Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
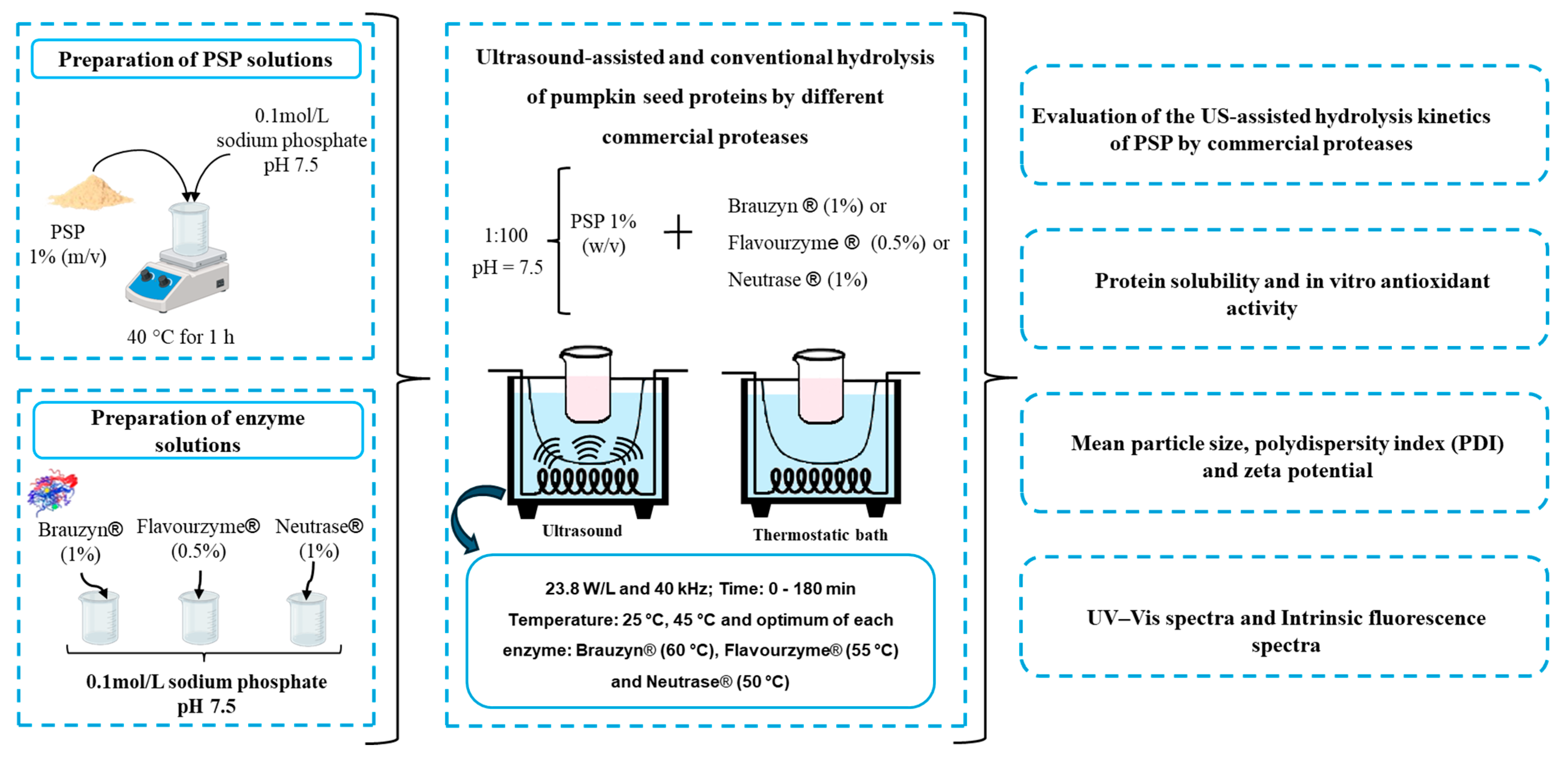
2.1. Enzymes and Pumpkin Seed Protein (PSP)
2.2. PSP Hydrolysis by Commercial Proteases Under US-Assisted Reaction
2.3. Evaluation of PSP Hydrolysis
2.3.1. Degree of Hydrolysis (DH)
2.3.2. Modeling of Hydrolysis Kinetics
2.4. Macrostructure, Solubility, and Antioxidant Activity of the Produced Hydrolysates
2.4.1. Evaluation of Protein Solubility and In Vitro Antioxidant Activity
2.4.2. Mean Particle Size, Polydispersity Index (PDI) and Zeta Potential
2.4.3. UV–Vis Spectra and Intrinsic Fluorescence Spectra
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. PSP Hydrolysis by Commercial Proteases Under US-Assisted Reaction
3.2. Evaluation of Protein Solubility
3.3. Evaluation of In Vitro Antioxidant Activity
3.4. Mean Particle Size, Polydispersity Index (PDI) and Zeta Potential
3.5. UV–Vis Spectra and Intrinsic Fluorescence Spectra
3.6. Comparative Study Between Ultrasound Treatments for Each Enzyme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, B.; Peng, Z.; Chen, B.; Rao, J. Unconventional sources of vegetable proteins: Technological properties. Curr. Opin. Food Sci. 2024, 57, 101150. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Leon, M.J.; Millan-Linares, M.C.; Montserrat-De la Paz, S. Antimicrobial plant-derived peptides obtained by enzymatic hydrolysis and fermentation as components to improve current food systems. Trends Food Sci. Technol. 2023, 135, 32–42. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Nalon, G.A.; Cunha, J.S.; Andressa, I.; Paiva, P.H.C.; Tribst, A.A.L.; Leite Júnior, B.R.C. Impact of ultrasonic pretreatment on pumpkin seed protein: Effect on protease activities, protein structure, hydrolysis kinetics and functional properties. Food Res. Int. 2025, 201, 115538. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Villanueva, A.; Pedroche, J.; Millan, F.; Martin, M.E.; Millan-Linares, M.C. Antioxidant and anti-inflammatory properties of bioavailable protein hydrolysates from lupin-derived agri-waste. Biomolecules 2021, 11, 1458. [Google Scholar] [CrossRef]
- Ahmad, I.; Xiong, Z.; Xiong, H.; Aadil, R.M.; Khalid, N.; Lakhoo, A.B.J.; Din, Z.; Nawaz, A.; Walayat, N.; Khan, R.S. Physicochemical, rheological and antioxidant profiling of yogurt prepared from non-enzymatically and enzymatically hydrolyzed potato powder under refrigeration. Food Sci. Hum. Wellness 2023, 12, 69–78. [Google Scholar] [CrossRef]
- Guimarães, A.D.B.; Magalhães, I.S.; Tribst, A.A.L.; de Oliveira, E.B.; Leite Júnior, B.R.C. Goat milk casein and protease sonication as a strategy to improve the proteolysis and functional properties of hydrolysates. Food Chem. Adv. 2023, 3, 100425. [Google Scholar] [CrossRef]
- Santos, F.R.; Cunha, J.S.; Pacheco, F.C.; Andressa, I.; Martins, C.C.N.; Pacheco, A.F.C.; Leite Júnior, B.R.C. Improvement of the production of pequi almond (Caryocar brasiliense Camb.) protein hydrolysates through ultrasound-assisted enzymolysis: Impact on hydrolysis kinetics, structure and functional properties of hydrolysates. Process Biochem. 2024, 147, 381–390. [Google Scholar] [CrossRef]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT—Food Sci. Technol. 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Pereira, G.Z.; Paiva, P.H.C.; Lelis, C.A.; Tribst, A.A.L.; Leite Júnior, B.R.C. Structural changes induced by ultrasound in proteases and their consequences on the hydrolysis of pumpkin seed proteins and the multifunctional properties of hydrolysates. Food Bioprod. Process. 2024, 144, 13–21. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, L.; Wang, Z.; Cai, J.; Toldrá, F.; Zhang, W. Study on the anti-inflammatory activity of the porcine bone collagen peptides prepared by ultrasound-assisted enzymatic hydrolysis. Ultrason. Sonochem. 2023, 101, 106697. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Gani, A.; Shah, A.; Gani, A.; Punoo, H.A. Ultrasonication assisted enzymatic hydrolysis for generation of pulses protein hydrolysate having antioxidant and ACE-inhibitory activity. Int. J. Biol. Macromol. 2024, 278, 134647. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, W.; Zhu, X.; Zhang, X.; Wei, Y.; Huang, J.; Yang, F.; Yang, F. Ultrasound-assisted enzymatic digestion for efficient extraction of proteins from quinoa. LWT—Food Sci. Technol. 2024, 194, 115784. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Z.; Wang, Z.; Hou, Z.; Liu, B.; Guo, X.; Zhu, B.; Hu, Y. Study on the antioxidant and antiosteoporotic activities of the oyster peptides prepared by ultrasound-assisted enzymatic hydrolysis. Ultrason. Sonochem. 2025, 112, 107211. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, I.S.; Guimarães, A.D.B.; Tribst, A.A.L.; de Oliveira, E.B.; Leite Júnior, B.R.C. Ultrasound-assisted enzymatic hydrolysis of goat milk casein: Effects on hydrolysis kinetics and on the solubility and antioxidant activity of hydrolysates. Food Res. Int. 2022, 157, 111310. [Google Scholar] [CrossRef]
- O’donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Vinatoru, M. Ultrasonically assisted extraction (UAE) of natural products some guidelines for good practice and reporting. Ultrason. Sonochem. 2015, 25, 94–95. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Influence of process conditions on enzymatic hydrolysis kinetics of chicken meat. Food Sci. Technol. 2009, 29, 557–566. [Google Scholar] [CrossRef]
- Cai, H.; Tao, L.; Liu, Y.; Sun, D.; Ma, Q.; Yu, Z.; Jiang, W. Effect of different pretreatments on the hydrolysis efficiency and flavor of squid viscera (Dosidicus gigas). Int. J. Gastron. Food Sci. 2024, 36, 100919. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Chen, W.; Wang, J.; Wang, S. Influence of ultrasound-assisted ionic liquid pretreatments on the functional properties of soy protein hydrolysates. Ultrason. Sonochem. 2021, 73, 105546. [Google Scholar] [CrossRef]
- Quaisie, J.; Ma, H.; Yiting, G.; Tuly, J.A.; Igbokwe, C.J.; Zhang, X.; Ekumah, J.N.; Akpabli-Tsigbe, N.D.K.; Nianzhen, S. Impact of sonication on slurry shear-thinning of protein from sea cucumber (Apostichopus japonicus): Proteolytic reaction kinetics, thermodynamics, and conformational modification. Innov. Food Sci. Emerg. Technol. 2021, 70, 102678. [Google Scholar] [CrossRef]
- Habinshuti, I.; Nsengumuremyi, D.; Muhoza, B.; Ebenezer, F.; Aregbe, A.Y.; Ndisanze, M.A. Recent and novel processing technologies coupled with enzymatic hydrolysis to enhance the production of antioxidant peptides from food proteins: A review. Food Chem. 2023, 423, 136313. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, H.N.; Zhang, M.; Mu, T.H.; Khan, N.M. Production, identification and characterization of antioxidant peptides from potato protein by energy-divergent and gathered ultrasound assisted enzymatic hydrolysis. Food Chem. 2023, 405, 134873. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.N.; Zhang, M.; Mu, T.H. Production and characterization of α-glucosidase inhibitory peptides from sweet potato protein by ultrasound-assisted enzymatic hydrolysis and in vitro gastrointestinal digestion. Eur. Food Res. Technol. 2024, 251, 257–267. [Google Scholar] [CrossRef]
- Cunha, J.S.; Pacheco, F.C.; Martins, C.C.N.; Pacheco, A.F.C.; Tribst, A.A.L.; Leite Júnior, B.R.C. Use of ultrasound to improve the activity of cyclodextrin glycosyltransferase in the producing of β-cyclodextrins: Impact on enzyme activity, stability and insights into changes on enzyme macrostructure. Food Res. Int. 2024, 191, 114662. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Clemente, A.; Pedroche, J.; del Mar Yust, M.; Millán, F. Obtention and uses of protein hydrolysates. Grasas Aceites 2001, 52, 132–136. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Qian, J.; Chen, D.; Zhang, Y.; Gao, X.; Xu, L.; Guan, G.; Wang, F. Ultrasound-assisted enzymatic protein hydrolysis in food processing: Mechanism and parameters. Foods 2023, 12, 4027. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Sarabandi, K.; Akbarbaglu, Z. Insights into enzymatic hydrolysis: Exploring effects on antioxidant and functional properties of bioactive peptides from chlorella proteins. J. Agric. Food Res. 2024, 16, 101129. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Popović, L.; Petrović, L.; Milinković, J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016, 60, 271–278. [Google Scholar] [CrossRef]
- Li, H.; Ren, C.; Hou, D.; Qiu, X.; Zhang, J.; Chen, X.; Zou, Y.; Sun, G.; Li, K.; Li, H.; et al. Effect of combined treatments of hydrolysis and succinylation on the solubility and emulsion stability of rennet casein and micellar casein. Food Bioprod. Process. 2024, 148, 108–117. [Google Scholar] [CrossRef]
- Hayta, M.; Benli, B.; İşçimen, E.M.; Kaya, A. Optimization of antihypertensive and antioxidant hydrolysate extraction from rice bran proteins using ultrasound assisted enzymatic hydrolysis. J. Food Meas. Charact. 2020, 14, 2578–2589. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Tavano, O.; Murcia, Á.B.; Torrestina-Sánchez, B.; Fernandez-Lafuente, R. Peptides with biological and technofunctional properties produced by bromelain hydrolysis of proteins from different sources: A review. Int. J. Biol. Macromol. 2023, 253, 127244. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, H.; Ahmadi Gavlighi, H.; Nikoo, M.; Udenigwe, C.C.; Khodaiyan, F. Relation of amino acid composition, hydrophobicity, and molecular weight with antidiabetic, antihypertensive, and antioxidant properties of mixtures of corn gluten and soy protein hydrolysates. Food Sci. Nutr. 2023, 11, 1257–1271. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT—Food Sci. Technol. 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wu, T.; You, H.; Liu, H.; Liu, X.; Wang, L.; Ding, L. Preparation of quinoa protein with ultrasound pretreatment and its effects on the physicochemical properties, structural and digestion characterizations. Int. J. Biol. Macromol. 2023, 238, 124202. [Google Scholar] [CrossRef]
- Huo, J.; Cui, Z.; Zhang, R.; Ouyang, H.; Liu, X.; Wang, P.; Yu, X.; Xie, T.; Gao, S.; Li, S. Study on the effect and mechanism of ultrasonic-assisted enzymolysis on antioxidant peptide activity in walnuts. Ultrason. Sonochem. 2025, 112, 107159. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Ozkan, G.; Tataroglu, P.; Gulec, S.; Capanoglu, E. Modification of pea protein isolates by high-intensity ultrasonication: Functional, structural and nutritional properties. Food Chem. Adv. 2024, 5, 100793. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, D.; Yin, C.; Li, Z.; Hao, J.; Li, Y.; Zhang, S. The biological activity, functionality, and emulsion stability of soybean meal hydrolysate–proanthocyanidin conjugates. Food Chem. 2024, 432, 137159. [Google Scholar] [CrossRef]
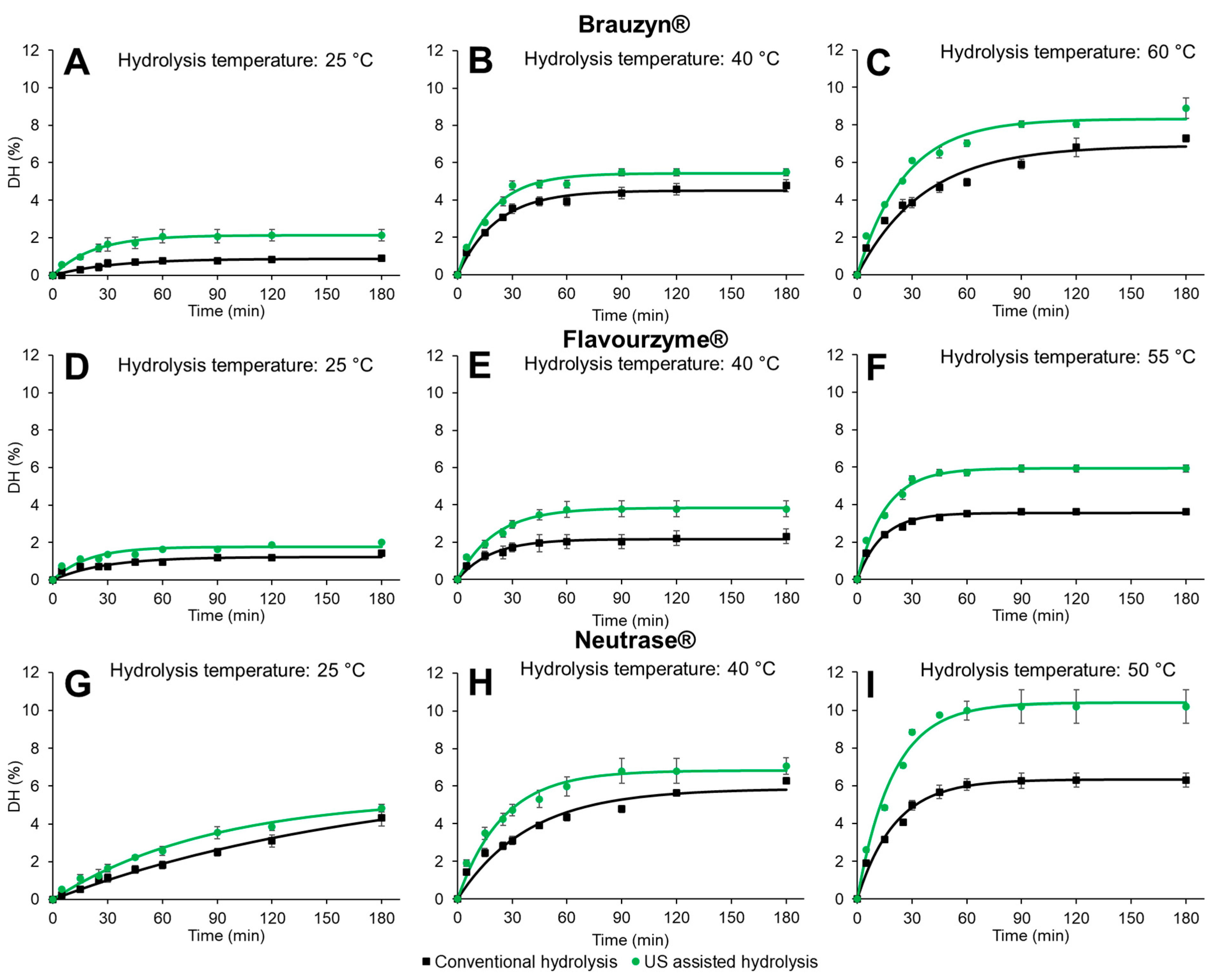
| Enzyme | Hydrolysis | T (°C) | k (min−1) | DH∞ (%) | R2 | DH45 min (%) | DH180 min (%) |
|---|---|---|---|---|---|---|---|
| Brauzyn® | Conventional hydrolysis | 25 | 0.031 ± 0.012 cd | 0.9 ± 0.0 f | 0.966 | 0.7 ± 0.1 e | 0.9 ± 0.1 e |
| 40 | 0.047 ± 0.003 ab | 4.5 ± 0.3 d | 0.994 | 3.9 ± 0.2 c | 4.8 ± 0.3 c | ||
| 60 | 0.027 ± 0.001 d | 6.9 ± 0.3 b | 0.982 | 4.7 ± 0.3 b | 7.3 ± 0.2 b | ||
| US assisted hydrolysis | 25 | 0.045 ± 0.003 abc | 2.1 ± 0.3 e | 0.991 | 1.7 ± 0.3 d | 2.1 ± 0.3 d | |
| 40 | 0.055 ± 0.003 a | 5.4 ± 0.2 c | 0.993 | 4.9 ± 0.2 b | 5.5 ± 0.2 c | ||
| 60 | 0.039 ± 0.003 bcd | 8.3 ± 0.4 a | 0.991 | 6.5 ± 0.3 a | 8.9 ± 0.5 a | ||
| Flavourzyme® | Conventional hydrolysis | 25 | 0.036 ± 0.000 d | 1.2 ± 0.0 d | 0.937 | 0.9 ± 0.0 d | 1.4 ± 0.0 d |
| 40 | 0.054 ± 0.008 bc | 2.2 ± 0.4 c | 0.984 | 2.0 ± 0.5 c | 2.3 ± 0.4 c | ||
| 55 | 0.074 ± 0.006 a | 3.6 ± 0.1 b | 0.993 | 3.3 ± 0.1 b | 3.6 ± 0.1 b | ||
| US assisted hydrolysis | 25 | 0.053 ± 0.000 c | 1.8 ± 0.0 cd | 0.949 | 1.4 ± 0.0 cd | 2.0 ± 0.0 cd | |
| 40 | 0.049 ± 0.005 c | 3.8 ± 0.4 b | 0.992 | 3.5 ± 0.3 b | 3.8 ± 0.4 b | ||
| 55 | 0.066 ± 0.002 ab | 5.9 ± 0.2 a | 0.994 | 5.7 ± 0.2 a | 5.9 ± 0.2 a | ||
| Neutrase® | Conventional hydrolysis | 25 | 0.006 ± 0.001 d | 6.7 ± 0.2 bc | 0.996 | 1.6 ± 0.2 d | 4.3 ± 0.4 c |
| 40 | 0.026 ± 0.002 c | 5.9 ± 0.1 bc | 0.977 | 3.9 ± 0.2 c | 6.3 ± 0.2 b | ||
| 50 | 0.048 ± 0.003 ab | 6.3 ± 0.4 bc | 0.994 | 5.7 ± 0.4 b | 6.3 ± 0.4b | ||
| US assisted hydrolysis | 25 | 0.012 ± 0.002 d | 5.4 ± 0.4 c | 0.994 | 2.2 ± 0.1 d | 4.8 ± 0.2 c | |
| 40 | 0.041 ± 0.002 b | 6.8 ± 0.6 b | 0.990 | 5.3 ± 0.4 b | 7.1 ± 0.4 b | ||
| 50 | 0.051 ± 0.006 a | 10.4 ± 0.8 a | 0.993 | 9.7 ± 0.1 a | 10.2 ± 0.9 a |
| Enzyme | Hydrolysis | Hydrolysis Time (min) | Protein Solubility (%) | ||||
|---|---|---|---|---|---|---|---|
| pH 2.0 | pH 4.0 | pH 6.0 | pH 8.0 | pH 10.0 | |||
| Brauzyn® | Conventional hydrolysis | 45 | 69 ± 3 cd | 23 ± 3 e | 17 ± 3 e | 56 ± 3 de | 84 ± 5 a |
| 180 | 76 ± 3 b | 37 ± 3 cd | 32 ± 3 cd | 68 ± 4 bc | 88 ± 3 a | ||
| US assisted hydrolysis | 45 | 77 ± 2 b | 37 ± 2 cd | 32 ± 4 cd | 70 ± 2 bc | 87 ± 3 a | |
| 180 | 81 ± 2 a | 46 ± 3 b | 41 ± 2 b | 77 ± 3 a | 88 ± 3 a | ||
| Flavourzyme® | Conventional hydrolysis | 45 | 63 ± 4 de | 18 ± 2 ef | 15 ± 3 e | 52 ± 3 e | 82 ± 4 a |
| 180 | 68 ± 3 cd | 21 ± 2 e | 15 ± 4 e | 55 ± 3 e | 83 ± 4 a | ||
| US assisted hydrolysis | 45 | 71 ± 5 bcd | 32 ± 2 d | 26 ± 3 d | 63 ± 4 cd | 86 ± 3 a | |
| 180 | 74 ± 4 bcd | 34 ± 2 d | 30 ± 4 cd | 67 ± 2 bc | 88 ± 3 a | ||
| Neutrase® | Conventional hydrolysis | 45 | 76 ± 3 b | 36 ± 3 d | 30 ± 4 cd | 66 ± 3 bc | 85 ± 4 a |
| 180 | 79 ± 4 ab | 42 ± 2 bc | 36 ± 2 c | 72 ± 3 ab | 89 ± 3 a | ||
| US assisted hydrolysis | 45 | 83 ± 2 a | 52 ± 2 a | 48 ± 3 a | 77 ± 3 a | 88 ± 4 a | |
| 180 | 84 ± 2 a | 54 ± 2 a | 50 ± 2 a | 79 ± 4 a | 90 ± 4 a | ||
| Native PSP—non-hydrolyzed | 60 ± 3 e | 14 ± 3 f | 12 ± 3 e | 50 ± 4 e | 81 ± 6 a | ||
| Enzyme | Hydrolysis | Hydrolysis Time (min) | DPPH Inhibition (%) | ABTS Inhibition (%) |
|---|---|---|---|---|
| Brauzyn® | Conventional hydrolysis | 45 | 34 ± 2 c | 56 ± 2 c |
| 180 | 37 ± 3 c | 58 ± 2 c | ||
| US assisted hydrolysis | 45 | 43 ± 2 ab | 63 ± 3 bc | |
| 180 | 46 ± 2 a | 74 ± 4 a | ||
| Flavourzyme® | Conventional hydrolysis | 45 | 15 ± 2 f | 46 ± 2 de |
| 180 | 21 ± 1 e | 47 ± 3 de | ||
| US assisted hydrolysis | 45 | 28 ± 3 d | 58 ± 4 c | |
| 180 | 32 ± 3 cd | 67 ± 4 ab | ||
| Neutrase® | Conventional hydrolysis | 45 | 26 ± 3 d | 43 ± 1 e |
| 180 | 28 ± 4 d | 42 ± 3 e | ||
| US assisted hydrolysis | 45 | 38 ± 3 bc | 50 ± 3 de | |
| 180 | 40 ± 3 bc | 54 ± 2 c | ||
| Native PSP—non-hydrolyzed | 6 ± 1 g | 16 ± 1 f | ||
| Enzyme | Hydrolysis | Hydrolysis Time (min) | Mean Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| Brauzyn® | Conventional hydrolysis | 45 | 566 ± 27 a | 0.911 ± 0.036 a | −32.7 ± 1.3 a |
| 180 | 302 ± 18 de | 0.643 ± 0.021 de | −49.8 ± 1.9 cd | ||
| US assisted hydrolysis | 45 | 419 ± 54 c | 0.792 ± 0.058 bc | −47.3 ± 1.0 bc | |
| 180 | 223 ± 39 f | 0.512 ± 0.020 f | −60.4 ± 1.7 gh | ||
| Flavourzyme® | Conventional hydrolysis | 45 | 377 ± 12 c | 0.736 ± 0.023 bcd | −52.1 ± 2.0 de |
| 180 | 168 ± 4 g | 0.592 ± 0.060 ef | −58.8 ± 0.5 fgh | ||
| US assisted hydrolysis | 45 | 211 ± 20 f | 0.618 ± 0.051 ef | −57.6 ± 0.5 fg | |
| 180 | 124 ± 6 h | 0.574 ± 0.029 ef | −62.4 ± 1.5 h | ||
| Neutrase® | Conventional hydrolysis | 45 | 513 ± 13 b | 0.838 ± 0.040 ab | −44.8 ± 0.9 b |
| 180 | 255 ± 30 ef | 0.607 ± 0.020 ef | −55.4 ± 1.7 ef | ||
| US assisted hydrolysis | 45 | 324 ± 24 d | 0.683 ± 0.018 cde | −52.5 ± 1.1 de | |
| 180 | 224 ± 20 f | 0.600 ± 0.048 ef | −61.7 ± 1.6 gh |
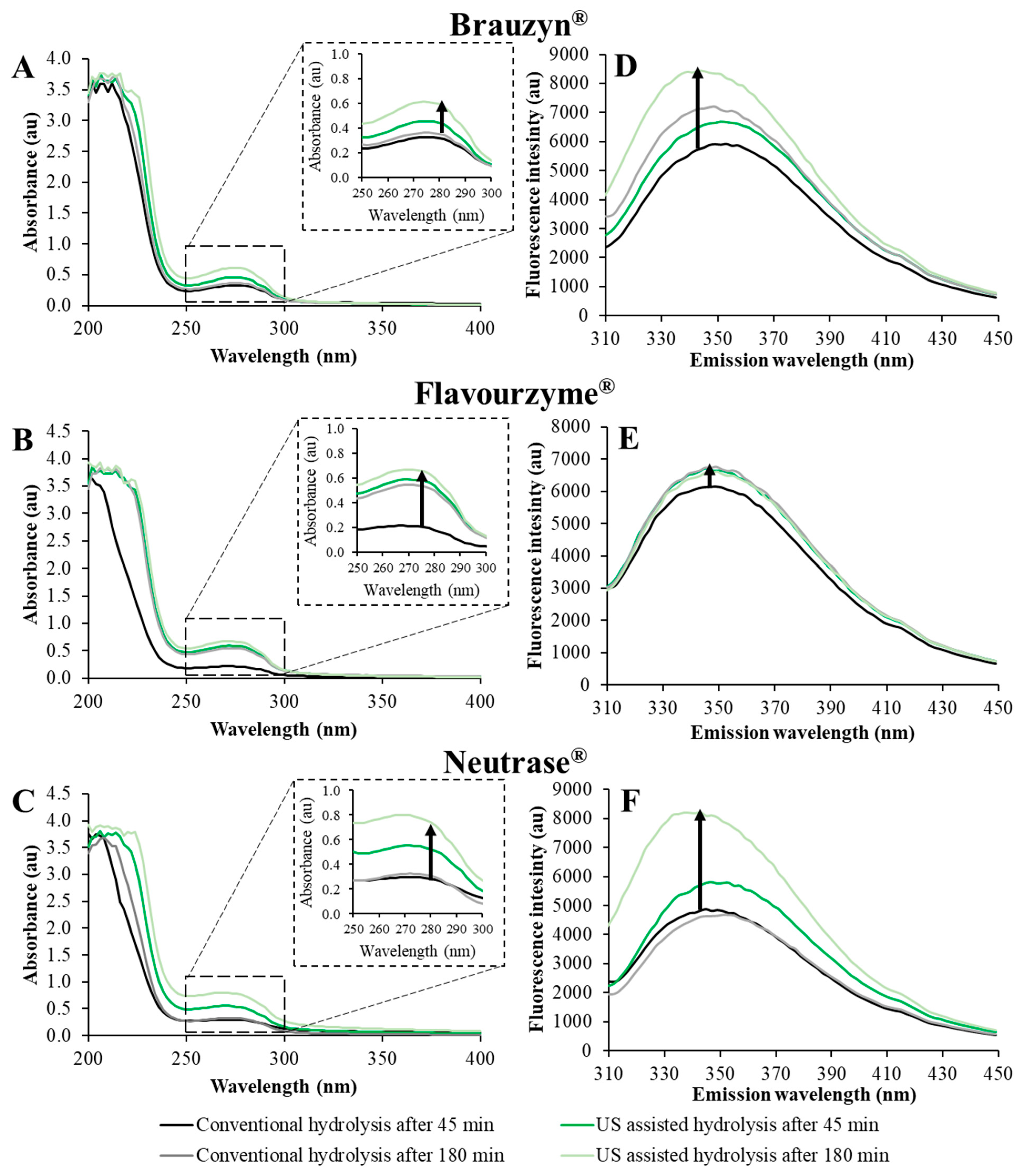
| Enzyme | Hydrolysis | Hydrolysis Time (min) | UV Intensity at 280 nm (au) | Maximum Fluorescence Intesinty (au) |
|---|---|---|---|---|
| Brauzyn® | Conventional hydrolysis | 45 | 0.321 ± 0.043 fg | 5919 ± 73 de |
| 180 | 0.354 ± 0.028 f | 7048 ± 171 b | ||
| US assisted hydrolysis | 45 | 0.444 ± 0.034 e | 6698 ± 98 c | |
| 180 | 0.595 ± 0.032 bc | 8451 ± 201 a | ||
| Flavourzyme® | Conventional hydrolysis | 45 | 0.205 ± 0.034 h | 6156 ± 52 d |
| 180 | 0.523 ± 0.042 cd | 6773 ± 89 bc | ||
| US assisted hydrolysis | 45 | 0.564 ± 0.029 cd | 6725 ± 63 c | |
| 180 | 0.638 ± 0.020 b | 6612 ± 92 c | ||
| Neutrase® | Conventional hydrolysis | 45 | 0.285 ± 0.032 g | 4888 ± 95 f |
| 180 | 0.310 ± 0.052 fg | 4674 ± 121 f | ||
| US assisted hydrolysis | 45 | 0.511 ± 0.028 d | 5811 ± 94 e | |
| 180 | 0.742 ± 0.032 a | 8206 ± 172 a |
| Enzyme | T (°C) | US Treatment | Gains (%): DH∞ | Hydrolysis Time (min) | Gains (%): Soluble Protein | Gains (%): Antioxidant Activity | ||
|---|---|---|---|---|---|---|---|---|
| pH 4 | pH 6 | DPPH | ABTS | |||||
| Brauzyn® | 60 | Enzyme | 8.6 | 45 | 61.3 | 83.8 | 26.6 | 4.3 |
| 180 | 15.2 | 14.9 | 25.9 | 28.8 | ||||
| Substrate | 32.4 | 45 | 57.3 | 78.6 | 29.6 | 3.9 | ||
| 180 | 20.6 | 24.7 | 32.2 | 15.5 | ||||
| US-assisted | 20.3 | 45 | 60.9 | 88.2 | 26.5 | 12.5 | ||
| 180 | 24.3 | 28.1 | 24.3 | 27.6 | ||||
| Flavourzyme® | 55 | Enzyme | 58.5 | 45 | 83.3 | 86.9 | 40.7 | 29.5 |
| 180 | 66.7 | 94.8 | 38.2 | 65.7 | ||||
| Substrate | 48.7 | 45 | 44.4 | 72.4 | 71.3 | 29.5 | ||
| 180 | 52.4 | 87.0 | 32.4 | 42.7 | ||||
| US-assisted | 63.9 | 45 | 77.8 | 73.3 | 86.7 | 26.1 | ||
| 180 | 61.9 | 100.0 | 52.4 | 42.6 | ||||
| Neutrase® | 50 | Enzyme | 42.2 | 45 | 39.8 | 53.8 | 39.8 | 28.6 |
| 180 | 26.7 | 38.4 | 52.5 | 39.4 | ||||
| Substrate | 89.1 | 45 | 33.9 | 52.5 | 56.8 | 15.0 | ||
| 180 | 25.1 | 36.4 | 39.4 | 19.0 | ||||
| US-assisted | 65.1 | 45 | 44.4 | 60.0 | 46.2 | 16.3 | ||
| 180 | 28.6 | 38.9 | 42.9 | 28.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, A.F.C.; Pacheco, F.C.; Cunha, J.S.; Nalon, G.A.; Gusmão, J.V.F.; Santos, F.R.d.; Andressa, I.; Paiva, P.H.C.; Tribst, A.A.L.; Leite Junior, B.R.d.C. Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis. Foods 2025, 14, 782. https://doi.org/10.3390/foods14050782
Pacheco AFC, Pacheco FC, Cunha JS, Nalon GA, Gusmão JVF, Santos FRd, Andressa I, Paiva PHC, Tribst AAL, Leite Junior BRdC. Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis. Foods. 2025; 14(5):782. https://doi.org/10.3390/foods14050782
Chicago/Turabian StylePacheco, Ana Flávia Coelho, Flaviana Coelho Pacheco, Jeferson Silva Cunha, Gabriela Aparecida Nalon, Jhonathan Valente Ferreira Gusmão, Fábio Ribeiro dos Santos, Irene Andressa, Paulo Henrique Costa Paiva, Alline Artigiani Lima Tribst, and Bruno Ricardo de Castro Leite Junior. 2025. "Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis" Foods 14, no. 5: 782. https://doi.org/10.3390/foods14050782
APA StylePacheco, A. F. C., Pacheco, F. C., Cunha, J. S., Nalon, G. A., Gusmão, J. V. F., Santos, F. R. d., Andressa, I., Paiva, P. H. C., Tribst, A. A. L., & Leite Junior, B. R. d. C. (2025). Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis. Foods, 14(5), 782. https://doi.org/10.3390/foods14050782








