Investigation of Self-Assembled Flexible Zein Nanoparticles and Their Sensitivity to Complex Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Flexibility
2.4. Deamidation Degree (DD) Measurement
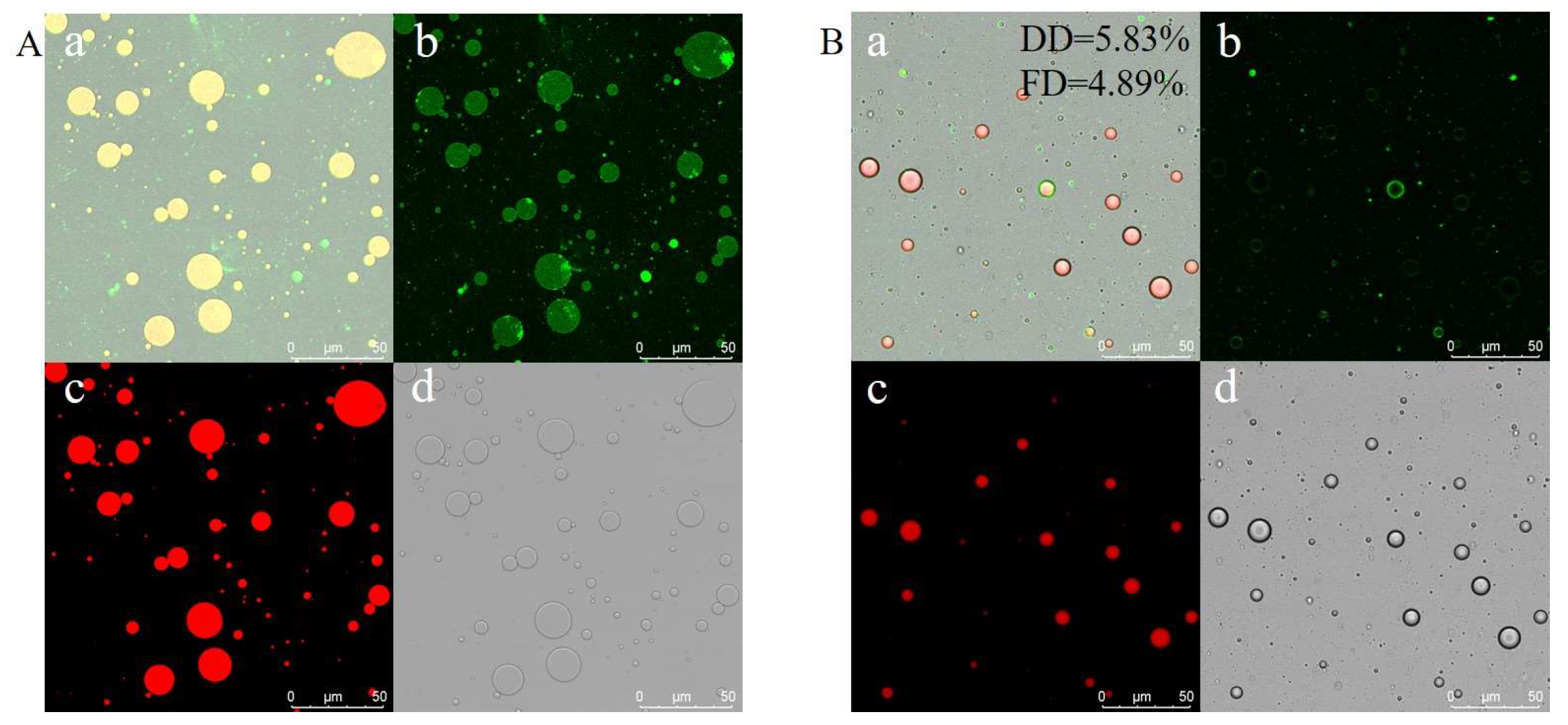
2.5. Confocal Laser Scanning Microscopy (CLSM) Determination
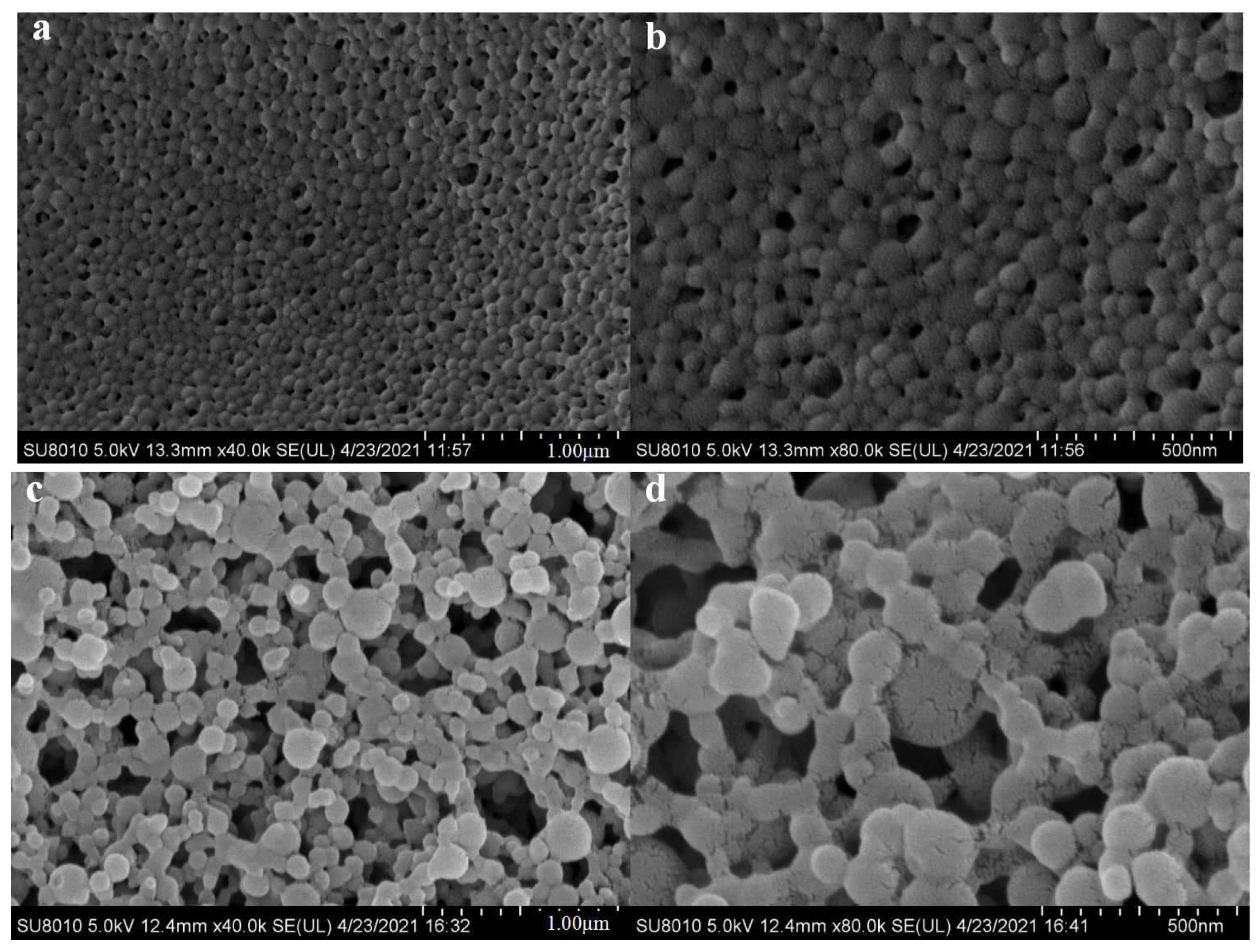
2.6. Scanning Electron Microscope (SEM) Experiment
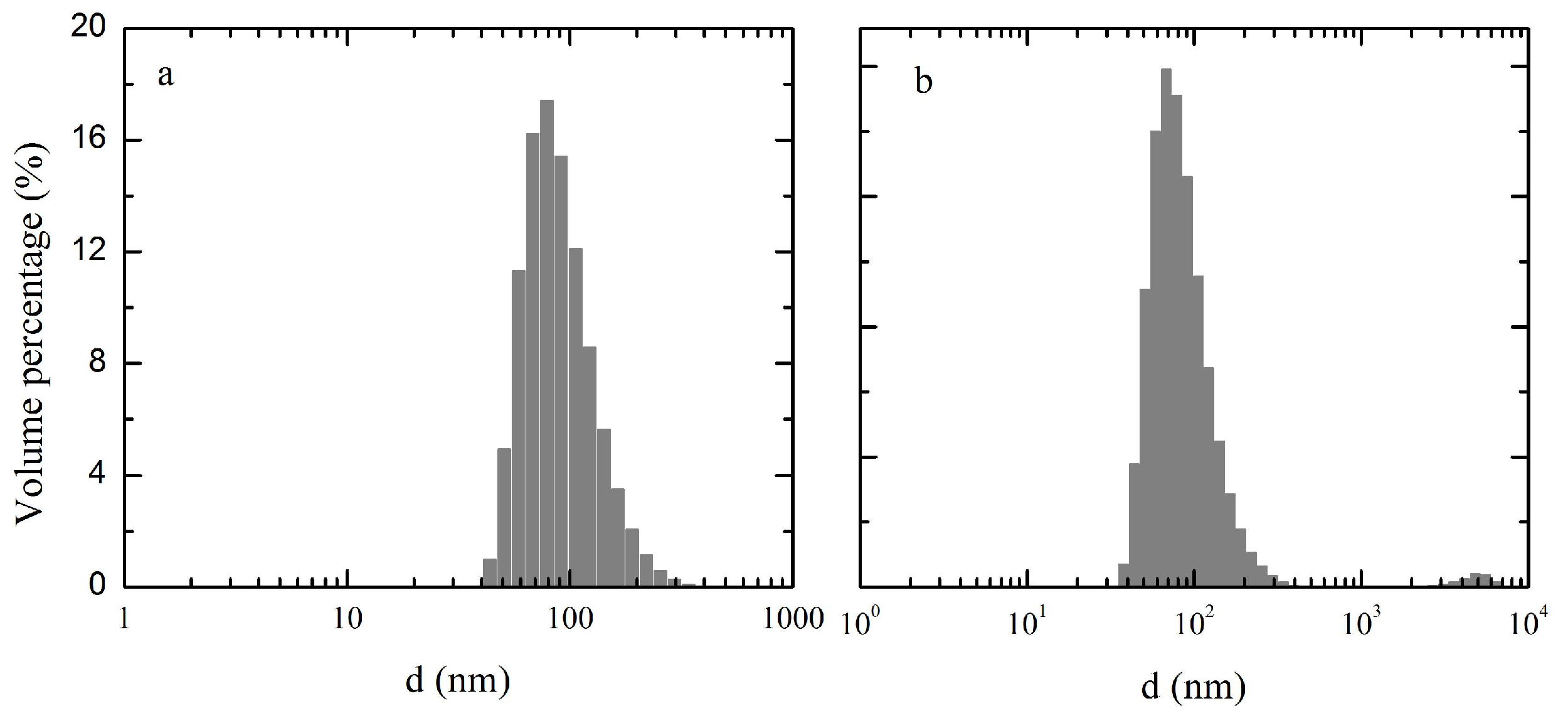
2.7. Particle Size and Zeta Potential Measurement
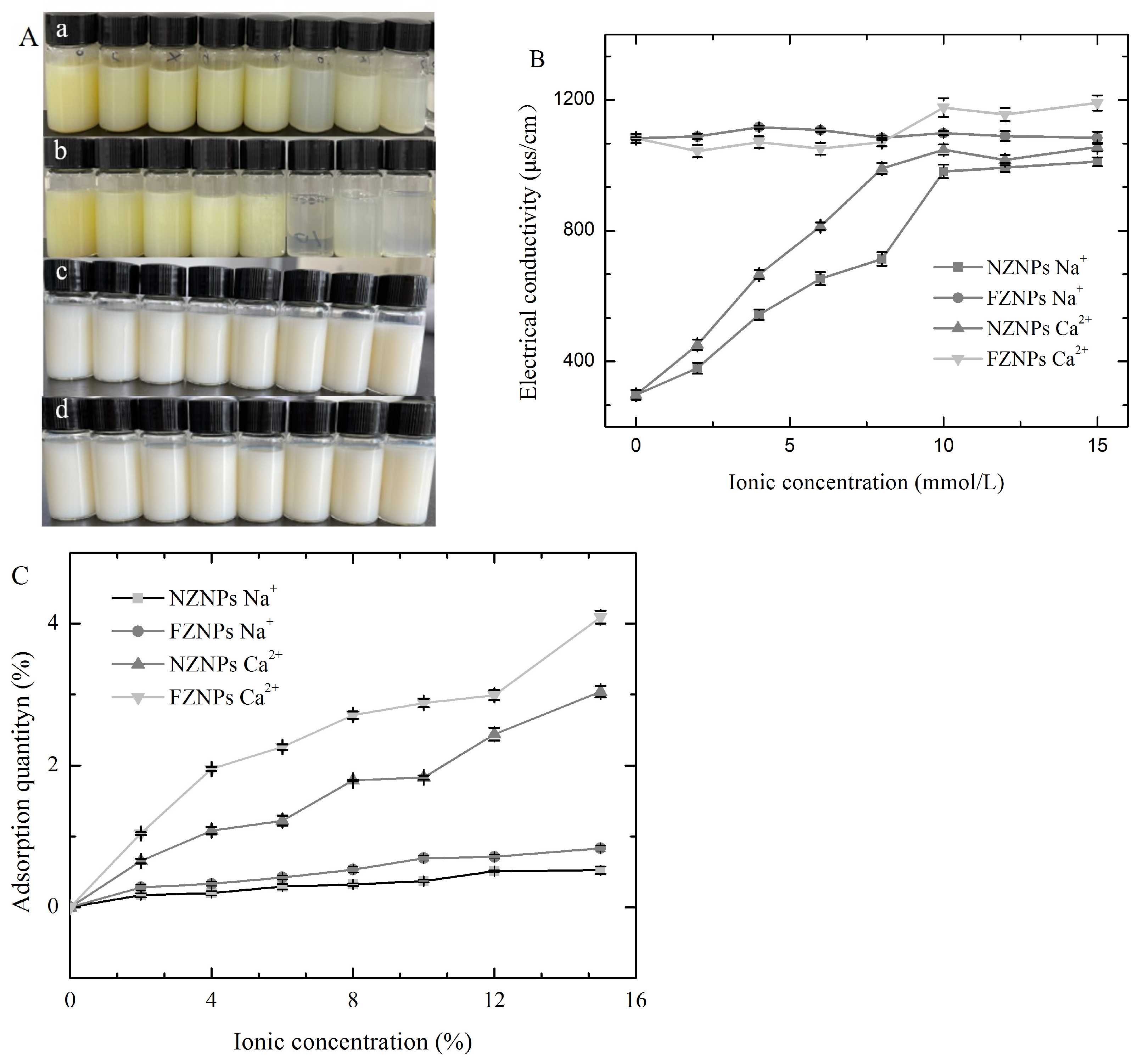
2.8. Electrical Conductivity and Adsorption Quantity Measurement
2.9. X-Ray Photoelectron Spectrum (XPS) Measurement
2.10. X-Ray Diffraction (XRD)
2.11. Circular Dichroism (CD) Analysis
2.12. Amino Acid Analysis
2.13. Statistical Analysis
3. Results
3.1. Flexibility of the Flexible Zein
3.2. SEM Analysis
3.3. Particle Size and Zeta Potential Analysis
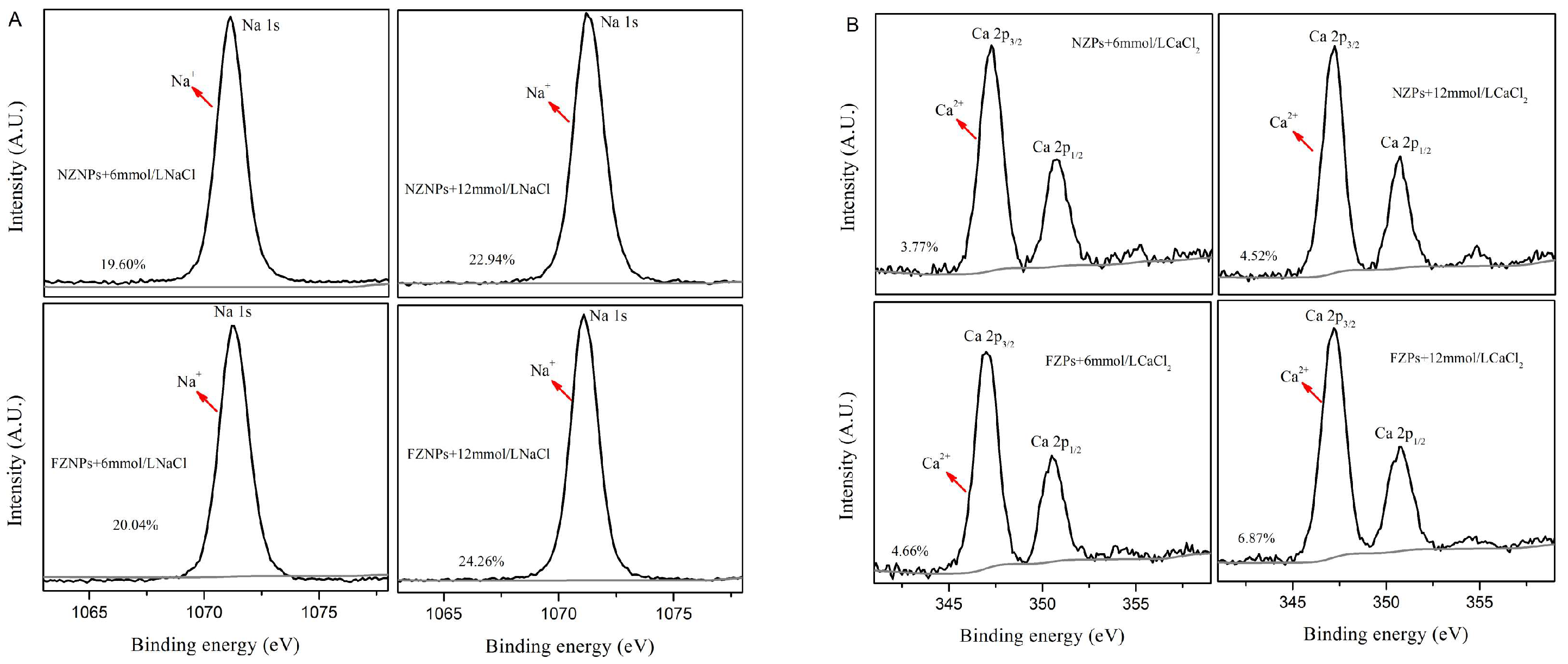
3.4. Sensitivity of the FZNPs to a Complex Environment
3.5. XPS Analysis
3.6. XRD Analysis
3.7. CD Analysis
3.8. Amino Acid Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Afreen, S.; Kong, Q.; Wang, J. Study on self-assembled morphology and structure regulation of α-zein in ethanol-water mixtures. Langmuir 2020, 36, 11975–11984. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yang, Y.; Lei, L.; Lei, X.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Interactions and structural properties of zein/ferulic acid: The effect of calcium chloride. Food Chem. 2022, 373, 131489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wang, J.M.; Guo, J.; Wan, Z.L.; Yang, X.Q. Amphiphilic zein hydrolysate as a delivery vehicle: The role of xanthophylls. LWT Food Sci. Technol. 2017, 79, 463–470. [Google Scholar] [CrossRef]
- Hu, K.; Mcclements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Li, R.; Mao, L.; Liu, F.; Gao, Y. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Jin, M. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocoll. 2009, 23, 2380–2387. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef]
- Dong, S.R.; Han, Q.; Xu, W.; Bian, C. Effect of solvent polarity on the formation of flexible zein nanoparticles and their environmental adaptability. J. Cereal Sci. 2021, 102, 103340. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Cheng, C.J.; Jones, O.G. Stabilizing zein nanoparticle dispersions with ι-carrageenan. Food Hydrocoll. 2017, 69, 28–35. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, W.; Zhao, Q.; Cui, C.; Zhao, M. Emulsifying and surface properties of citric acid deamidated wheat gliadin. J. Cereal Sci. 2013, 58, 68–75. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Lundin, L.; Wooster, T.J. Interfacial properties of deamidated wheat protein in relation to its ability to stabilise oil-in-water emulsions. Food Hydrocoll. 2009, 23, 2158–2167. [Google Scholar] [CrossRef]
- Dong, S.R.; Xu, H.H.; Ma, J.Y.; Gao, Z.W. Enhanced molecular flexibility of α-zein in different polar solvents. J. Cereal Sci. 2020, 96, 103097. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Matsushima, N.; Danno, G.I.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize α-zein proteins studied by small-angle X-ray scattering. Biochim. Biophys. Acta 1997, 1339, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Cabra, V.; Arreguin, R.; Vazquezduhalt, R.; Farres, A. Effect of alkaline deamidation on the structure, surface hydrophobicity, and emulsifying properties of the Z19 α-zein. J. Agric. Food Chem. 2007, 55, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.H.; Yamaguchi, S.; Gu, Y.S.; Mori, T.; Matsumura, Y. Effects of enzymatic deamidation by protein-glutaminase on structure and functional properties of alpha-zein. J. Agric. Food Chem. 2004, 52, 7094–7100. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, M.; Kolinski, A.; Kmiecik, S. CABS-flex: Server for fast simulation of protein structure fluctuations. Nucleic Acids Res. 2013, 41, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T. Interorgan amino acid transport and its regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Komatsu, K.; Fujimoto, K.; Kobayashi, K. Relationship between surface functional properties and flexibility of proteins detected by the protease susceptibility. J. Agric. Food Chem. 1985, 33, 931–934. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Guan, C.; Bing, S.; Yang, X.; Guo, R.; Chen, Y.; Xu, H.; Yu, G. Homogeneous nuclei-induced, secondary nuclei-induced, and spontaneous whey protein concentrate nanofibril formation through different pathways. J. Dairy Sci. 2022, 1055, 600–5609. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, P.; Wei, Y.; Lei, Y.; Guo, X.; Deng, X.; Zhang, J. Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin. Foods 2024, 13, 3111. [Google Scholar] [CrossRef] [PubMed]
- Paraman, I.; Lamsal, B.P. Recovery and characterization of α-zein from corn fermentation coproducts. J. Agric. Food Chem. 2011, 59, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Wu, C.S.C.; Martinez, H.M. Calculation of protein conformation from circular dichroism. Method Enzym. 1986, 130, 208–269. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Wang, Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem. 2011, 124, 210–220. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, S.; Wu, C.; Qi, B.; Teng, F.; Wang, Z.; Li, Y.; Jiang, L. The investigation of protein flexibility of various soybean cultivars in relation to physicochemical and conformational properties. Food Hydrocoll. 2020, 103, 105709. [Google Scholar] [CrossRef]
- Tang, C.H.; Shen, L. Dynamic adsorption and dilatational properties of BSA at oil/water interface: Role of conformational flexibility. Food Hydrocoll. 2015, 43, 388–399. [Google Scholar] [CrossRef]
- Tang, C.H.; Shen, L. Role of conformational flexibility in the emulsifying properties of bovine serum albumin. J. Agric. Food Chem. 2013, 61, 3097–3110. [Google Scholar] [CrossRef]
- Song, T.; Xiong, Z.; Shi, T.; Yuan, L.; Gao, R. Effect of glutamic acid on the preparation and characterization of Pickering emulsions stabilized by zein. Food Chem. 2021, 366, 130598. [Google Scholar] [CrossRef]
- Garavand, F.; Khodaei, D.; Mahmud, N.; Islam, J.; Khan, I.; Jafarzadeh, S.; Tahergorabi, R.; Cacciotti, I. Recent progress in using zein nanoparticles-loaded nanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 3639–3659. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Xu, Y.; Wang, D. Surface coating of zein nanoparticles to improve the application of bioactive compounds: A review. Trends Food Sci. Technol. 2022, 120, 1–15. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Zhang, L.; Dai, L.; Yang, S.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Influence of calcium ions on the stability, microstructure and in vitro digestion fate of zein-propylene glycol alginate-tea saponin ternary complex particles for the delivery of resveratrol. Food Hydrocoll. 2020, 106, 105886. [Google Scholar] [CrossRef]
- Ramosa, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Vicent, A.A. Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food. Crit. Rev. Food Technol. 2015, 57, 1377–1393. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of Peptide-calcium complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef]
- Yadav, D.; Suri, S.; Choudhary, A.A.; Sikender, M.; Kardam, H.; Beg, N.M.; Garg, V.; Ahmad, A.; Asif, M. A Novel Approach: Herbal Remedies and Natural Products in Pharmaceutical Science as Nano Drug Delivery Systems. Int. J. Pharm. Technol. 2011, 3, 3092–3116. [Google Scholar]
- Wang, Q.; Yin, L.; Padua, G.W. Effect of hydrophilic and lipophilic compounds on zein microstructures. Food Biophys. 2008, 3, 174–181. [Google Scholar] [CrossRef]
- Selling, G.W.; Sah, H.; Sessa, D.J. Effect of solvent and temperature on secondary and tertiary structure of zein by circular dichroism. Cereal Chem. 2007, 84, 265–270. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Nanoscale characterization of zein self-assembly. Langmuir 2012, 28, 2429–2435. [Google Scholar] [CrossRef] [PubMed]



| Samples d Range | D < 200nm | 200–1000 nm | >1000 nm | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| NZNPs | 98.00 ± 0.03 a | 2.00 ± 0.05 a | 0 | 0.145 ± 0.02 a | 37.63 ± 0.17 a |
| FZNPs | 96.49 ± 0.06 b | 2.17 ± 0.02 b | 1.34 ± 0.07 a | 0.220 ± 0.01 b | 34.67 ± 0.66 b |
| Samples | Amino Acid Composition (%) | ||
|---|---|---|---|
| Acidic * | Basic ** | Hydrophobic *** | |
| NZNPs | 0 | 15.64 ± 1.24 a | 38.76 ± 0.46 a |
| FZNPs | 7.18 ± 0.70 a | 15.89 ± 0.85 a | 36.61 ± 0.56 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Mu, G. Investigation of Self-Assembled Flexible Zein Nanoparticles and Their Sensitivity to Complex Environments. Foods 2025, 14, 859. https://doi.org/10.3390/foods14050859
Dong S, Mu G. Investigation of Self-Assembled Flexible Zein Nanoparticles and Their Sensitivity to Complex Environments. Foods. 2025; 14(5):859. https://doi.org/10.3390/foods14050859
Chicago/Turabian StyleDong, Shirong, and Guangqing Mu. 2025. "Investigation of Self-Assembled Flexible Zein Nanoparticles and Their Sensitivity to Complex Environments" Foods 14, no. 5: 859. https://doi.org/10.3390/foods14050859
APA StyleDong, S., & Mu, G. (2025). Investigation of Self-Assembled Flexible Zein Nanoparticles and Their Sensitivity to Complex Environments. Foods, 14(5), 859. https://doi.org/10.3390/foods14050859










