Recent Advances in Polysaccharide-Based Nanocomposite Films for Fruit Preservation: Construction, Applications, and Challenges
Abstract
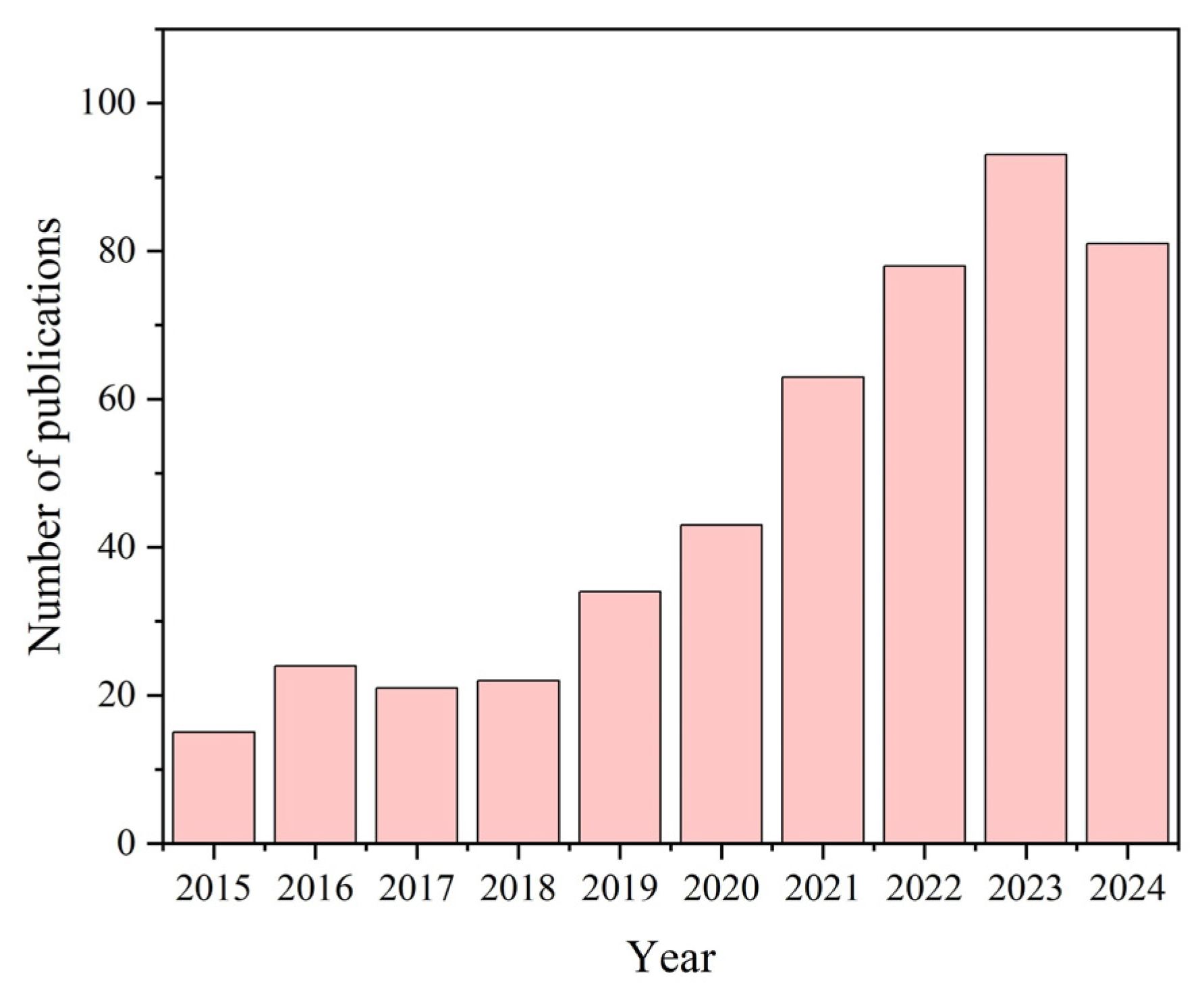
1. Introduction
2. Factors and Mechanisms Affecting Quality Deterioration of Water Fruits and Preservation Methods
2.1. Fruit Ripening
2.2. Fruit Deterioration
2.3. Methods of Fruit Preservation
Fundamental Mechanisms of Polysaccharide-Based Packaging
3. Polysaccharide-Based Nanocomposite Film Design and Preparation Strategy
3.1. Polysaccharide Types
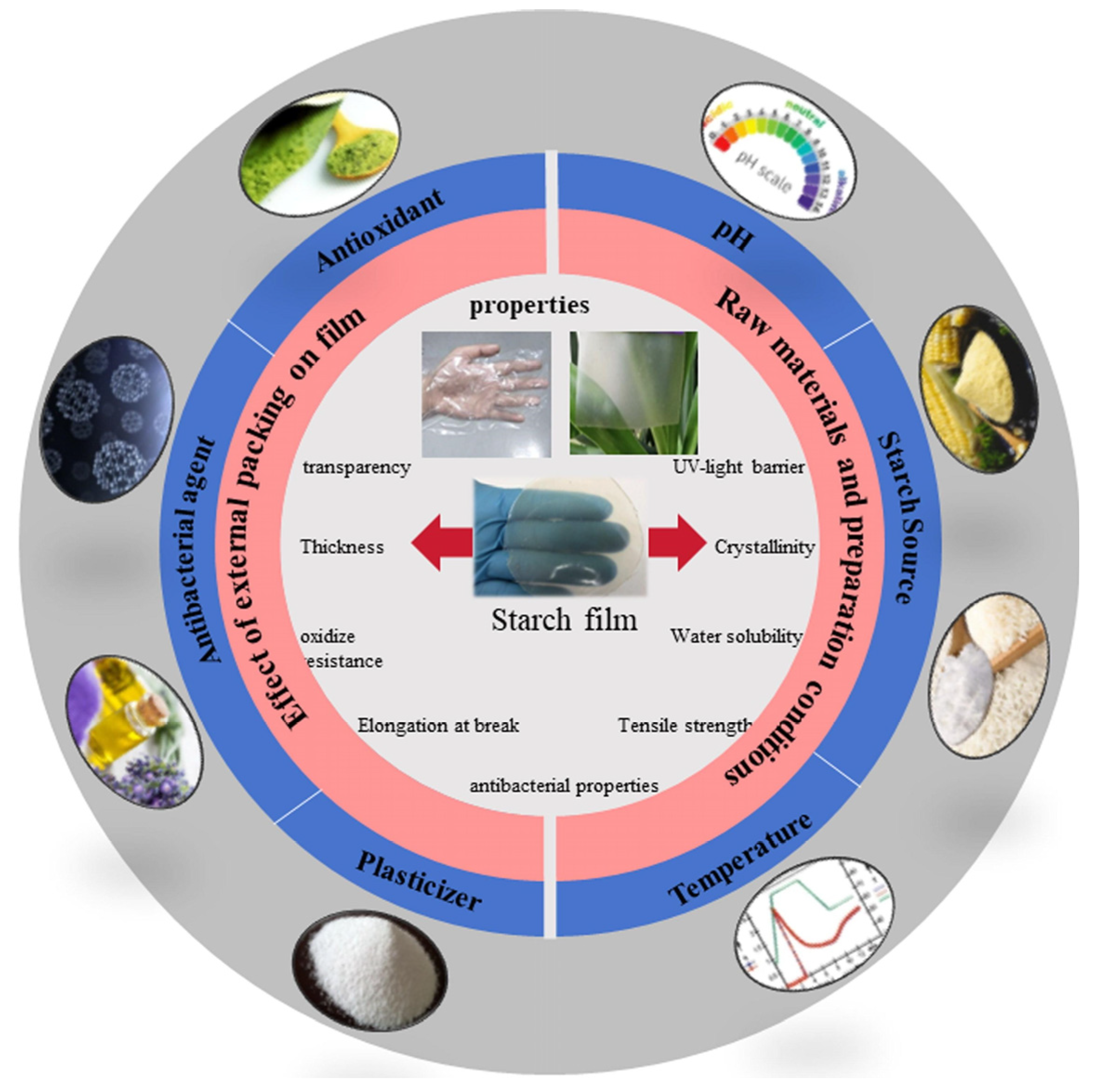
3.1.1. Starch
3.1.2. Cellulose
3.1.3. Chitosan
3.1.4. Seaweed Polysaccharides
3.2. Addition of Nanomaterials
3.2.1. Metal and Metal Oxide Nanomaterials
Silver Nanoparticles (AgNPs)
3.2.2. Non-Metallic Nanomaterials
Graphene Oxide
Cellulose Nanocrystals
3.2.3. Controlled Release of Nanomaterials
3.3. Loaded Active Substances
4. Freshness Preservation Mechanism and Safety of Polysaccharide-Based Nanocomposite Films
Safety of Polysaccharide-Based Nanofilms
5. Applications
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial Spoilage of Fruits: A Review on Causes and Prevention Methods. Food Rev. Int. 2020, 38, 225–246. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Jayakumar, A.; de Souza, C.K.; Rhim, J.W.; Kim, J.T. Advances in strawberry postharvest preservation and packaging: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13417. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-Based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, N.T.M.; Rawi, N.F.M.; Hassan, N.A.A.; Saharudin, N.I.; Kassim, M.H.M. Seaweed polysaccharide nanocomposite films: A review. Int. J. Biol. Macromol. 2023, 245, 125486. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Song, P. Recent advances in polysaccharide-based carbon aerogels for environmental remediation and sustainable energy. Sustain. Mater. Technol. 2021, 27, e00240. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Abdiryim, T.; Liu, X. Polysaccharides and their derivatives for solar-driven water evaporators. Cellulose 2024, 31, 7251–7280. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Y.; Fu, G.; Feng, C.; Hu, J.; Haegeman, W.; Liu, H. Calcareous silt earthen construction using biopolymer reinforcement. J. Build. Eng. 2023, 72, 106571. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Lu, R.; Xu, J.; Hu, K.; Liu, Y. Preparation of Chitosan/Corn Starch/Cinnamaldehyde Films for Strawberry Preservation. Foods 2019, 8, 423. [Google Scholar] [CrossRef]
- Xue, C.; Linqin, C.; Xue, Y.; Yilin, W.; Jiaxin, X.; Rongfan, Z.; Shaole, L.; Yuanyuan, L. Active curcumin-loaded γ-cyclodextrin-metal organic frameworks as nano respiratory channels for reinforcing chitosan/gelatin films in strawberry preservation. Food Hydrocoll. 2024, 159, 110656. [Google Scholar]
- Nadar, S.S.; Vaidya, L.; Maurya, S.; Rathod, V.K. Polysaccharide based metal organic frameworks (polysaccharide–MOF): A review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Li, Y.; Bian, B.; Tang, R.; Zhang, K. Characterization of a Clove Essential Oil Slow-Release Microencapsulated Composite Film and Its Preservation Effects on Blueberry. ACS Omega 2024, 9, 12643–12656. [Google Scholar] [CrossRef]
- Geng, C.; Jiang, Y.; Bian, H.; Huang, G. Selective gas-permeation films with nanoMOFs as gas “Switches” for mango preservation. Chem. Eng. J. 2024, 481, 148757. [Google Scholar] [CrossRef]
- Xu, M.; Fang, D.; Shi, C.; Xia, S.; Wang, J.; Deng, B.; Kimatu, B.M.; Guo, Y.; Lyu, L.; Wu, Y.; et al. Anthocyanin-loaded polylactic acid/quaternized chitosan electrospun nanofiber as an intelligent and active packaging film in blueberry preservation. Food Hydrocoll. 2024, 158, 110586. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, S.E.; Kim, Y.-D.; Park, M.-K. Industrial-scale blown active packaging film with essential oils: Properties, dual-functional performance, and box packaging application of instant noodles. Food Packag. Shelf Life 2024, 43, 101276. [Google Scholar] [CrossRef]
- Wu, X.; Gong, D.; Zhao, K.; Chen, D.; Dong, Y.; Gao, Y.; Wang, Q.; Hao, G.-F. Research and development trends in plant growth regulators. Adv. Agrochem. 2024, 3, 99–106. [Google Scholar] [CrossRef]
- Li, B.-J.; Grierson, D.; Shi, Y.; Chen, K.-S. Roles of abscisic acid in regulating ripening and quality of strawberry, a model non-climacteric fruit. Hortic. Res. 2022, 9, uhac089. [Google Scholar] [CrossRef]
- Martín-Pizarro, C.; Vallarino, J.G.; Osorio, S.; Meco, V.; Urrutia, M.; Pillet, J.; Casañal, A.; Merchante, C.; Amaya, I.; Willmitzer, L.; et al. The NAC transcription factor FaRIF controls fruit ripening in strawberry. Plant Cell 2021, 33, 1574–1593. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Ma, D.; Liu, Z.; Qi, P.; Wang, Z.; Di, S.; Wang, X. Review of fruits flavor deterioration in postharvest storage: Odorants, formation mechanism and quality control. Food Res. Int. 2024, 182, 114077. [Google Scholar] [CrossRef]
- An, X.; Zhu, P.; Li, Z.; Fadiji, T.; Wani, A.A. Effect of expanded polyethylene (EPE) foam packing net design on the mechanical damage resistance of strawberry fruit during transportation. Food Packag. Shelf Life 2023, 40, 101193. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 59, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Y.; Fang, Z.; Wang, Z.-C.; Nan, Y.; Shi, H.; Zhang, H.; Song, W.; Gu, H. Modified atmosphere packaging and plant extracts synergistically enhance the preservation of meat: A review. Food Control 2024, 164, 110622. [Google Scholar] [CrossRef]
- Bisht, B.; Bhatnagar, P.; Gururani, P.; Kumar, V.; Tomar, M.S.; Sinhmar, R.; Rathi, N.; Kumar, S. Food irradiation: Effect of ionizing and non-ionizing radiations on preservation of fruits and vegetables—A review. Trends Food Sci. Technol. 2021, 114, 372–385. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Hermes, V.S.; Pagno, C.; Castagna, A.; Mannucci, A.; Sgherri, C.; Pinzino, C.; Ranieri, A.; Flôres, S.H.; Rios, A.d.O. Phenolic enrichment in apple skin following post-harvest fruit UV-B treatment. Postharvest Biol. Technol. 2018, 138, 37–45. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Gabaldón, J.A.; Gómez-López, V.M. Effect of pH on pulsed light inactivation of polyphenol oxidase. Enzym. Microb. Technol. 2021, 148, 109812. [Google Scholar] [CrossRef]
- Salazar-Zúñiga, M.N.; Lugo-Cervantes, E.; Rodríguez-Campos, J.; Sanchez-Vega, R.; Rodríguez-Roque, M.J.; Valdivia-Nájar, C.G. Pulsed Light Processing in the Preservation of Juices and Fresh-Cut Fruits: A Review. Food Bioprocess Technol. 2022, 16, 510–525. [Google Scholar] [CrossRef]
- Cheigh, C.-I.; Hwang, H.-J.; Chung, M.-S. Intense pulsed light (IPL) and UV-C treatments for inactivating Listeria monocytogenes on solid medium and seafoods. Food Res. Int. 2013, 54, 745–752. [Google Scholar] [CrossRef]
- Gyawali, R.; Degala, H.L.; Biswal, A.K.; Bardsley, C.A.; Mahapatra, A.K. Effects of intense pulsed light on inactivation of Salmonella Typhimurium and quality characteristics of pecan halves. LWT—Food Sci. Technol. 2024, 203, 116344. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jubinville, E.; Goulet-Beaulieu, V.; Jean, J. Inactivation of murine norovirus and hepatitis A virus on various frozen fruits using pulsed light. Int. J. Food Microbiol. 2024, 424, 110851. [Google Scholar] [CrossRef]
- Karaoglan, H.A.; Keklik, N.M.; Develi Işikli, N. Modeling Inactivation ofCandida inconspicuaIsolated from Turnip Juice using Pulsed UV Light. J. Food Process Eng. 2016, 40, e12418. [Google Scholar] [CrossRef]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of starch-based coatings incorporated with antimicrobial agents for preservation of fruits and vegetables: A review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, D.; Belwal, T.; Li, L.; Chen, H.; Xu, T.; Luo, Z. Effect of Nano-SiOx/Chitosan Complex Coating on the Physicochemical Characteristics and Preservation Performance of Green Tomato. Molecules 2019, 166, 106800. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shi, B.; Luan, X.; Wang, Y.; Song, H. Chitosan/cellulose nanocrystal biocomposite coating for fruit postharvest preservation. Ind. Crops Prod. 2023, 205, 117543. [Google Scholar] [CrossRef]
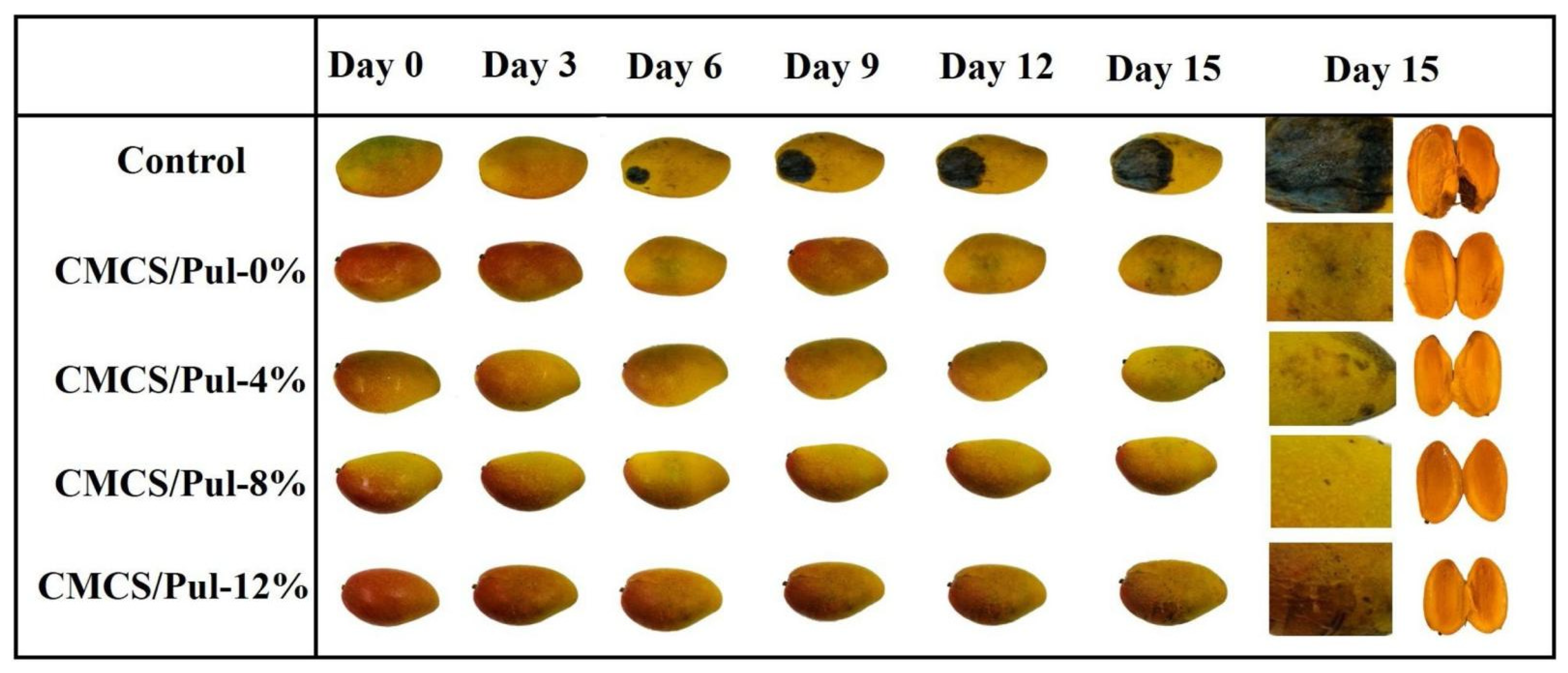
- Wu, J.; Zhang, J.; Ni, W.; Xu, X.; George, M.S.; Lu, G. Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage. Foods 2023, 12, 4352. [Google Scholar] [CrossRef]
- Li, Y.; Hua, Z.; Li, Y.; Chen, T.; Alamri, A.S.; Xu, Y.; Gong, W.; Hou, Y.; Alhomrani, M.; Hu, J. Development of multifunctional chitosan-based composite film loaded with tea polyphenol nanoparticles for strawberry preservation. Int. J. Biol. Macromol. 2024, 275, 133648. [Google Scholar] [CrossRef]
- Tang, J.; Huang, C.; Liu, W.; Zeng, X.; Zhang, J.; Liu, W.; Pang, J.; Wu, C. Preparation and characterization of a konjac glucomannan-based bio-nanocomposite film and its application in cherry tomato preservation. Food Hydrocoll. 2024, 159, 110689. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Wang, F.; Lu, C.; Liu, Y.; Zhang, S.; Ma, S.; Wang, L. Development of cellulose-based self-healing hydrogel smart packaging for fish preservation and freshness indication. Carbohydr. Polym. 2025, 348, 122806. [Google Scholar] [CrossRef]
- Yin, C.; Ding, X.; Lin, Z.; Cao, J.; Shi, W.; Wang, J.; Xu, D.; Xu, D.; Liu, Y.; Liu, G. Preparation and characterization of quercetin@ZIF-L/GO@AgNPs nanocomposite film for room-temperature strawberry preservation. Food Chem. 2024, 450, 139411. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Zhao, M.; Ruan, C.-Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohydr. Polym. 2023, 321, 121267. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 94, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, R.; Zhang, S.; Bai, Y.; Zhao, P.; Zhou, H.; Long, M.; Wang, X.; Meng, Y.H.; Guo, Y. Pectin-containing lignocellulosic nanofibers isolated from young apples enhance chitosan preservation film with robust mechanical and barrier properties. Food Hydrocoll. 2024, 162, 110978. [Google Scholar] [CrossRef]
- Ying Ben, Z.; Samsudin, H.; Firdaus Yhaya, M. Glycerol: Its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar]
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef]
- Shao, H.; Sun, H.; Yang, B.; Zhang, H.; Hu, Y. Facile and green preparation of hemicellulose-based film with elevated hydrophobicity via cross-linking with citric acid. RSC Adv. 2019, 9, 2395–2401. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Wang, Z.; Hu, G.; Yang, W.; Hu, Y. Chitosan/PCL fibrous membranes loaded potassium cinnamate/β-CD clathrate compounds developed by ELS for fruit preservation. Food Hydrocoll. 2024, 156, 110341. [Google Scholar] [CrossRef]
- Lai, W.; Wang, L.; Pang, Y.; Xin, M.; Li, M.; Shi, L.; Mao, Y. Preparation of citric acid cross-linked chitosan quaternary phosphonium/polyvinyl alcohol composite film and its application in strawberry preservation. Food Chem. 2024, 455, 139908. [Google Scholar] [CrossRef]
- Qin, Z.; Huang, Y.; Xiao, S.; Zhang, H.; Lu, Y.; Xu, K. Preparation and Characterization of High Mechanical Strength Chitosan/Oxidized Tannic Acid Composite Film with Schiff Base and Hydrogen Bond Crosslinking. Int. J. Mol. Sci. 2022, 23, 9284. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, G.; Li, K.; Ye, J.; Chen, J.; Zhang, J. Preparation, characterization, and antibacterial application of cross-linked nanoparticles composite films. Food Chem. X 2024, 25, 102057. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, Q.; Liu, K.; Chang, Y.; Wang, L.; Zhang, S.; Yu, Y. Characterization and Antioxidant and Antibacterial Activities of Carboxymethylated Tamarind Seed Polysaccharide Composite Films Incorporated with ε-Polylysine and Their Application in Fresh-Cut Green Bell Pepper Preservation. J. Agric. Food Chem. 2024, 72, 8805–8816. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Ji, R.; Li, K.; Zhang, W. Preparation and Characterization of Pullulan/Carboxymethyl Cellulose/Nano-TiO2 Composite Films for Strawberry Preservation. Food Biophys. 2021, 16, 460–473. [Google Scholar] [CrossRef]
- Yu, H.C.; Zhang, H.; Ren, K.; Ying, Z.; Zhu, F.; Qian, J.; Ji, J.; Wu, Z.L.; Zheng, Q. Ultrathin κ-Carrageenan/Chitosan Hydrogel Films with High Toughness and Antiadhesion Property. ACS Appl. Mater. Interfaces 2018, 10, 9002–9009. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, X.; Jia, R.; Fang, Y.; Qin, Z. Development of antibacterial film based on alginate fiber, and peanut red skin extract for food packaging. J. Food Eng. 2022, 330, 111106. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, H.; Dong, C.; He, Z.; Long, Z. Eco-friendly and flexible polysaccharide-based packaging films for fruit preservation. Int. J. Biol. Macromol. 2024, 281, 136132. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Souza, A.G.; Quispe, Y.M.; Rosa, D.S. Essential oils loaded-chitosan nanocapsules incorporation in biodegradable starch films: A strategy to improve fruits shelf life. Int. J. Biol. Macromol. 2021, 188, 628–638. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Cheng, G.; Cheng, F.; Lin, Y.; Zhu, P.-X. Fabrication and characterization of starch-based nanocomposites reinforced with montmorillonite and cellulose nanofibers. Carbohydr. Polym. 2019, 210, 429–436. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef]
- Tang, H.; Han, Z.; Zhao, C.; Jiang, Q.; Tang, Y.; Li, Y.; Cheng, Z. Preparation and characterization of Aloe vera polysaccharide-based packaging film and its application in blueberry preservation. Prog. Org. Coat. 2023, 177, 107445. [Google Scholar] [CrossRef]
- Tastan, Ö.; Ferrari, G.; Baysal, T.; Donsì, F. Understanding the effect of formulation on functionality of modified chitosan films containing carvacrol nanoemulsions. Food Hydrocoll. 2016, 61, 756–771. [Google Scholar] [CrossRef]
- Kundu, S.; Das, A.; Basu, A.; Abdullah, M.F.; Mukherjee, A. Guar gum benzoate nanoparticle reinforced gelatin films for enhanced thermal insulation, mechanical and antimicrobial properties. Carbohydr. Polym. 2017, 170, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Yu, J.; Xu, S.; Chen, R.; Shao, P. A promising bioactive chitosan film in strawberry fresh-keeping: Plasticized with tomato processing by-product extract of deep eutectic solvent. Food Hydrocoll. 2024, 151, 109859. [Google Scholar] [CrossRef]
- Liu, Z.; Du, M.; Liu, H.; Zhang, K.; Xu, X.; Liu, K.; Tu, J.; Liu, Q. Chitosan films incorporating litchi peel extract and titanium dioxide nanoparticles and their application as coatings on watercored apples. Prog. Org. Coat. 2020, 151, 106103. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, W.; Meng, F.; Wang, L. Fabricating an anti-shrinking κ-carrageenan/sodium carboxymethyl starch film by incorporating carboxylated cellulose nanofibrils for fruit preservation. Int. J. Biol. Macromol. 2021, 191, 706–713. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y.; Liu, Y.; Li, Y.; Xu, C.; Fu, L.; Lin, B. Curcumin-loaded HKUST-1@ carboxymethyl starch-based composites with moisture-responsive release properties and synergistic antibacterial effect for perishable fruits. Int. J. Biol. Macromol. 2022, 241, 181–191. [Google Scholar] [CrossRef]
- Wang, M.; Nian, L.; Wu, J.; Cheng, S.; Yang, Z.; Cao, C. Visible light-responsive chitosan/sodium alginate/QDs@ZIF-8 nanocomposite films with antibacterial and ethylene scavenging performance for kiwifruit preservation. Food Hydrocoll. 2023, 145, 109073. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, W.; Kong, Y.; Wang, S.; Yao, B.; Wang, Y.; Wang, Z. Truxillic calcium supramolecular skeleton fortified pH responsive and biodegradable alginate hydrogel films promoting fruit preservation. Int. J. Biol. Macromol. 2024, 287, 138423. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, J.; Diao, Y.; Zhao, F.; Yang, Q. The application of starch-based edible film in food preservation: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2024, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Gopi, S.; Sreekala, M.S.; Thomas, S. UV resistant transparent bionanocomposite films based on potato starch/cellulose for sustainable packaging. Starch 2017, 70, 1700139. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef]
- Ali, A.; Xie, F.; Yu, L.; Liu, H.; Meng, L.; Khalid, S.; Chen, L. Preparation and characterization of starch-based composite films reinfoced by polysaccharide-based crystals. Compos. Part B Eng. 2018, 133, 122–128. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Liu, Y.; Chen, J.; Wang, C.; Hu, Y.; Hu, K. Production of biodegradable potato starch films containing Lycium barbarum polysaccharide and investigation of their physicochemical properties. Food Packag. Shelf Life 2024, 44, 101320. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, H.; Ma, Q.; Cheng, D.; Zhang, Y.; Wang, W.; Wang, J.; Sun, J. Development of chitosan/potato peel polyphenols nanoparticles driven extended-release antioxidant films based on potato starch. Food Packag. Shelf Life 2022, 31, 100793. [Google Scholar] [CrossRef]
- Yue, Z.; Ziheng, L.; Jie, Z.; Haiyan, G.; Junru, Q. Barrier properties characterization and release kinetics study of citrus fibers-reinforced functional starch composites. Food Hydrocoll. 2025, 164, 111195. [Google Scholar]
- Chen, K.; Tian, R.; Jiang, J.; Xiao, M.; Wu, K.; Kuang, Y.; Deng, P.; Zhao, X.; Jiang, F. Moisture loss inhibition with biopolymer films for preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2024, 263, 130337. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; von Borries-Medrano, E.; Aguilar-Méndez, M.A. Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int. J. Biol. Macromol. 2022, 206, 1012–1025. [Google Scholar] [CrossRef]
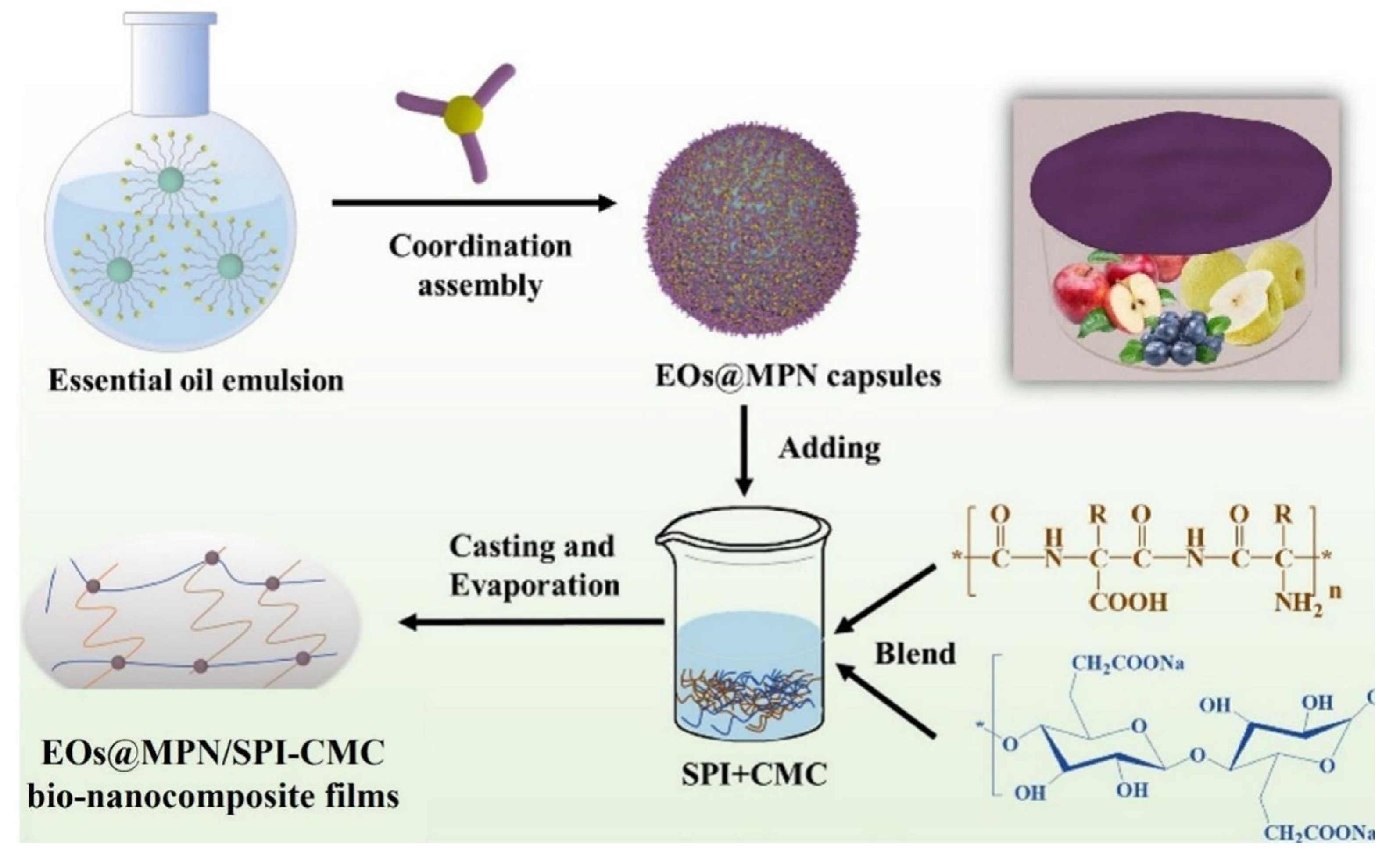
- Liu, M.; Wang, Y.; Su, S.; Long, F.; Zhong, L.; Hu, J. Multifunctional bio-nanocomposite films integrated with essential oils@metal-phenolic network nanocapsules for durable fruit preservation. Int. J. Biol. Macromol. 2024, 278, 134916. [Google Scholar] [CrossRef] [PubMed]
- Nongnual, T.; Butprom, N.; Boonsang, S.; Kaewpirom, S. Citric acid crosslinked carboxymethyl cellulose edible films: A case study on preserving freshness in bananas. Int. J. Biol. Macromol. 2024, 267, 131135. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti Khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A.; Noshirvani, N.; Hosseini, M. The optimization of gelatin-CMC based active films containing chitin nanofiber and Trachyspermum ammi essential oil by Response Surface Methodology. Carbohydr. Polym. 2019, 208, 457–468. [Google Scholar] [CrossRef]
- Shen, Y.; Seidi, F.; Ahmad, M.; Liu, Y.; Saeb, M.R.; Akbari, A.; Xiao, H. Recent Advances in Functional Cellulose-based Films with Antimicrobial and Antioxidant Properties for Food Packaging. J. Agric. Food Chem. 2023, 71, 16469–16487. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qin, S.; Han, S.; Qi, H. Antimicrobial, UV blocking, water-resistant and degradable coatings and packaging films based on wheat gluten and lignocellulose for food preservation. Compos. Part B Eng. 2022, 238, 109868. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Bai, Y.; Fang, Z.; Zhang, S.; Wang, X.; Yang, Y.; Guo, Y. Recent Advances in Cellulose Nanofiber Modification and Characterization and Cellulose Nanofiber-Based Films for Eco-Friendly Active Food Packaging. Foods 2024, 13, 3999. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Xu, H.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the production of TEMPO-mediated oxidation cellulose nanofibrils by kneading. Int. J. Biol. Macromol. 2024, 261, 129612. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Zhang, S.; Tang, J.; Shi, X.; Qin, S.; Pan, L.; Xiao, H. Enhancing the applicability of gelatin-carboxymethyl cellulose films by cold plasma modification for the preservation of fruits. LWT—Food Sci. Technol. 2023, 178, 114612. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, D.; Gu, Z.; Li, Z.; Hong, Y.; Li, C. Preparation of acetylated nanofibrillated cellulose from corn stalk microcrystalline cellulose and its reinforcing effect on starch films. Int. J. Biol. Macromol. 2018, 111, 959–966. [Google Scholar] [CrossRef]
- Shen, H.; Chen, J.; Tan, K.B. Ethyl cellulose matrixed poly(sulfur-co-sorbic acid) composite films: Regulation of properties and application for food preservation. Int. J. Biol. Macromol. 2024, 279, 135183. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Pagnocelli, M.; Baldasso, C.; Zattera, A.J. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose 2016, 24, 769–779. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Z.; Gu, Y.; Di, Y.; Liu, Y.; Srinivasan, V.; Lian, C.; Li, Y. Acetylated nanocellulose reinforced hydroxypropyl starch acetate realizing polypropylene replacement for green packaging application. Carbohydr. Polym. 2024, 331, 121886. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Wang, Q.; Lin, G.; Yang, H.; Yu, D.; Cui, S.W.; Xia, W. Antimicrobial and antioxidant films formed by bacterial cellulose, chitosan and tea polyphenol—Shelf life extension of grass carp. Food Packag. Shelf Life 2022, 33, 100866. [Google Scholar] [CrossRef]
- Ding, K.; Xie, Y.; Xu, H.; Xu, S.; Ge, S.; Li, H.; Chang, X.; Chen, J.; Wang, R.; Shan, Y.; et al. Visible light-responsive TiO2-based hybrid nanofiller reinforced multifunctional chitosan film for effective fruit preservation. Food Chem. 2024, 460, 140539. [Google Scholar] [CrossRef]
- Chen, K.; Brennan, C.; Cao, J.; Cheng, G.; Li, L.; Qin, Y.; Chen, H. Characterization of chitosan/eugenol-loaded IRMOF-3 nanoparticles composite films with sustained antibacterial activity and their application in postharvest preservation of strawberries. LWT—Food Sci. Technol. 2023, 186, 115270. [Google Scholar] [CrossRef]
- Ji, Q.; Jin, Z.; Ding, W.; Wu, Y.; Liu, C.; Yu, K.; Zhang, N.; Jin, G.; Lu, P.; Bao, D.; et al. Chitosan composite films based on tea seed oil nano-microcapsules: Antibacterial, antioxidant and physicochemical properties. Food Packag. Shelf Life 2023, 40, 101212. [Google Scholar] [CrossRef]
- Fu, H.; Huang, R.; Li, J.; Lin, Z.; Wei, F.; Lin, B. Multifunctional cinnamaldehyde-tannic acid nano-emulsion/chitosan composite film for mushroom preservation. Food Hydrocoll. 2023, 145, 109111. [Google Scholar] [CrossRef]
- Khan, S.; Hashim, S.B.H.; Arslan, M.; Zhang, K.; Siman, L.; Mukhtar, A.; Zhihua, L.; Tahir, H.E.; Zhai, X.; Shishir, M.R.I.; et al. Development of an active biogenic silver nanoparticles composite film based on berry wax and chitosan for rabbit meat preservation. Int. J. Biol. Macromol. 2024, 275, 133128. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Cho Rok, L.; Su Jin, L.; Tae In, K.; Kiramage, C.; Jong Soo, L.; Sangsik, K.; Min Hee, K.; Won Ho, P. Chitosan-gallic acid conjugate edible coating film for perishable fruits. Food Chem. 2024, 463, 141322. [Google Scholar]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Development of Multifunctional Pullulan/Chitosan-Based Composite Films Reinforced with ZnO Nanoparticles and Propolis for Meat Packaging Applications. Foods 2021, 10, 2789. [Google Scholar] [CrossRef] [PubMed]
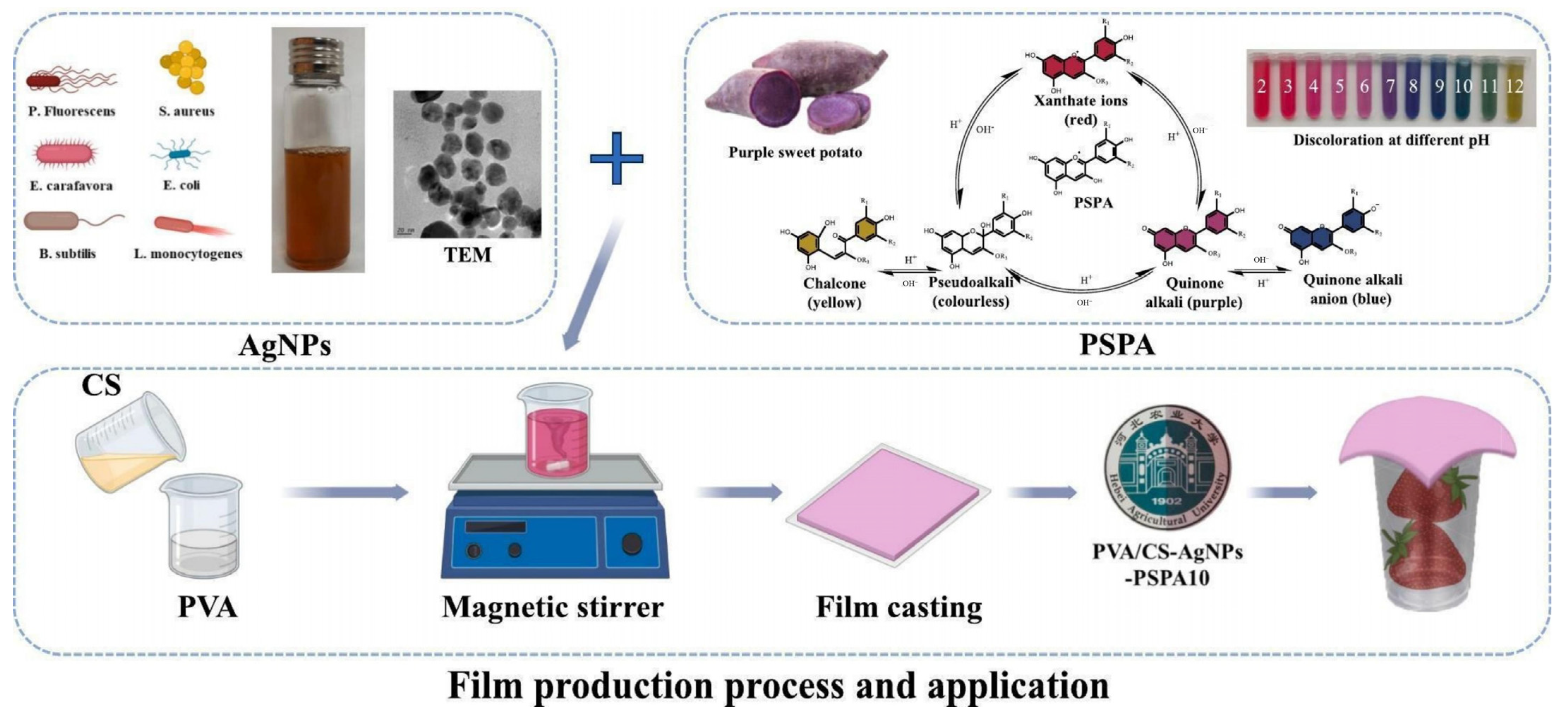
- Wu, J.; Zhang, Y.; Zhang, F.; Mi, S.; Yu, W.; Sang, Y.; Wang, X. Preparation of chitosan/polyvinyl alcohol antibacterial indicator composite film loaded with AgNPs and purple sweet potato anthocyanins and its application in strawberry preservation. Food Chem. 2024, 463, 101442. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Liang, H.; Zhou, J.; Zong, M.; Cao, Y.; Lou, W. A review of advancements in chitosan-essential oil composite films: Better and sustainable food preservation with biodegradable packaging. Int. J. Biol. Macromol. 2024, 274, 133242. [Google Scholar] [CrossRef]
- Nesren, M.E.-B.; Soliman, M.A.S.; Neveen, M.K.; Mohamed, N.A.E.-G. Chitosan and alginate/Aspergillus flavus-mediated nanocomposite films for preservation of postharvest tomatoe. Int. J. Biol. Macromol. 2025, 297, 139559. [Google Scholar]
- Wang, Y.; Zhang, Y.; Ma, Y.; Liu, J.; Zhang, R. Preparation and application of chitosan/nano-TiO2/daisy essential oil composite films in the preservation of Actinidia arguta. Food Chem. X 2025, 26, 102303. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, C.; Fan, H.; Fang, J.; Wu, X.; Qi, J.; Li, H. Chitosan/PVA composite film enhanced by ZnO/lignin with high-strength and antibacterial properties for food packaging. Int. J. Biol. Macromol. 2025, 306, 141658. [Google Scholar] [CrossRef]
- He, J.; Zhang, W.; Goksen, G.; Khan, M.R.; Ahmad, N.; Cong, X. Functionalized sodium alginate composite films based on double-encapsulated essential oil of wampee nanoparticles: A green preservation material. Food Chem. X 2024, 24, 101842. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; Deng, J.; Cheng, C.; Xu, Z.; Hou, W.; Yi, Y.; McClements, D.J.; Chen, S. Intelligent carrageenan-based composite films containing color indicator-loaded nanoparticles for monitoring fish freshness. Food Packag. Shelf Life 2024, 47, 101420. [Google Scholar] [CrossRef]
- Orsuwan, A.; Shankar, S.; Wang, L.-F.; Sothornvit, R.; Rhim, J.-W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Krishnan, L.; Ravi, N.; Kumar Mondal, A.; Akter, F.; Kumar, M.; Ralph, P.; Kuzhiumparambil, U. Seaweed-based polysaccharides—Review of extraction, characterization, and bioplastic application. Green Chem. 2024, 26, 5790–5823. [Google Scholar] [CrossRef]
- Giriyappa Thimmaiah, P.; Mudinepalli, V.R.; Thota, S.R.; Obireddy, S.R.; Lai, W.-F. Preparation, Characterization and Dielectric Properties of Alginate-Based Composite Films Containing Lithium Silver Oxide Nanoparticles. Front. Chem. 2022, 9, 777079. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, X.; Zhang, S.; Zhao, J.; Wang, R.; Wang, L.; Wang, X.; Yuan, Y.; Yue, T.; Cai, R.; et al. A versatile bilayer smart packaging based on konjac glucomannan/alginate for maintaining and monitoring seafood freshness. Carbohydr. Polym. 2024, 340, 122244. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.; Holmes, M.; Povey, M.; et al. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2021, 123, 107187. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh Nguyen, T.-T.; Tran Pham, B.-T.; Van Tran, T.; Bach, L.G.; Bui Thi, P.Q.; Ha Thuc, C.N. Development of poly (vinyl alcohol)/agar/maltodextrin coating containing silver nanoparticles for banana (Musa acuminate) preservation. Food Packag. Shelf Life 2021, 29, 100740. [Google Scholar] [CrossRef]
- Sun, S.; Wang, N.; Ali, E.; Qiao, L.; Guo, Q.; Lu, L. Glucose-responsive carboxymethyl chitosan/ sodium alginate film protects against mechanical wound on postharvest blueberry. Food Hydrocoll. 2024, 159, 110648. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Li, M.; Li, Z.; Shi, J.; Huang, X.; Zou, X.; Yan, M.; Qian, W.; Gong, Y.; et al. Saccharomyces cerevisiae-incorporated and sucrose-rich sodium alginate film: An effective antioxidant packaging film for longan preservation. Int. J. Biol. Macromol. 2022, 223, 673–683. [Google Scholar] [CrossRef]
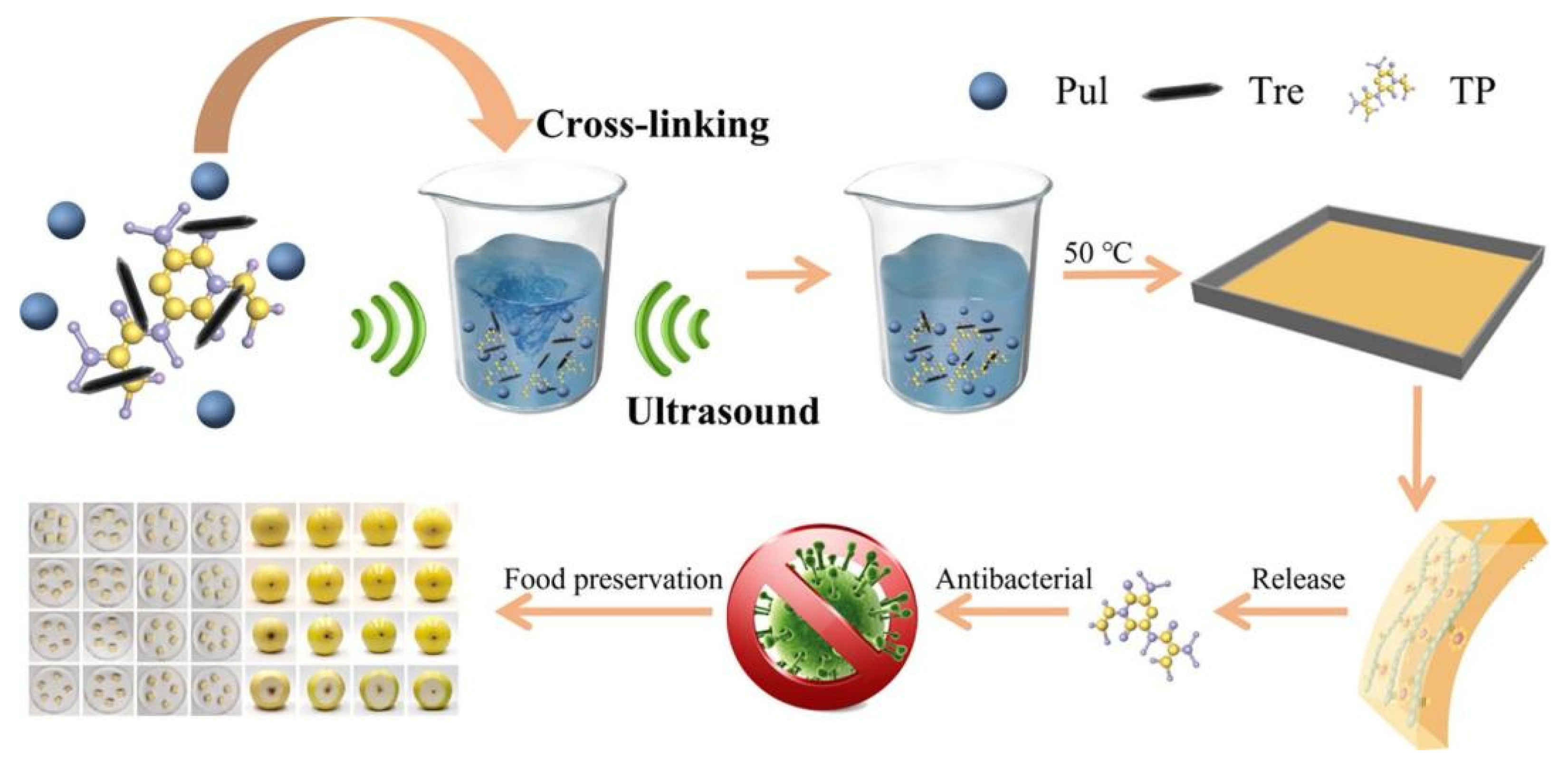
- Kang, L.; Liang, Q.; Rashid, A.; Qayum, A.; Chi, Z.; Ren, X.; Ma, H. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason. Sonochem. 2022, 92, 106242. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Scamparini, A.R.P. Sucrose and inverted sugar as plasticizer. Effect on cassava starch–gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007, 103, 255–262. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Souza, V.G.L.; Silva, J.A.; Esteves, A.; Pastrana, L.M.; Saraiva, C.; Cerqueira, M.A. Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology. Foods 2023, 12, 4076. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Liu, H.; Lu, H.; Xu, X.; Shen, M. Sodium alginate-camellia seed cake protein active packaging film cross-linked by electrostatic interactions for fruit preservation. Int. J. Biol. Macromol. 2024, 288, 138627. [Google Scholar] [CrossRef]
- Ruchika; Sharma, S.K.; Kumar, R.; Yadav, S.K.; Saneja, A. Development of a multifunctional and sustainable pterostilbene nanoemulsion incorporated chitosan-alginate food packaging film for shiitake mushroom preservation. Int. J. Biol. Macromol. 2024, 293, 139241. [Google Scholar] [CrossRef]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.; Bi, X.; Liu, X.; Shui, Y.; Lin, H.; et al. Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef]
- Zhang, W.; Sani, M.A.; Zhang, Z.; McClements, D.J.; Jafari, S.M. High performance biopolymeric packaging films containing zinc oxide nanoparticles for fresh food preservation: A review. Int. J. Biol. Macromol. 2023, 230, 123188. [Google Scholar] [CrossRef]
- Wang, G.; Yang, X.; Chen, X.; Huang, J.; He, R.; Zhang, R.; Zhang, Y. Construction and antibacterial activities of walnut green husk polysaccharide based silver nanoparticles (AgNPs). Int. J. Biol. Macromol. 2024, 276, 133798. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zheng, X.; Chen, M.; Tang, K. Soluble soybean polysaccharide/nano zinc oxide antimicrobial nanocomposite films reinforced with microfibrillated cellulose. Int. J. Biol. Macromol. 2020, 159, 793–803. [Google Scholar] [CrossRef]
- Xu, H.; Quan, Q.; Chang, X.; Ge, S.; Xu, S.; Wang, R.; Xu, Y.; Luo, Z.; Shan, Y.; Ding, S. A new nanohybrid particle reinforced multifunctional active packaging film for efficiently preserving postharvest fruit. Food Hydrocoll. 2023, 144, 109017. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Mehmood, A.; Khan, M.A.; Ahmad, K.S.; Nabi, A.G. Green synthesis of silver nanoparticles for their antifungal activity against anthracnose disease causing Colletotrichumcapsici. Biocatal. Agric. Biotechnol. 2024, 58, 103178. [Google Scholar] [CrossRef]
- Ton-That, P.; Dinh, T.A.; Thanh Gia-Thien, H.; Van Minh, N.; Nguyen, T.; Huynh, K.P.H. Novel packaging chitosan film decorated with green-synthesized nanosilver derived from dragon fruit stem. Food Hydrocoll. 2024, 158, 110496. [Google Scholar] [CrossRef]
- Xiang, F.; Xia, Y.; Wang, Y.; Wang, Y.; Wu, K.; Ni, X. Preparation of konjac glucomannan based films reinforced with nanoparticles and its effect on cherry tomatoes preservation. Food Packag. Shelf Life 2021, 29, 100701. [Google Scholar] [CrossRef]
- Surendhiran, D.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Fabrication of chitosan-based food packaging film impregnated with turmeric essential oil (TEO)-loaded magnetic-silica nanocomposites for surimi preservation. Int. J. Biol. Macromol. 2022, 203, 650–660. [Google Scholar] [CrossRef]
- Rodríguez-González, C.; Martínez-Hernández, A.L.; Castaño, V.M.; Kharissova, O.V.; Ruoff, R.S.; Velasco-Santos, C. Polysaccharide Nanocomposites Reinforced with Graphene Oxide and Keratin-Grafted Graphene Oxide. Ind. Eng. Chem. Res. 2012, 51, 3619–3629. [Google Scholar] [CrossRef]
- Dat, N.M.; Nam, N.T.H.; Cong, C.Q.; Huong, L.M.; Hai, N.D.; Tai, L.T.; An, H.; Duy, B.T.; Dat, N.T.; Viet, V.N.D.; et al. Chitosan membrane drafting silver-immobilized graphene oxide nanocomposite for banana preservation: Fabrication, physicochemical properties, bioactivities, and application. Int. J. Biol. Macromol. 2023, 242, 124607. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Zhang, N.; Liu, X.; Ding, J.; Xu, Q.; Cui, K.; Wang, Y.; Zhang, Q.; Cong, H. Novel graphene oxide modified LDH@CEO antibacterial materials capable of NIR stimuli-responsive control release for food preservation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 709, 136081. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, C.; Xu, Z.; Shan, P.; Fu, C.; Tao, Y.; Hu, J.; Wang, H.; Du, J. Eco-friendly chitosan-based composite film with anti-dissolution capacity as active packaging for fruit preservation. Food Hydrocoll. 2025, 163, 111146. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, S.; Li, C.; Li, M.; Li, Y.; Wu, P.; Li, H.; Ai, S. Functionalized cellulose nanocrystals reinforced polysaccharide matrix for the preparation of active packaging for perishable fruits preservation. Int. J. Biol. Macromol. 2024, 290, 138902. [Google Scholar] [CrossRef]
- Yang, J.; Dahlström, C.; Edlund, H.; Lindman, B.; Norgren, M. pH-responsive cellulose–chitosan nanocomposite films with slow release of chitosan. Cellulose 2019, 26, 3763–3776. [Google Scholar] [CrossRef]
- Du, C.; Li, S.; Fan, Y.; Lu, Y.; Sheng, J.; Song, Y. Preparation of gelatin-chitosan bilayer film loaded citral nanoemulsion as pH and enzyme stimuli-responsive antibacterial material for food packaging. Int. J. Biol. Macromol. 2023, 254, 127620. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, S.; Zhang, Z.; Cao, X.; Zhang, B.; Wu, D.; Chen, K.; Wang, W.-J.; Liu, P. Grafting Hollow Covalent Organic Framework Nanoparticles with Thermal-Responsive Polymers for the Controlled Release of Preservatives. ACS Appl. Mater. Interfaces 2022, 14, 22982–22988. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Li, F.; Zhou, X.; Lu, C.; Zhang, T. Characterization and sustained release study of starch-based films loaded with carvacrol: A promising UV-shielding and bioactive nanocomposite film. LWT—Food Sci. Technol. 2023, 180, 114719. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Tang, T.; Yang, L.; Chen, X.; Meng, S.; Zheng, R.; Wu, H. Carboxymethyl chitosan coating infused with linalool-loaded molten globular β-Lactoglobulin nanoparticles for extended preservation of fresh-cut apples. Food Chem. 2024, 460, 140578. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmad, T.; Belwal, T.; Aadil, R.M.; Siddique, M.; Pang, L.; Xu, Y. A review on protein based nanocarriers for polyphenols: Interaction and stabilization mechanisms. Food Innov. Adv. 2023, 2, 193–202. [Google Scholar] [CrossRef]
- Rehman Sheikh, A.; Wu-Chen, R.A.; Matloob, A.; Mahmood, M.H.; Javed, M. Nanoencapsulation of volatile plant essential oils: A paradigm shift in food industry practices. Food Innov. Adv. 2024, 3, 305–319. [Google Scholar] [CrossRef]
- Bi, J.; Fang, H.; Zhang, J.; Lu, L.; Gu, X.; Zheng, Y. A review on the application, phytochemistry and pharmacology of Polygonatum odoratum, an edible medicinal plant. J. Future Foods 2023, 3, 240–251. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, W.; Zhang, S.; Niu, Y.; Zhang, Y.; Liu, T. Pressure shear assisted extraction of polysaccharide from Auricularia auricula and its biological activities. J. Future Foods 2023, 3, 29–36. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Nayak, A.; Mukherjee, A.; Kumar, S.; Dutta, D. Exploring the potential of jujube seed powder in polysaccharide based functional film: Characterization, properties and application in fruit preservation. Int. J. Biol. Macromol. 2024, 260, 129450. [Google Scholar] [CrossRef]
- Huang, G.; Huang, L.; Geng, C.; Lan, T.; Huang, X.; Xu, S.; Shen, Y.; Bian, H. Green and multifunctional chitosan-based conformal coating as a controlled release platform for fruit preservation. Int. J. Biol. Macromol. 2022, 219, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Zhang, W.; Khan, M.R.; Ahmad, N.; Tian, W. Pectin film fortified with zein nanoparticles and Fe3+-Encapsulated propolis extract for enhanced fruit preservation. Food Hydrocoll. 2024, 157, 110405. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Hu, P.; Wang, W.; Luo, L.; Li, B.; Pan, B.; Zhu, W.; Wang, Y.; Wang, J. Self-assembly technology engineering multi-functional slow-release packaging system with self-starting program for prolonged preservation of perishable products. Food Hydrocoll. 2024, 155, 110230. [Google Scholar] [CrossRef]
- Zou, J.; Zhong, H.; Jiang, C.; Zhu, G.; Lin, X.; Huang, Y. Ginkgo biloba leaf polysaccharide-stabled selenium nanozyme as an efficient glutathione peroxidase mimic for the preservation of bananas and cherry tomatoes. Food Chem. 2024, 459, 140443. [Google Scholar] [CrossRef]
- Qi, C.; Dianpeng, H.; Xiaoyu, Q.; Wen, Z.; Yuan, P.; Shuang, L.; Kang, Q.; Shuyue, R.; Yu, W.; Huanying, Z.; et al. Self-enhanced multifunctional composite membranes based on metal-organic frameworks embedded with thymol nanoparticles for post-harvest preservation of fruits. Food Hydrocoll. 2025, 164, 111208. [Google Scholar]
- Jiang, T.-S.; Li, X.-Y.; Zhu, C.-H.; Yu, T.-R.; Zhao, H.-Q. Bismuth selenide nanosheet layer materials with peroxidase activity for antimicrobial applications. Adv. Agrochem. 2024, 3, 308–315. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, Q.; Li, H.; Li, Y.; Wu, P.; Ai, S. Polydopamine-coated lignin nanoparticles in polysaccharide-based films: A plasticizer, mechanical property enhancer, anti-ultraviolet agent and bioactive agent. Food Hydrocoll. 2024, 147, 109325. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Ding, X.; Lin, H.; Zhou, J.; Lin, Z.; Huang, Y.; Chen, G.; Zhang, Y.; Lv, J.; Chen, J.; Liu, G.; et al. Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects. Nanomaterials 2024, 14, 1244. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Y.; Zhang, Q.; Li, H.; Chen, L. Activity and safety evaluation of natural preservatives. Food Res. Int. 2024, 190, 114548. [Google Scholar] [CrossRef]
- Costa, R.R.; Neto, A.I.; Calgeris, I.; Correia, C.R.; Pinho, A.C.M.; Fonseca, J.; Öner, E.T.; Mano, J.F. Adhesive nanostructured multilayer films using a bacterial exopolysaccharide for biomedical applications. J. Mater. Chem. B 2013, 1, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, J.; Chen, Q.; Xie, F.; Zhang, D.; He, Z.; Cheng, S.; Cai, J. Self-reinforced multifunctional starch nanocomposite film for litchi fruit postharvest preservation. Chem. Eng. J. 2024, 486, 150262. [Google Scholar] [CrossRef]
- Qin, C.C.; Abdalkarim, S.Y.H.; Yang, M.C.; Dong, Y.J.; Yu, H.-Y.; Ge, D. All-naturally structured tough, ultrathin, and washable dual-use composite for fruits preservation with high biosafety evaluation. Int. J. Biol. Macromol. 2023, 247, 125828. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.-G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of biodegradable films using sunflower protein isolates and bacterial nanocellulose as innovative food packaging materials for fresh fruit preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2023, 437, 137710. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Zhang, X.; Tang, W.; Huang, M.; Huang, Q.; Tu, Z. Interaction mechanisms of edible film ingredients and their effects on food quality. Curr. Res. Food Sci. 2024, 8, 100696. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A Critical Overview on the Synthesis and Properties Generated by Quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Wu, K.C.; Freedman, B.R.; Kwon, P.S.; Torre, M.; Kent, D.O.; Bi, W.L.; Mooney, D.J. A tough bioadhesive hydrogel supports sutureless sealing of the dural membrane in porcine and ex vivo human tissue. Sci. Transl. Med. 2024, 16, eadj0616. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Tabbasum, K.; Mukherjee, R.; Garg, P.; Haldar, J. Functionalized chitosan based antibacterial hydrogel sealant for simultaneous infection eradication and tissue closure in ocular injuries. Int. J. Biol. Macromol. 2024, 273, 132838. [Google Scholar] [CrossRef]
- Eshkol-Yogev, I.; Gilboa, E.; Giladi, S.; Zilberman, M. Formulation—Properties effects of novel dual composite hydrogels for use as medical sealants. Eur. Polym. J. 2021, 152, 110470. [Google Scholar] [CrossRef]
- Dilara, D.; Mustafa, T.; Funda, K. Antifungal Activities of Different Essential Oils and Their Electrospun Nanofibers against Aspergillus and Penicillium Species Isolated from Bread. ACS Omega 2022, 7, 37943–37953. [Google Scholar]
- Wang, S.; Li, M.; He, B.; Yong, Y.; Zhu, J. Composite films of sodium alginate and konjac glucomannan incorporated with tea polyphenols for food preservation. Int. J. Biol. Macromol. 2023, 242, 124732. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Chang, Z.; E, Y.Y.; Feng, Y.; Yao, X.; Wang, M.; Ju, Y.; Wang, K.; Jiang, J.; Li, P.; et al. Electrospinning and electrospun polysaccharide-based nanofiber membranes: A review. Int. J. Biol. Macromol. 2024, 263, 130335. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Li, S.; Zhang, Q.; Chen, M.; Yuan, X.; Zhou, M.; Zhang, Z.; Chen, A. Incorporation of cellulose nanocrystals to improve the physicochemical and bioactive properties of pectin-konjac glucomannan composite films containing clove essential oil. Int. J. Biol. Macromol. 2024, 260, 129469. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2020, 256, 117579. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Yin, S.; Duan, M.; Fellner, M.; Wang, Z.; Lv, C.; Zang, J.; Zhao, G.; Zhang, T. pH/glucose dual-responsive protein-based hydrogels with enhanced adhesive and antibacterial properties for diabetic wound healing. Food Innov. Adv. 2024, 3, 332–343. [Google Scholar] [CrossRef]
- Huang, H.-L.; Tsai, I.L.; Lin, C.; Hang, Y.-H.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohydr. Polym. 2022, 299, 120133. [Google Scholar] [CrossRef]
- Puttipan, R.; Khankaew, S. Novel bio-based, time-temperature dependent colorimetric ink and film containing colorants from the red pitaya (Hylocereus costaricensis). Prog. Org. Coat. 2023, 178, 107470. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, M.; Xiang, H.; Zhou, Z.; Yu, D.; Yan, R. A Biomass-Based Colorimetric Sulfur Dioxide Gas Sensor for Smart Packaging. ACS Nano 2023, 17, 6849–6856. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.-H.; Chen, M.-M.; Bing, L. An aerogel-based intelligent active packaging with the dual functions of spoilage detection and freshness preservation. Food Hydrocoll. 2024, 156, 110160. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mehrotra, S.; Sharma, S.K. Potential of sensing interventions in the life cycle assessment of fruits and fruit juices. Trends Food Sci. Technol. 2024, 151, 104614. [Google Scholar] [CrossRef]
- Zheng, Q.; Bai, X.; Chen, T.; Li, F.; Zhu, P.; Li, M.; Tang, Y. Carboxymethyl hemicellulose/sorbitol/gallic acid green composite films for fresh fruit preservation. Ind. Crops Prod. 2024, 218, 119013. [Google Scholar] [CrossRef]
- Guo, X.; Li, L.; Qi, Y.; Su, J.; Ou, X.; Lv, M.; Jin, Y.; Han, X.; Zhang, Y.; Wu, H.; et al. Green-synthesized antibacterial and unidirectional water-permeable polylactic acid/ZnO composite film for enhanced preservation of perishable fruits. Mater. Today Chem. 2024, 40, 102284. [Google Scholar] [CrossRef]
- Xi, W.; Liu, P.; Ling, J.; Xian, D.; Wu, L.; Yuan, Y.; Zhang, J.; Xie, F. Pre-gelatinized high-amylose starch enables easy preparation of flexible and antimicrobial composite films for fresh fruit preservation. Int. J. Biol. Macromol. 2023, 254, 127938. [Google Scholar] [CrossRef]
- Ali, M.H.; Dutta, S.K.; Sultana, M.S.; Habib, A.; Dhar, P.K. Green synthesized CeO2 nanoparticles-based chitosan/PVA composite films: Enhanced antimicrobial activities and mechanical properties for edible berry tomato preservation. Int. J. Biol. Macromol. 2024, 280, 135976. [Google Scholar] [CrossRef]
- Heras, M.; Huang, C.-C.; Chang, C.-W.; Lu, K.-H. Trends in chitosan-based films and coatings: A systematic review of the incorporated biopreservatives, biological properties, and nanotechnology applications in meat preservation. Food Packag. Shelf Life 2024, 42, 101259. [Google Scholar] [CrossRef]
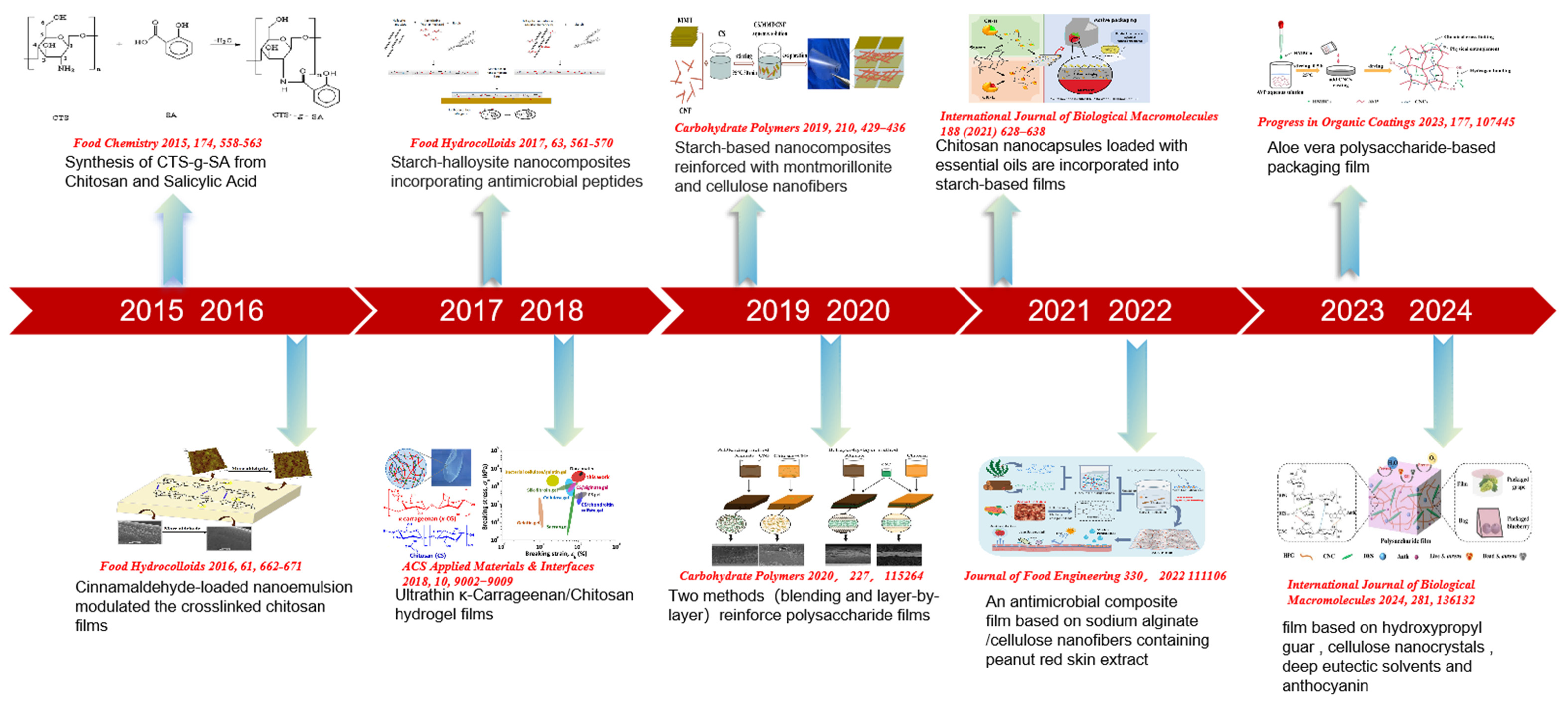
| Type of Films | Nanomaterials/Bioactive Substances | Date | Innovation | Reference |
|---|---|---|---|---|
| Chitosan films | Carvacrol nanoemulsions | 2016 | The addition of nanomaterials improves the homogeneity and surface hydrophobicity of the film | [62] |
| Gelatin films | Guar gum benzoate nanoparticles | 2017 | Superior barrier properties, reinforcing and thermal insulation effects | [63] |
| Chitosan films | TiO2 nanoparticles | 2018 | Ethylene photodegradation to prolong shelf life of fruit | [64] |
| Starch-PVA composite films | Zinc-oxide nanoparticles/phytochemicals | 2019 | The fruit in the film is intelligently monitored by the color of the film changes with the pH | [65] |
| Chitosan films | Nano-TiO2/litchi peel extract | 2020 | The activity of polyphenol oxidase, electrolyte leakage, and malondialdehyde accumulation were inhibited | [66] |
| κ-carrageenan and sodium carboxymethyl starch | Carboxylated cellulose nanocrystals | 2021 | This material resists shrinking effectively and maintains durability even with regular use | [67] |
| Carboxymethyl starch films | MOF-199/curcumin | 2022 | The nanomaterial is decomposed by water, and curcumin is released to prolong shelf life | [68] |
| Chitosan sodium alginate composite films | ZIF-8/quantum dots | 2023 | Efficient sterilization under light conditions and ethylene removal effect | [69] |
| Alginate films | Nanometer-calcium | 2024 | Bodegradable, pH-triggered hydrogel films promoted enhanced polyphenol–calcium binding | [70] |
| Bioactive Substance | Types of Films | Applied Fresh Food Types | Apply Effect | Reference |
|---|---|---|---|---|
| Jujube Seed Powder | Pectin–chitosan composite film | Grapes | No browning effect was observed in the grapes kept in the films during 10 days. | [150] |
| Curcumin | Chitosan-based films | Lychee, Strawberry, Mango and Plum | After 9 days, there was no mold growth on the outside of the samples and the pulp was still shiny and translucent. | [151] |
| Propolis Extract | Pectin-based films | Strawberry | Showed a lower rate of spoilage from day 3 onwards. | [152] |
| Deep Eutectic Solvent Extraction of Tomato Extract | Chitosan film | Strawberry | Within 9 days, strawberry rot was reduced by 55.41%. | [65] |
| Tannins and Cinnamaldehyde | Chitosan film | Mandarin Orange (Citrus Reticulata) | Removes more than 99.9% of bacteria and fungi, significantly extending the shelf life of citrus by approximately 1.9 times. | [153] |
| Ginkgo Biloba Extract | Chitosan film | Banana and Cherry Tomato | The treated banana and cherry tomatoes showed better sensory quality on day 8 and 20, respectively. | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Ding, X.; Huang, Y.; Zhao, Y.; Chen, G.; Xu, X.; Xu, D.; Jiao, B.; Zhao, X.; Liu, G. Recent Advances in Polysaccharide-Based Nanocomposite Films for Fruit Preservation: Construction, Applications, and Challenges. Foods 2025, 14, 1012. https://doi.org/10.3390/foods14061012
Chen X, Ding X, Huang Y, Zhao Y, Chen G, Xu X, Xu D, Jiao B, Zhao X, Liu G. Recent Advances in Polysaccharide-Based Nanocomposite Films for Fruit Preservation: Construction, Applications, and Challenges. Foods. 2025; 14(6):1012. https://doi.org/10.3390/foods14061012
Chicago/Turabian StyleChen, Xin, Xin Ding, Yanyan Huang, Yiming Zhao, Ge Chen, Xiaomin Xu, Donghui Xu, Bining Jiao, Xijuan Zhao, and Guangyang Liu. 2025. "Recent Advances in Polysaccharide-Based Nanocomposite Films for Fruit Preservation: Construction, Applications, and Challenges" Foods 14, no. 6: 1012. https://doi.org/10.3390/foods14061012
APA StyleChen, X., Ding, X., Huang, Y., Zhao, Y., Chen, G., Xu, X., Xu, D., Jiao, B., Zhao, X., & Liu, G. (2025). Recent Advances in Polysaccharide-Based Nanocomposite Films for Fruit Preservation: Construction, Applications, and Challenges. Foods, 14(6), 1012. https://doi.org/10.3390/foods14061012









