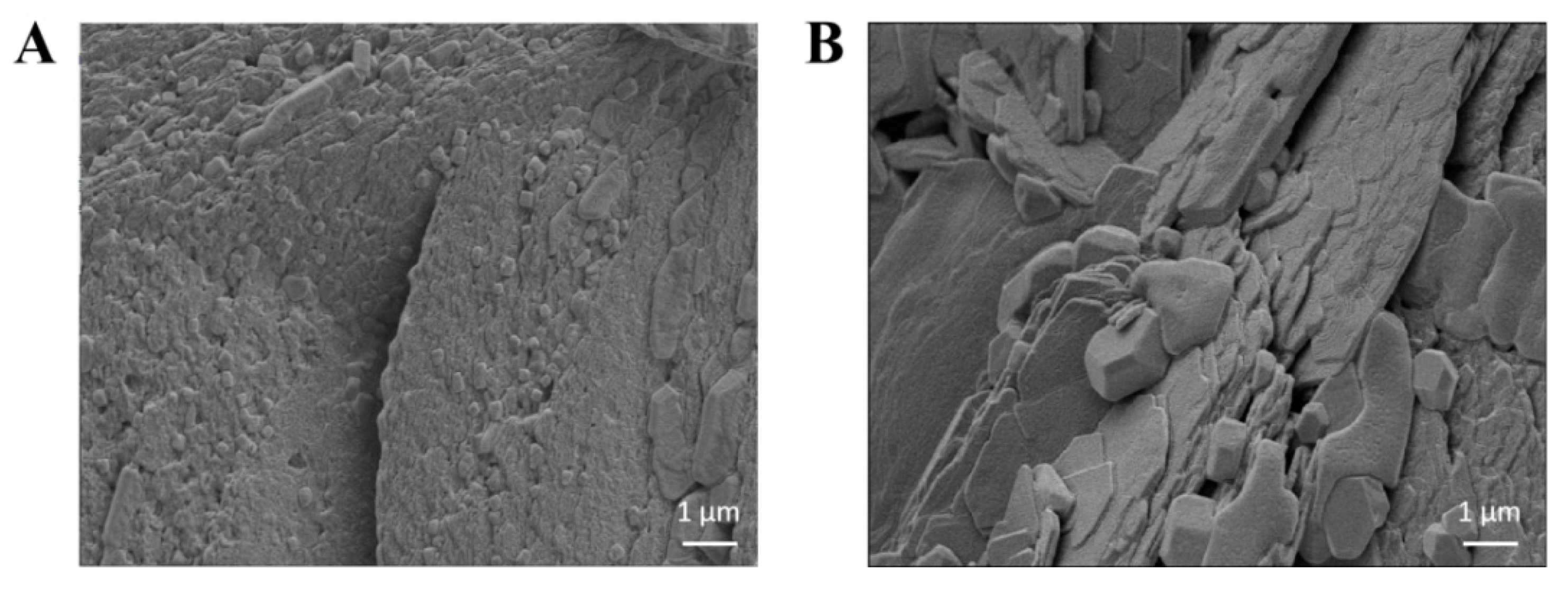
3.6. Microscopic Structures
3.7. In Vitro Antioxidant Activity
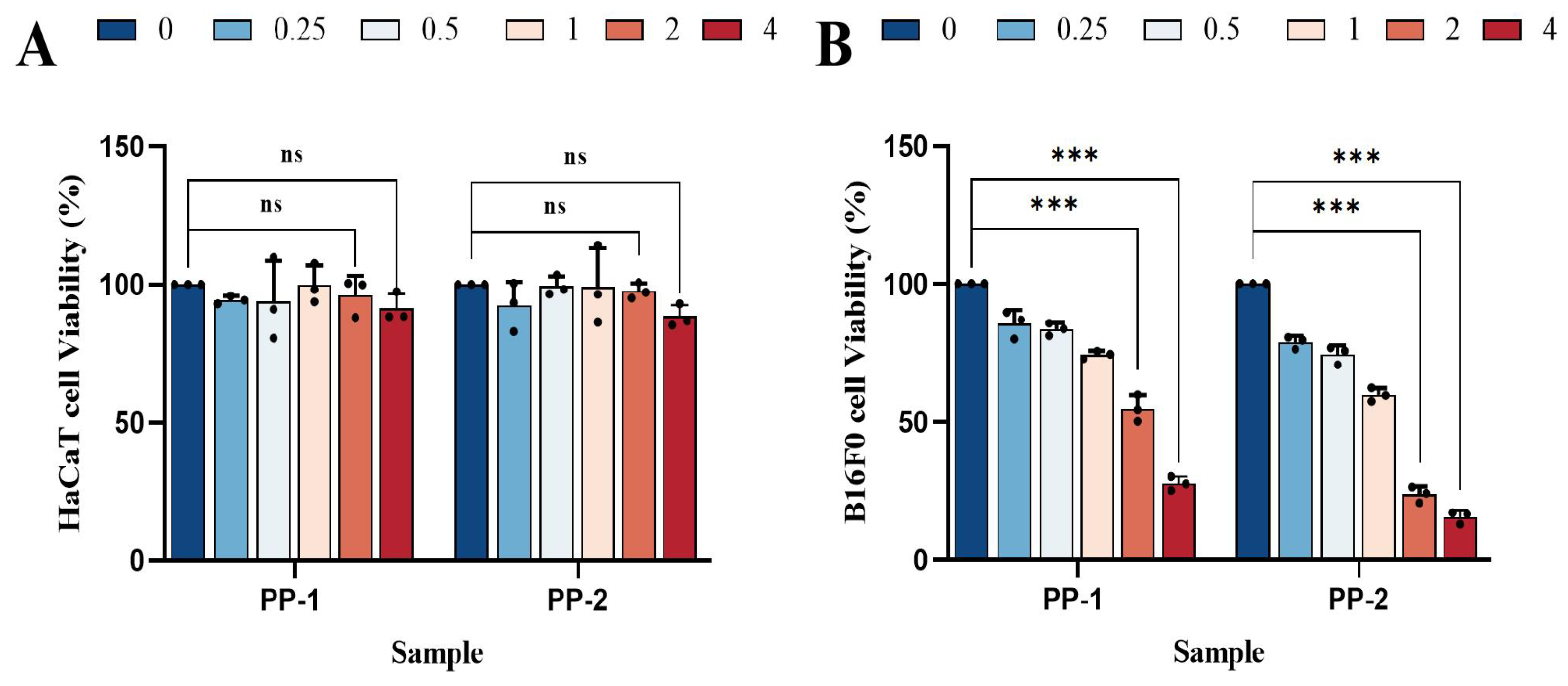
3.8. Cell Cytotoxicity Study
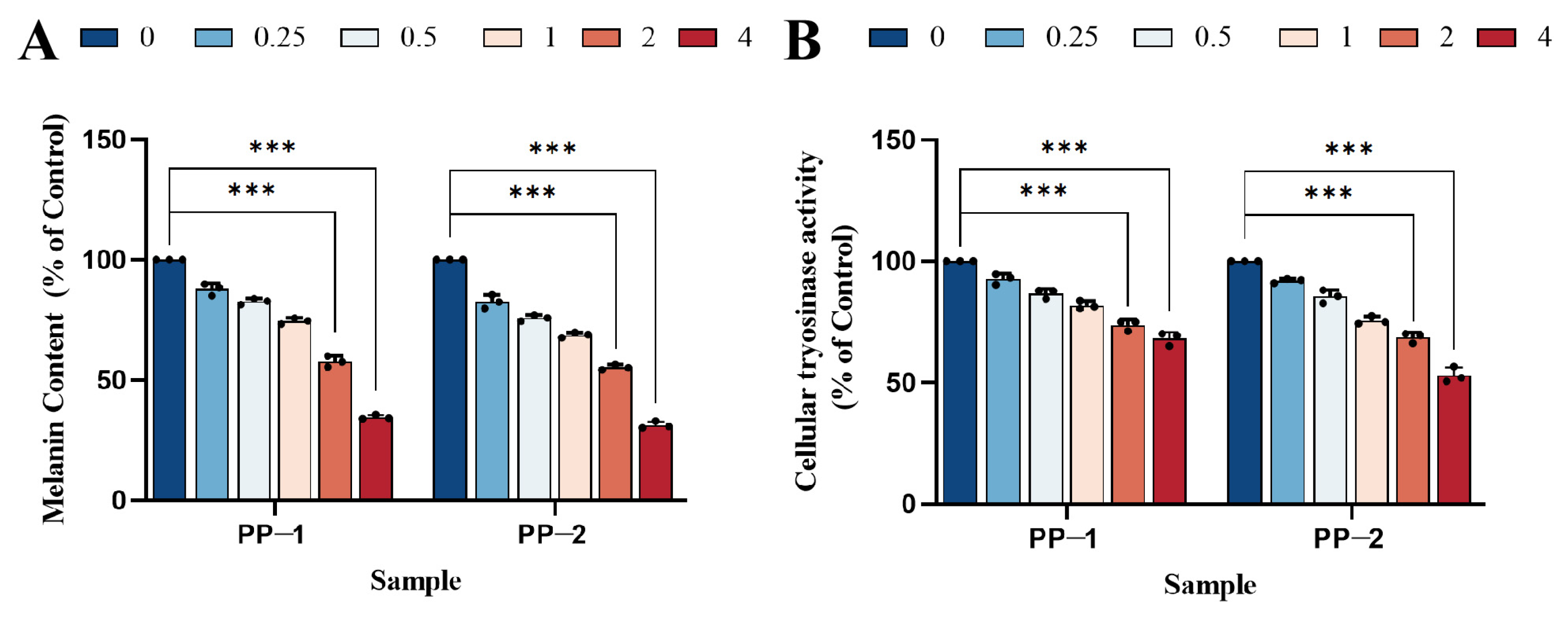
3.9. Measurement of Cellular Melanin Content
3.10. Cellular Tyrosinase Inhibition Study
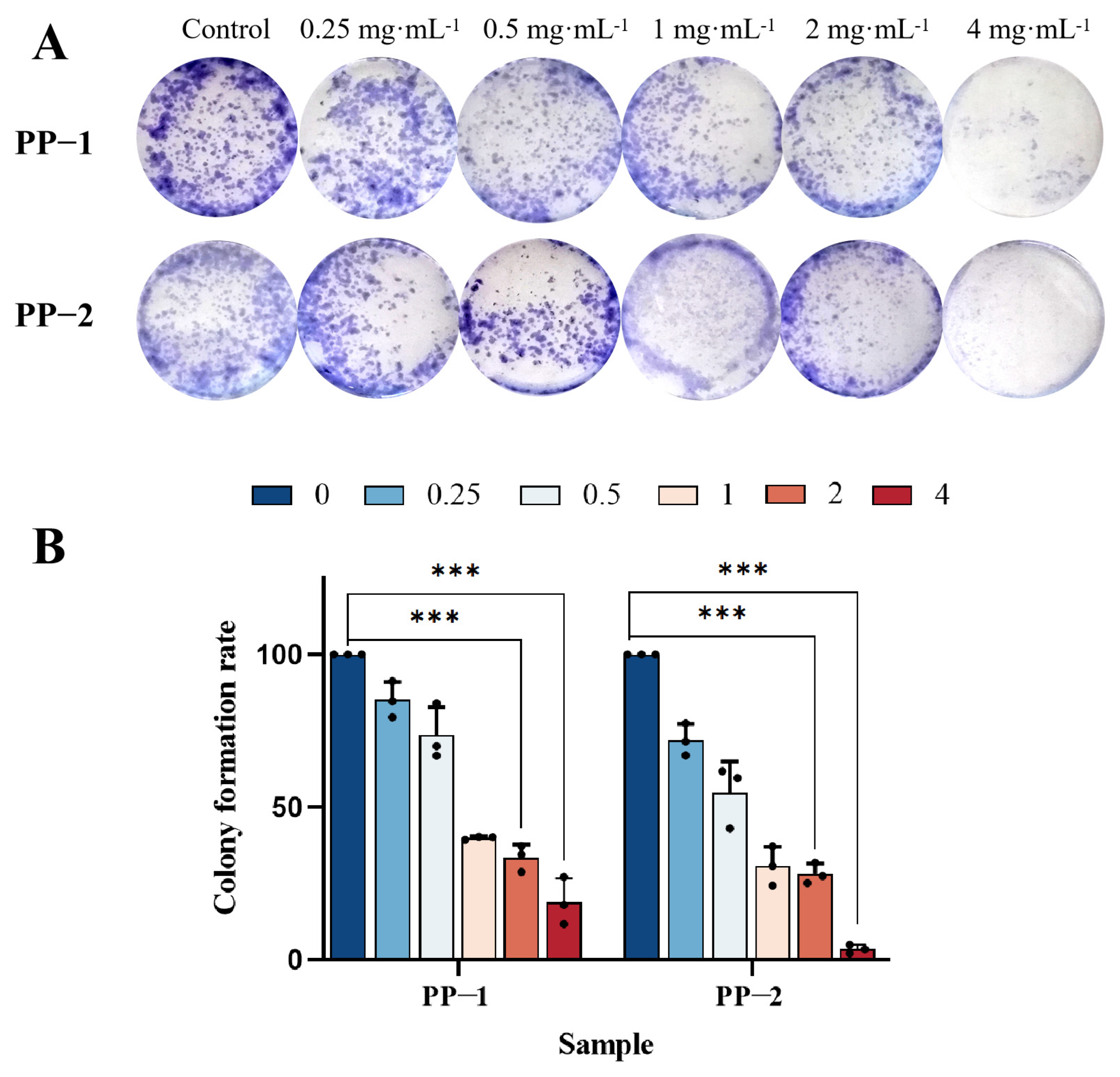
3.11. Cloning Experiment
3.12. Cell Wound Scratch Assay
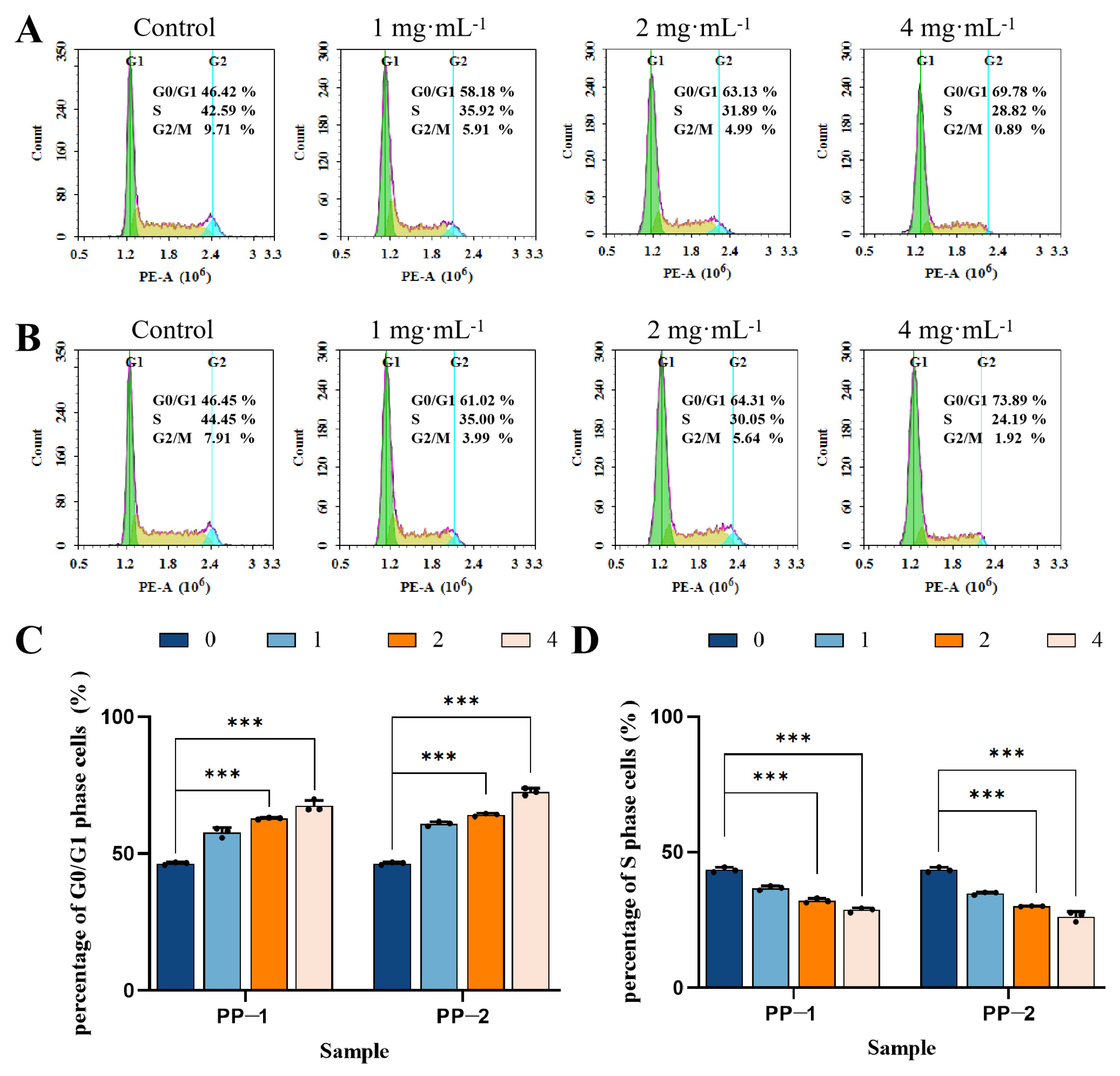
3.13. Cell Cycle
3.14. Flow Cytometry Detection of Cell Apoptosis
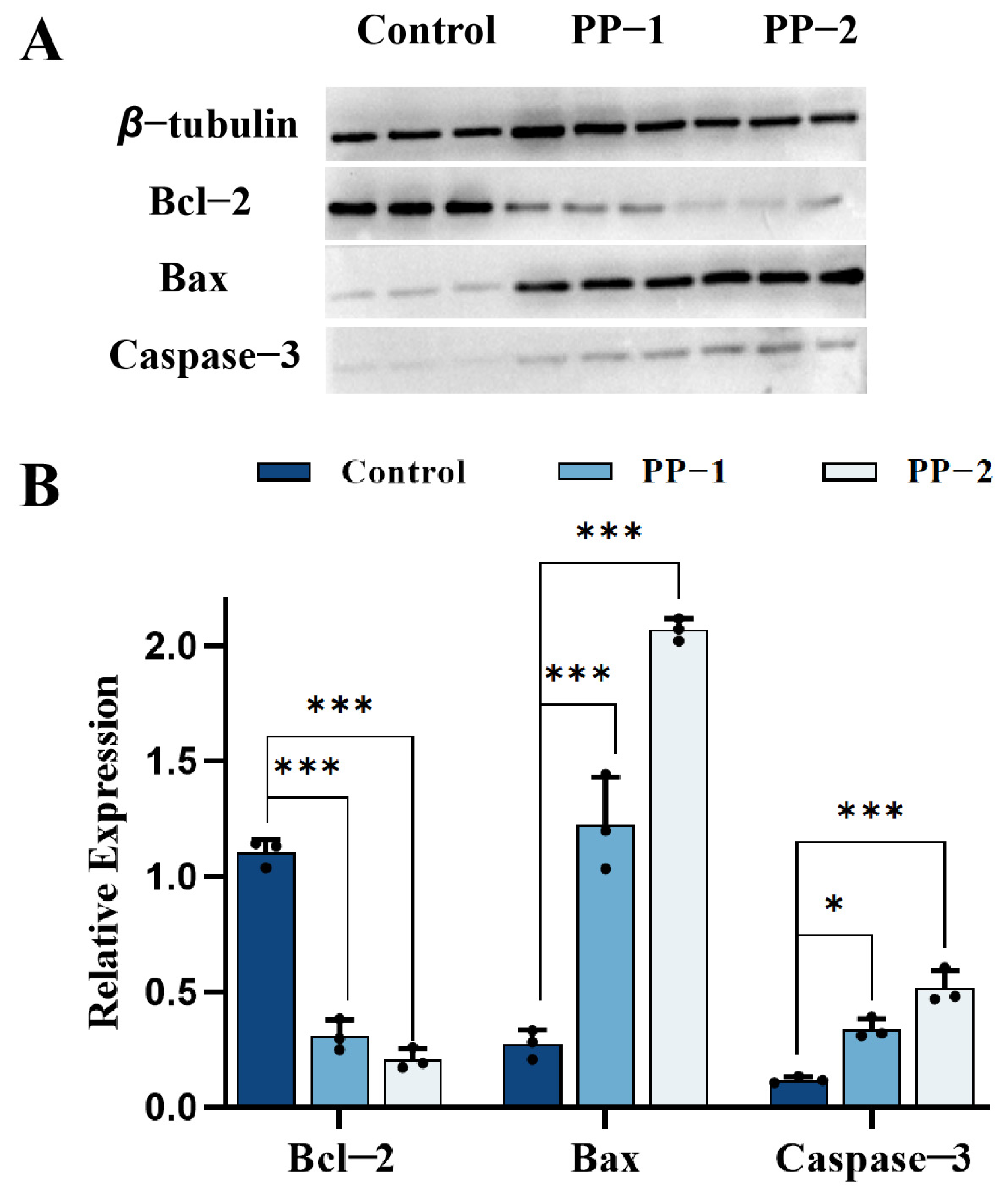
3.15. Western Blotting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, J.A.; Robinson, W.A. Malignant melanoma. Annu. Rev. Med. 1993, 44, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Y.; Liu, Q.; Zhang, H.; Jia, L.; Chai, Y.; Jiang, H.; Wu, M.; Li, Y. Global Trends in Melanoma Burden: A Comprehensive Analysis from the Global Burden of Disease Study, 1990–2021. J. Am. Acad. Dermatol. 2024, 92, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wang, L.; Yin, P.; Lin, Y.; Zhou, M. Burden of melanoma in China, 1990-2017: Findings from the 2017 global burden of disease study. Int. J. Cancer 2020, 147, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Le, A.; Lam, F.C.; Scherrer, E.; Kerr, R.G.; Lau, A.C.; Han, J.; Jiang, R.; Diede, S.J.; Shui, I.M. Current Treatment Approaches and Global Consensus Guidelines for Brain Metastases in Melanoma. Front. Oncol. 2022, 12, 885472. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths among US Whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef]
- Misir, A.F.; Durmuslar, M.C.; Zerener, T.; Gun, B.D. Primary malignant melanoma. Saudi. Med. J. 2016, 37, 446–449. [Google Scholar] [CrossRef]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.H.; Berking, C.; Sunderkoetter, C.; Kaatz, M.; Schatton, K.; Lehmann, P.; et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients with Melanoma with Positive Sentinel Node. J. Clin. Oncol. 2019, 37, 3000–3008. [Google Scholar] [CrossRef]
- Dumitras, D.; Andrei, S. Recent Advances in the Antiproliferative and Proapoptotic Activity of Various Plant Extracts and Constituents Against Murine Malignant Melanoma. Molecules 2022, 27, 2585. [Google Scholar] [CrossRef]
- Gupta, M.; Asfaha, D.M.; Ponnaiah, G. Millets: A Nutritional Powerhouse with Anti-cancer Potential. Cureus 2023, 15, e47769. [Google Scholar] [CrossRef]
- Rakashanda, S.; Qazi, A.K.; Majeed, R.; Andrabi, S.M.; Hamid, A.; Sharma, P.R.; Amin, S. Plant-derived Protease Inhibitors LC-pi (Lavatera cashmeriana) Inhibit Human Lung Cancer Cell Proliferation in Vitro. Nutr. Cancer 2014, 67, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume Seeds and Colorectal Cancer Revisited: Protease Inhibitors Reduce MMP-9 Activity and Colon Cancer Cell Migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pots, A.; Gruppen, H.; Van Diepenbeek, R.; Van Der Lee, J.; Van Boekel, M.; Wijngaards, G.; Voragen, A. The Effect of Storage of Whole Potatoes of Three Cultivars on the Patatin and Protease Inhibitor Content; A Study Using Capillary Electrophoresis and Maldi-Tof Mass Spectrometry. J. Sci. Food. Agric. 1999, 79, 1557–1564. [Google Scholar] [CrossRef]
- Brta, J.; Hemanov, V.; Divi, J.I. Effect of low-molecular additives on precipitation of potato fruit juice proteins under different temperature regimes. J. Food Process Eng. 2008, 31, 533. [Google Scholar] [CrossRef]
- Al-Mughrabi, K.I.; Coleman, W.K.; Vikram, A.; Poirier, R.; Jayasuriya, K.E. Effectiveness of Essential Oils and Their Combinations with Aluminum Starch Octenylsuccinate on Potato Storage Pathogens. J. Essent. Oil-Bear. Plants 2013, 16, 23–31. [Google Scholar] [CrossRef]
- Mu, T.; Tan, S.; Xue, Y. The Amino Acid Composition, Solubility and Emulsifying Properties of Sweet Potato Protein. Food Chem 2009, 112, 1002–1005. [Google Scholar] [CrossRef]
- Lokra, S.; Schuller, R.B.; Egelandsdal, B.; Engebretsen, B.; Straetkvern, K.O. Comparison of Composition, Enzyme Activity and Selected Functional Properties of Potato Proteins Isolated from Potato Juice with Two Different Expanded Bed Resins. LWT-Food Sci. Technol. 2009, 42, 906–913. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Luo, Z.; Tamer, T.M. Stability of Trypsin Inhibitor Isolated from Potato Fruit Juice Against Ph and Heating Treatment and in Vitro Gastrointestinal Digestion. Food Chem. 2020, 328, 127152. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Jiang, L.; Zhang, G.; Li, G.; Wu, N. Preparation, Identification, Structure, and in Vitro Anti-Obesity Effects of Protease Inhibitors Isolated from Potato Fruit Juice. Eur. Food Res. Technol. 2013, 237, 149–157. [Google Scholar] [CrossRef]
- Hill, A.J.; Peikin, S.R.; Ryan, C.A.; Blundell, J.E. Oral Administration of Proteinase Inhibitor II from Potatoes Reduces Energy Intake in Man. Physiol. Behav. 1990, 48, 241–246. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.Y.; Ryan, C.A.; Dong, Z. Proteinase Inhibitors I and Ii From Potatoes Specifically Block Uv-Induced Activator Protein-1 Activation Through a Pathway That Is Independent of Extracellular Signal-Regulated Kinases, C-Jun N-Terminal Kinases, and P38 Kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 11957–11962. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Park, S.I.; Kang, S.J.; Kim, J.W.; Lee, E.K.; Song, C.H.; Han, C.H.; Lee, Y.J.; Ku, S.K. In vitro antioxidant and anti-adipogenic effects of slendestatm, standard potato extracts contains 5% protease inhibitor Ii. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 164. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, Y.; Bai, Y.; Yu, S.; Tian, Y.; Li, J.; Li, S.; Li, T. Potato Protease Inhibitors, a Functional Food Material with Antioxidant and Anticancer Potential. Food Sci. Hum. Wellness 2023, 12, 1762–1771. [Google Scholar] [CrossRef]
- Ying, S.; Ya-ping, T. Study on the Interaction with DNA and Antioxidant Activity of the Aspartic Protease Inhibitors from Potato. J. Food Sci. Biotechnol. 2011, 30, 435–439. [Google Scholar]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and Quantification of Protein Glycosylation. Int. J. Carbohydr. Chem. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and Identification of Antioxidant Peptides from Chickpea (Cicer arietinum L.) Albumin Hydrolysates. LWT-Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Siswoyo, T.A.; Mardiana, E.; Lee, K.O.; Hoshokawa, K. Isolation and Characterization of Antioxidant Protein Fractions from Melinjo (Gnetum gnemon) Seeds. J. Agric. Food Chem. 2011, 59, 5648–5656. [Google Scholar] [CrossRef]
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and Identification of Antioxidative Peptides from Porcine Collagen Hydrolysate by Consecutive Chromatography and Electrospray Ionization–mass Spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, P.; Su, W.; Wang, Y.; Wu, H.; Peng, W.; Li, P. Antioxidant Activity and Hepatoprotective Potential of Quercetin 7-Rhamnoside in Vitro and in Vivo. Molecules 2018, 23, 1188. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Alves, C.; Maia, D.S.V.; Krumreich, F.D.; Crizel-Cardoso, M.M.; Fioravante, J.B.; Silva, W.P.D.; Borges, C.D.; Zambiazi, R.C. Propolis Microparticles Produced with Pea Protein: Characterization and Evaluation of Antioxidant and Antimicrobial Activities. Food Hydrocoll. 2019, 87, 703–711. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2011, 5, 2342–2352. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Rout, S.; Banerjee, R. Free Radical Scavenging, Anti-Glycation and Tyrosinase Inhibition Properties of a Polysaccharide Fraction Isolated from the Rind from Punica granatum. Bioresour. Technol. 2006, 98, 3159–3163. [Google Scholar] [CrossRef]
- Gao, J. Recovery and Properties of Protein from Potato Starch Wastewater; Shanxi University of Science and Technology: Xi’an, China, 2012. [Google Scholar]
- Liu, W. Research on Protein Recovery Methods and Process Design in Potato Starch Production Wastewater; Heilongjiang Oriental College: Harbin, China, 2022. [Google Scholar]
- Bartova, V.; Barta, J. Chemical Composition and Nutritional Value of Protein Concentrates Isolated from Potato (Solanum tuberosum L.) Fruit Juice by Precipitation with Ethanol or Ferric Chloride. J. Agric. Food Chem. 2009, 57, 9028–9034. [Google Scholar] [CrossRef]
- Zwijnenberg, H.J.; Kemperman, A.J.B.; Boerrigter, M.E.; Lotz, M.; Dijksterhuis, J.F.; Poulsen, P.E.; Koops, G. Native protein recovery from potato fruit juice by ultrafiltration. Desalination 2002, 144, 331–334. [Google Scholar] [CrossRef]
- Tkvern, K.O.S.; Schwarz, J.G. Recovery of Native Potato Protein Comparing Expanded Bed Adsorption and Ultrafiltration. Food Bioprocess Technol. 2011, 5, 1939–1949. [Google Scholar]
- Liu, Y.W.; Han, C.H.; Lee, M.H.; Hsu, F.L.; Hou, W.C. Patatin, the Tuber Storage Protein of Potato (Solanum tuberosum L.), Exhibits Antioxidant Activity in Vitro. J. Agric. Food Chem. 2003, 51, 4389–4393. [Google Scholar] [CrossRef]
- Ahlden, I.; Tragardh, G. A Study of Soluble Proteins from Four Potato Varieties Used in the Swedish Starch Industry. Food Chem. 1992, 44, 113–118. [Google Scholar] [CrossRef]
- Partsia, Z.; Kiosseoglou, V. Foaming Properties of Potato Proteins Recovered by Complexation with Carboxymethylcellulose. Colloids Surf. B Biointerfaces 2001, 21, 69–74. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Bai, Y.; Zhao, Q.; Yu, S.; Tian, Y.; Bian, Y.; Li, J.; Li, S.; Li, T. Preparation and Antioxidative Stability of the Potato Protease Inhibitors (ppis) from Potato Starch Waste-Water. LWT 2020, 134, 109963. [Google Scholar] [CrossRef]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Prabu, D.L.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, N.S.; Mathew, S. Antioxidant, Functional Properties and Amino Acid Composition of Pepsin-Derived Protein Hydrolysates from Whole Tilapia Waste As Influenced by Pre-Processing Ice Storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Mcclements, D.; Decker, E. Antioxidant Activity of Cysteine, Tryptophan, and Methionine Residues in Continuous Phase Beta-Lactoglobulin in Oil-In-Water Emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef]
- Stdal, H.; Davies, M.J.; Andersen, H.J. Reaction between protein radicals and other biomolecules. Free. Radic. Biol. Med. 2002, 33, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, Y.; Zhang, W.; He, J. Potato Proteins for Technical Applications: Nutrition, Isolation, Modification and Functional Properties-A Review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103533. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Wei, D. Partial Characterization, in Vitro Antioxidant and Antiproliferative Activities of Patatin Purified from Potato Fruit Juice. Food Funct. 2013, 4, 1502. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Y.; Hu, T.; Li, W.; Deng, Y.; Wang, X.; Tang, H.; Zhao, L.; Yan, G. Optimization of Cellulase-Assisted Extraction of Total Flavonoids from Equisetum Via Response Surface Methodology Based on Antioxidant Activity. Processes 2023, 11, 1978. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology Applied to Solid Lipid Nanoparticles and Nanostructured Lipid Carriers–A Systematic Review of in Vitro Data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Phagocytosis of polymer microspheres by macrophages. Adv. Polym. Sci. 1990, 94, 107–141. [Google Scholar]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G. Flow Cytometric Detection of Hyper-Polarized Mitochondria in Regulated and Accidental Cell Death Processes. Apoptosis 2020, 25, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Barar, J.; Hejazi, M.S.; Samadi, N. Doxorubicin Changes Bax/Bcl-Xl Ratio, Caspase-8 and 9 in Breast Cancer Cells. Adv. Pharm. Bull. 2015, 5, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of Apoptosis Induced by Doxorubicin Through the Generation of Hydrogen Peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar] [CrossRef]
- Sheih, I.; Fang, T.J.; Wu, T.; Lin, P. Anticancer and Antioxidant Activities of the Peptide Fraction from Algae Protein Waste. J. Agric. Food Chem. 2010, 58, 1202–1207. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Liu, R.H.; Regenstein, J.M. Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J. Agric. Food Chem. 2011, 59, 7948–7953. [Google Scholar] [CrossRef]
- Sedloev, D.; Chen, Q.; Unglaub, J.M.; Schanda, N.; Hao, Y.; Besiridou, E.; Neuber, B.; Schmitt, A.; Raffel, S.; Liu, Y.; et al. Proteasome Inhibition Enhances the Anti-Leukemic Efficacy of Chimeric Antigen Receptor (CAR) Expressing NK Cells Against Acute Myeloid Leukemia. J. Hematol. Oncol. 2024, 17, 1–22. [Google Scholar] [CrossRef]





| Amino Acid | Content (%) | |
|---|---|---|
| PP-1 | PP-2 | |
| Thr | 5.14 | 2.73 |
| Val | 6.00 | 9.88 |
| Met | 3.20 | 4.04 |
| Ile | 3.17 | 1.96 |
| Leu | 8.08 | 4.25 |
| Phe | 5.59 | 3.93 |
| His | 2.54 | 1.86 |
| Lys | 7.37 | 5.14 |
| EAAs/TAAs | 41.09 | 33.79 |
| Asp | 9.61 | 6.96 |
| Ser | 4.69 | 2.80 |
| Glu | 10.72 | 5.27 |
| Gly | 4.78 | 3.80 |
| Ala | 5.13 | 2.95 |
| Tyr | 6.07 | 5.30 |
| Arg | 3.86 | 2.38 |
| Pro | 4.18 | 4.63 |
| Cys | 9.87 | 32.13 |
| NEAAs/TAAs | 58.91 | 66.21 |
| Antioxidant Activity | IC50 * (mg·mL−1) | |||
|---|---|---|---|---|
| Vc | OVA | PP-1 | PP-2 | |
| Reducing power | --- | 7.74 ± 0.67 | 12.63 ± 2.62 | 3.13 ± 0.28 |
| Hydroxyl radical scavenging ability | 0.05 ± 0.01 | 69.99 ± 2.27 | 209.50 ± 5.90 | 104.43 ± 2.67 |
| DPPH radical scavenging ability | --- | 0.89 ± 0.05 | 1.97 ± 0.30 | 0.64 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Jiang, L.; Chen, N.; Wang, X.; Yao, J.; Su, Z.; Zhao, S. Anti-Melanoma Activity and Potential Mechanism of Purified Potato Protease Inhibitor. Foods 2025, 14, 1026. https://doi.org/10.3390/foods14061026
Li Q, Jiang L, Chen N, Wang X, Yao J, Su Z, Zhao S. Anti-Melanoma Activity and Potential Mechanism of Purified Potato Protease Inhibitor. Foods. 2025; 14(6):1026. https://doi.org/10.3390/foods14061026
Chicago/Turabian StyleLi, Qiuyan, Lu Jiang, Ni Chen, Xingzhi Wang, Jiajun Yao, Zhien Su, and Suqing Zhao. 2025. "Anti-Melanoma Activity and Potential Mechanism of Purified Potato Protease Inhibitor" Foods 14, no. 6: 1026. https://doi.org/10.3390/foods14061026
APA StyleLi, Q., Jiang, L., Chen, N., Wang, X., Yao, J., Su, Z., & Zhao, S. (2025). Anti-Melanoma Activity and Potential Mechanism of Purified Potato Protease Inhibitor. Foods, 14(6), 1026. https://doi.org/10.3390/foods14061026







