A Broad-Spectrum Monoclonal Antibody-Based Heterologous ic-ELISA for the Detection of Multiple Pyrethroids in Water, Milk, Celery, and Leek
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Cells
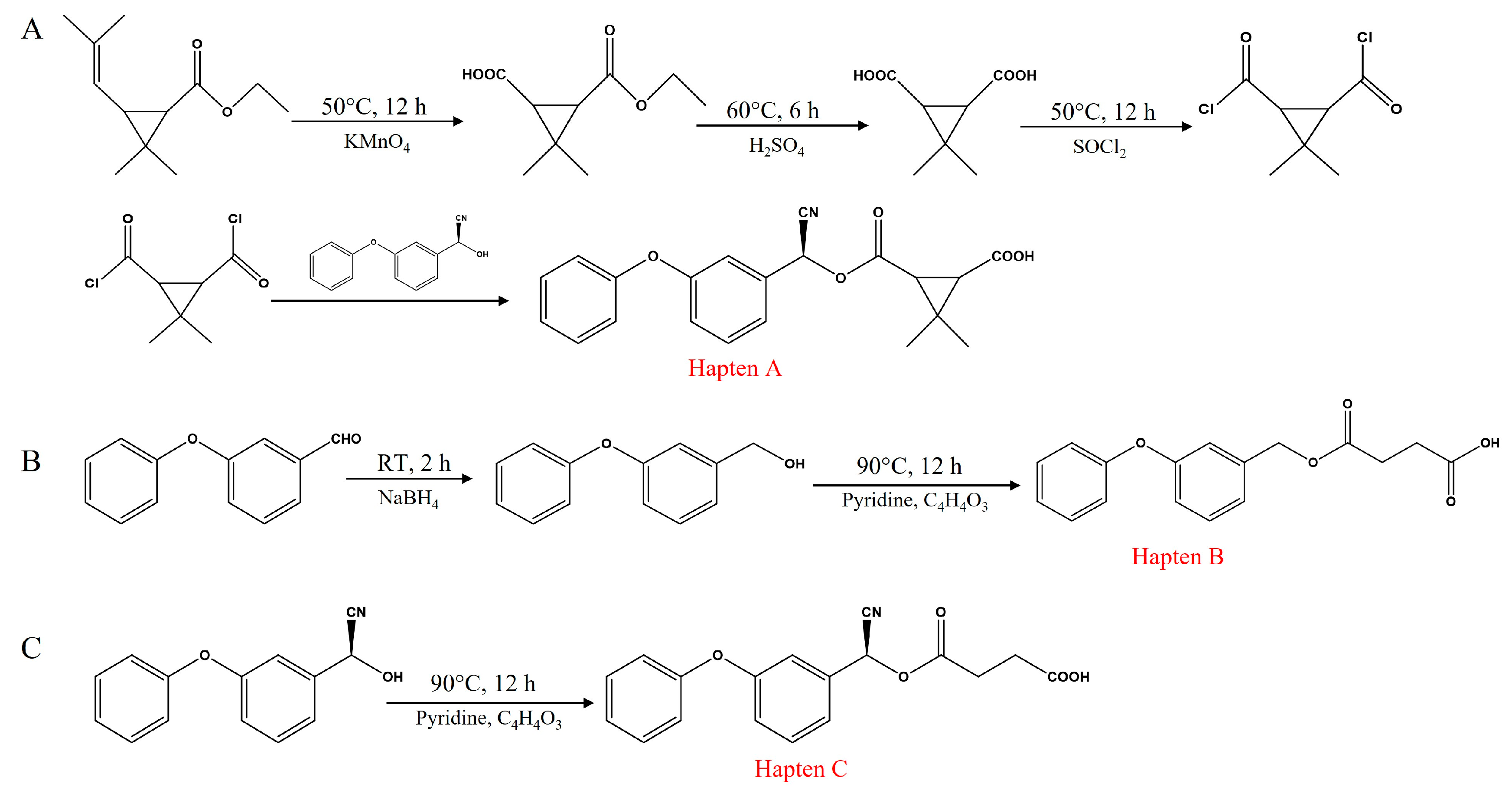
2.3. Synthesis of Haptens
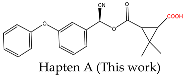
2.3.1. Synthesis of Hapten A
2.3.2. Synthesis of Hapten B
2.3.3. Synthesis of Hapten C
2.4. Synthesis of Antigens
2.4.1. Synthesis of A-DCC-KLH, A-DCC-BSA, and A-DCC-OVA
2.4.2. Synthesis of B-DCC-BSA
2.4.3. Synthesis of C-DCC-OVA
2.5. Preparation of mAb
2.6. ic-ELISA Procedure
2.7. Standard Curve and Cross-Reactivity (CR) for ic-ELISA
2.8. Sample Preparation
2.9. Validation of the ic-ELISA
3. Results and Discussion
3.1. Design and Characterization of Haptens
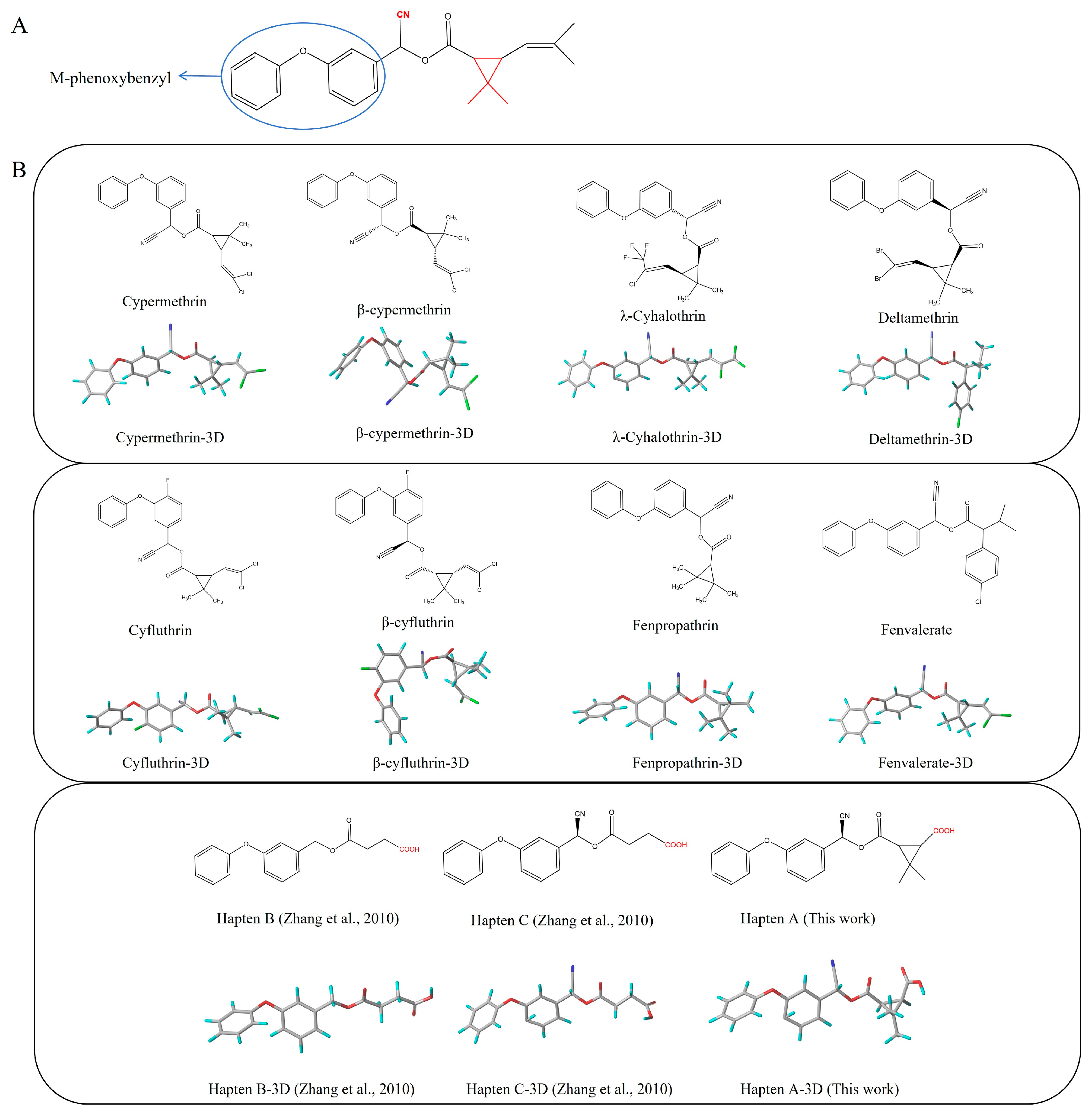
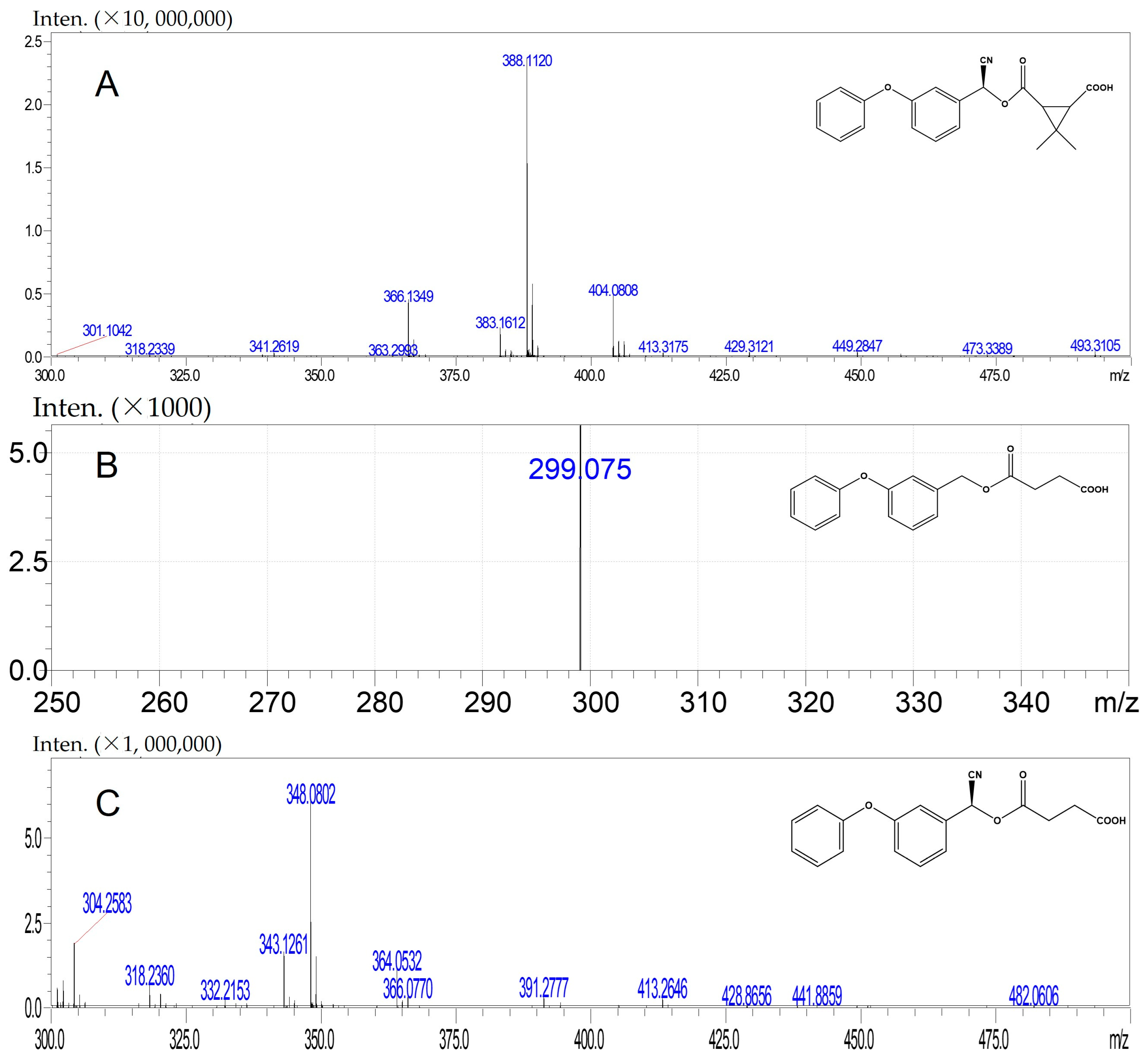
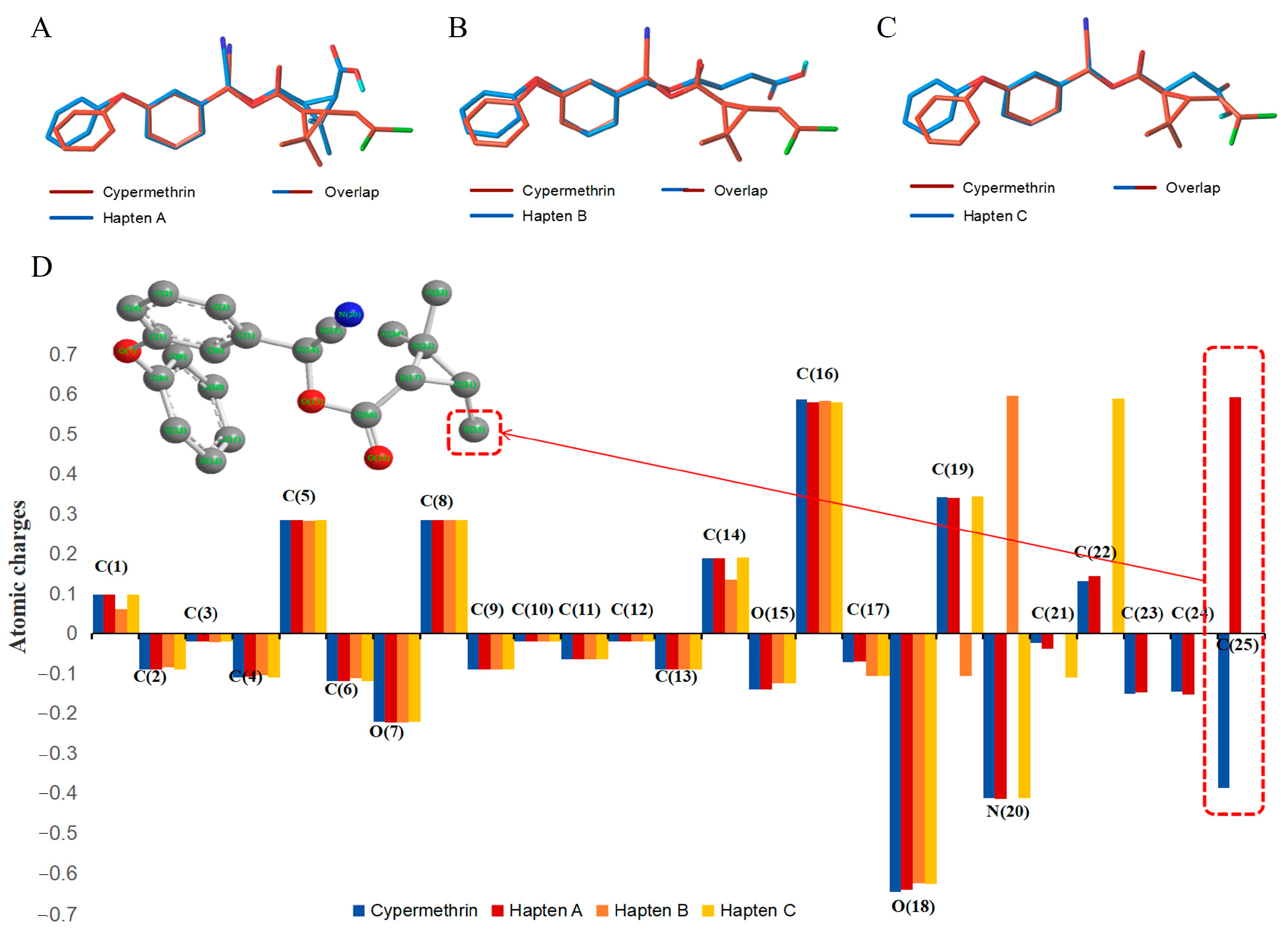
3.2. Characterization of Antigens
3.3. Identification of Antiserum and mAb
3.4. Development and Optimization of ic-ELISA
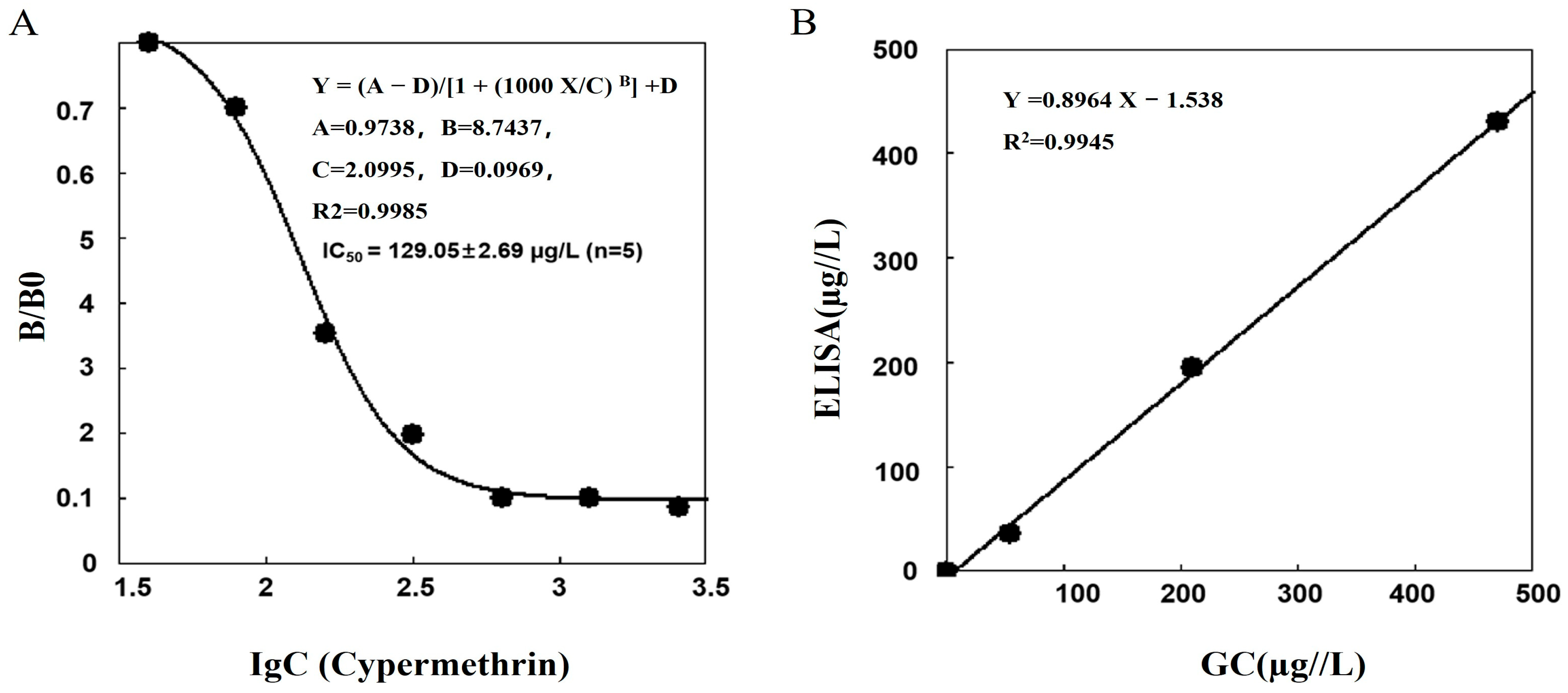
3.5. The Standard Curve and the CR for the ic-ELISA
3.6. Validation of the ic-ELISA Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. B-Phys. 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Renée Prater, M.; Gogal, R.M.; Blaylock, B.L.; Longstreth, J.; Holladay, S.D. Single-dose topical exposure to the pyrethroid insecticide, permethrin in C57BL/6N mice: Effects on thymus and spleen. Food Chem. Toxicol. 2002, 40, 1863–1873. [Google Scholar] [CrossRef]
- Deng, F.; Sun, J.; Dou, R.; Yu, X.; Wei, Z.; Yang, C.; Zeng, X.; Zhu, X. Contamination of pyrethroids in agricultural soils from the Yangtze River Delta, China. Sci. Total Environ. 2020, 731, 139181. [Google Scholar] [CrossRef]
- María, A.M.; Irma, A.; José, L.R.; Marta, M.; David, R.M.; Castellano, V.; Bernardo, L.T.; María, R.M.L.; Arturo, A. Pyrethroid insecticide lambda-cyhalothrin induces hepatic cytochrome P450 enzymes, oxidative stress and apoptosis in rats. Sci. Total Environ. 2018, 631–632, 1371–1382. [Google Scholar] [CrossRef]
- Nagarjuna, A.; Doss, P. Acute oral toxicity and histopathological studies of cypermethrin in rats. Indian J. Anim. Res. 2009, 43, 235–240. [Google Scholar]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Su, X.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and health effects–An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, X.; Huang, J.; Chen, C.; Chen, Z.; Liu, L.; Zhang, G.; Yang, J.; Zhang, Z.; Lin, Z.; et al. Fenpropathrin, a Widely Used Pesticide, Causes Dopaminergic Degeneration. Mol. Neurobiol. 2015, 53, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pan, W.; Zhao, S.; Zhao, Y.; Zhu, Y.; Liu, J.; Liu, W. Relationships of Pyrethroid Exposure with Gonadotropin Levels and Pubertal Development in Chinese Boys. Environ. Sci. Technol. 2017, 51, 6379–6386. [Google Scholar] [CrossRef]
- Xu, H.; Mao, Y.; Xu, B. Association between pyrethroid pesticide exposure and hearing loss in adolescents. Environ. Res. 2020, 187, 109640. [Google Scholar] [CrossRef]
- Shafer, T.J.; Meyer, D.A.; Crofton, K.M. Developmental Neurotoxicity of Pyrethroid Insecticides: Critical Review and Future Research Needs. Environ. Health Perspect. 2005, 113, 123–136. [Google Scholar] [CrossRef]
- Gatto, M.P.; Fioretti, M.; Fabrizi, G.; Gherardi, M.; Strafella, E.; Santarelli, L. Effects of potential neurotoxic pesticides on hearing loss: A review. Neuro Toxicol. 2014, 42, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Joode, B.W.; Mora, A.M.; Lindh, C.; Bonilla, D.H.; Córdoba, L.; Wesseling, C.; Hoppin, J.A.; Mergler, D. Pesticide exposure and neurodevelopment in children aged 6–9 years from Talamanca, Costa Rica. Cortex 2016, 85, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zang, X.; Wang, M.; Chang, Q.; Zhang, S.; Wang, C. Fibrous boron nitride nanocomposite for magnetic solid phase extraction of ten pesticides prior to the quantitation by gas chromatography. Microchim. Acta 2018, 185, 561. [Google Scholar] [CrossRef]
- Riaz, G.; Tabinda, A.B.; Kashif, M.; Yasar, A.; Mahmood, A.; Rasheed, R.; Khan, M.I.; Iqbal, J.; Siddique, S.; Mahfooz, Y. Monitoring and spatiotemporal variations of pyrethroid insecticides in surface water, sediment, and fish of the river Chenab Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 22584–22597. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Kurz, M.H.S.; Guimarães, M.C.M.; Martins, M.L.; Prestes, O.D.; Zanella, R.; Ribeiro, J.N.S.; Gonçalves, F.F. Development and validation of a method for the analysis of pyrethroid residues in fish using GC–MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef]
- Mao, X.; Wan, Y.; Li, Z.; Chen, L.; Lew, H.; Yang, H. Analysis of organophosphorus and pyrethroid pesticides in organic and conventional vegetables using QuEChERS combined with dispersive liquid-liquid microextraction based on the solidification of floating organic droplet. Food Chem. 2020, 309, 125755. [Google Scholar] [CrossRef]
- Tuck, S.; Furey, A.; Crooks, S.R.H.; Danaher, M. A review of methodology for the analysis of pyrethrin and pyrethroid residues in food of animal origin. Food Addit. Contam. Part A 2018, 35, 911–940. [Google Scholar] [CrossRef]
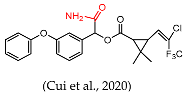
- Cui, N.; Cao, L.; Sui, J.; Hong, L.; Han, X.; Chen, X.; Xie, H.; Sun, X. Quick and convenient construction of lambda-cyhalothrin antigen for the generation of specific antibody. Anal. Biochem. 2020, 597, 113669. [Google Scholar] [CrossRef]
- Gao, H.; Ling, Y.; Xu, T.; Zhu, W.; Jing, H.; Sheng, W.; Li, Q.X.; Li, J. Development of an Enzyme-Linked Immunosorbent Assay for the Pyrethroid Insecticide Cyhalothrin. J. Agric. Food Chem. 2006, 54, 5284–5291. [Google Scholar] [CrossRef] [PubMed]
- Fruhmann, P.; Sanchís, A.; Mayerhuber, L.; Vanka, T.; Kleber, C.; Salvador, J.P.; Marco, M.P. Immunoassay and amperometric biosensor approaches for the detection of deltamethrin in seawater. Anal. Bioanal. Chem. 2018, 410, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, Q.; Zhang, W.; Gee, S.J.; Li, P. Development of a Monoclonal Antibody-Based Enzyme Immunoassay for the Pyrethroid Insecticide Deltamethrin. J. Agric. Food Chem. 2010, 58, 8189–8195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.K.; Cerasoli, D.M.; Lenz, D.E. Role of immunogen design in induction of soman-specific monoclonal antibodies. Immunol. Lett. 2005, 96, 121–127. [Google Scholar] [CrossRef]
- Fránek, M.; Diblíková, I.; Černoch, I.; Vass, M.; Hruška, K. Broad-Specificity Immunoassays for Sulfonamide Detection: Immunochemical Strategy for Generic Antibodies and Competitors. Anal. Chem. 2006, 78, 1559–1567. [Google Scholar] [CrossRef]
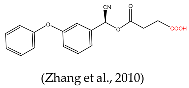
- Zhang, Q.; Zhang, W.; Wang, X.; Li, P. Immunoassay Development for the Class-Specific Assay for Types I and II Pyrethroid Insecticides in Water Samples. Molecules 2010, 15, 164–177. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Liu, Y.; Guo, Y.; Xie, R.; Gui, W.; Zhu, G. Development of a Mab-Based Heterologous Immunoassay for the Broad-Selective Determination of Organophosphorus Pesticides. J. Agric. Food Chem. 2010, 58, 5658–5663. [Google Scholar] [CrossRef]
- Shan, G.; Huang, H.; Stoutamire, D.W.; Gee, S.J.; Leng, G.; Hammock, B.D. A sensitive class specific immunoassay for the detection of pyrethroid metabolites in human urine. Chem. Res. Toxicol. 2004, 17, 218–225. [Google Scholar] [CrossRef]
- National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. 2021. Available online: http://www.trans1.cn/translation/show.php?itemid=2694 (accessed on 3 September 2022).
- European Union Pesticides Database. 2017. Available online: https://data.europa.eu/eli/reg/2017/626/oj (accessed on 3 September 2022).
| Immunogen | No. | Dose (μg) | Titer (1: X 1) | (1 − B/B0) % 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| A-DCC-KLH | 4 | 50 | 16,000 | 16,000 | 16,000 | 16,000 | 48 | 49 | 47 | 50 |
| A-DCC-KLH | 4 | 100 | 16,000 | 16,000 | 32,000 | 16,000 | 49 | 53 | 50 | 51 |
| A-DCC-BSA | 4 | 50 | 16,000 | 16,000 | 8000 | 8000 | 5 | 4 | 7 | 8 |
| A-DCC-BSA | 4 | 100 | 16,000 | 16,000 | 8000 | 8000 | 16 | 8 | 17 | 12 |
| Coating Antigen | Antiserum 1 | Antiserum 2 | Antiserum 3 | Antiserum 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Titer | (1 − B/B0)% 1 | Titer | (1 − B/B0)% 1 | Titer | (1 − B/B0)% 1 | Titer | (1 − B/B0)% 1 | |
| A-DCC-BSA | 16,000 | 15 | 16,000 | 11 | 16,000 | 22 | 16,000 | 20 |
| A-DCC-OVA | 8000 | 22 | 8000 | 32 | 8000 | 7 | 4000 | 5 |
| B-DCC-BSA | 16,000 | 48 | 16,000 | 50 | 32,000 | 58 | 16,000 | 51 |
| C-DCC-OVA | 800 | 15 | 800 | 10 | 4000 | 3 | 4000 | 2 |
| Hapten | Antibody | Analyte | IC50 (μg/L) | CR (%) |
|---|---|---|---|---|
 | mAb | Cypermethrin | 129.1 | 100.0 |
| β-Cypermethrin | 199.6 | 64.7 | ||
| Cyfluthrin | 215.5 | 59.9 | ||
| Fenpropathrin | 220.3 | 58.6 | ||
| λ-Cyhalothrin | 226.9 | 56.9 | ||
| β-Cyfluthrin | 241.7 | 53.4 | ||
| Deltamethrin | 591.2 | 21.8 | ||
| Fenvalerate | 763.1 | 16.9 | ||
 | pAb | λ-Cyhalothrin | 40.1 | 100.0 |
| Deltamethrin | 802.6 | 5.0 | ||
| Cypermethrin | 802.6 | 5.0 | ||
| Cyfluthrin | 401.3 | 10.0 | ||
 | mAb | Phenothrin | 204.0 | 100.0 |
| Permethrin | 325.0 | 62.8 | ||
| Deltamethrin | 52.0 | 392.3 | ||
| Cypermethrin | 49.0 | 416.3 | ||
| Cyhalothrin | 58.0 | 351.7 | ||
 | pAb | Cyhalothrin | 37.2 | 100.0 |
| Cypermethrin | 1488.0 | 2.5 | ||
 | mAb | Deltamethrin | 17.0 | 100.0 |
 | pAb | Phenoxybenzoic acid | 1.7 | 100.0 |
| 4-Fluoro-3-phenoxybenzoic acid | 2.3 | 72.0 |
| Samples | Compounds | LOD (μg/kg) | LOQ (μg/kg) | Spiked Level (μg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|---|---|---|
| Water | Cypermethrin | 25.2 | 44.4 | 40, 80, 160 | 77.4–96.6 | 6.1–9.8 |
| β-Cypermethrin | 24.4 | 36.6 | 40, 80, 160 | 90.5–102.7 | 5.5–12.7 | |
| Cyfluthrin | 36.0 | 54.7 | 60, 120, 240 | 92.7–101.8 | 9.0–11.0 | |
| λ-Cyhalothrin | 43.2 | 55.8 | 50, 100, 200 | 92.7–103.0 | 5.0–13.1 | |
| β-Cyfluthrin, | 46.0 | 58.3 | 50, 100, 200 | 74.1–105.4 | 3.9–14.8 | |
| Fenpropathrin | 40.0 | 56.1 | 50, 100, 200 | 93.3–106.7 | 3.0–14.8 | |
| Deltamethrin | 25.2 | 44.4 | 75, 150, 300 | 89.8–102.3 | 5.8–11.5 | |
| Fenvalerate | 45.7 | 75.1 | 75, 150, 300 | 81.9–91.0 | 5.3–7.3 | |
| Milk | Cypermethrin | 37.5 | 53.3 | 50(MRL), 100, 200 | 82.8–104.2 | 4.3–14.0 |
| β-Cypermethrin | 37.5 | 52.8 | 50(MRL), 100, 200 | 103.0–106.2 | 4.0–12.4 | |
| Cyfluthrin | 59.0 | 87.0 | 80, 160, 320 | 84.9–96.6 | 3.8–10.2 | |
| λ-Cyhalothrin | 52.7 | 82.8 | 80, 160, 200(MRL), 320 | 83.9–100.5 | 4.5–12.3 | |
| β-Cyfluthrin, | 57.4 | 80.6 | 80, 160, 320 | 79.4–102.6 | 6.7–12.5 | |
| Fenpropathrin | 52.0 | 81.0 | 80, 160, 320 | 66.4–92.5 | 5.5–10.8 | |
| Deltamethrin | 67.4 | 95.4 | 100, 200, 400 | 96.3–103.8 | 4.6–10.5 | |
| Fenvalerate | 68.9 | 96.9 | 100(MRL), 200, 400 | 86.4–103.7 | 6.2–12.3 | |
| Celery | Cypermethrin | 68.5 | 97.2 | 100, 200, 400, 1000(MRL) | 75.7–102.3 | 4.6–13.1 |
| β-Cypermethrin | 65.5 | 102.6 | 100, 200, 400, 1000(MRL) | 77.9–99.9 | 3.4–9.6 | |
| Cyfluthrin | 68.6 | 97.3 | 100, 200, 400, 500(MRL) | 82.9–108.5 | 2.9–11.4 | |
| λ-Cyhalothrin | 72.1 | 104.3 | 100, 200, 400, 500(MRL) | 88.0–102.5 | 1.1–3.7 | |
| β-Cyfluthrin, | 72.7 | 102.1 | 100, 200, 400, 500(MRL) | 79.1–92.0 | 3.3–7.9 | |
| Fenpropathrin | 64.1 | 96.3 | 100, 200, 400, 1000(MRL) | 72.6–93.5 | 3.9–5.1 | |
| Deltamethrin | 143.6 | 183.5 | 200, 400, 800, 2000 | 72.9–99.3 | 5.4–13.4 | |
| Fenvalerate | 148.4 | 185.5 | 200, 400, 800 | 93.0–98.8 | 2.1–8.6 | |
| Leek | Cypermethrin | 72.2 | 99.5 | 100, 200, 400, 1000(MRL) | 74.9–107.4 | 4.6–10.6 |
| β-Cypermethrin | 67.4 | 93.3 | 100, 200, 400, 1000(MRL) | 78.7–112.4 | 4.5–9.4 | |
| Cyfluthrin | 64.1 | 95.6 | 100, 200, 400, 500(MRL) | 65.1–108.2 | 4.2–7.9 | |
| λ-Cyhalothrin | 75.8 | 105.2 | 100, 200, 400, 500(MRL) | 85.9–100.5 | 3.2–10.8 | |
| β-Cyfluthrin, | 67.3 | 100.9 | 100, 200, 400, 500(MRL) | 73.1–106.2 | 5.2–8.1 | |
| Fenpropathrin | 65.6 | 97.1 | 100, 200, 400, 1000(MRL) | 71.9–99.2 | 2.6–8.9 | |
| Deltamethrin | 152.2 | 188.6 | 200, 400, 800 | 92.3–97.9 | 3.5–6.6 | |
| Fenvalerate | 142.1 | 181.5 | 200, 400, 800 | 94.6–105.7 | 1.5–7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Zhang, D.; Xu, Z.; Shen, Y.; Wang, Y. A Broad-Spectrum Monoclonal Antibody-Based Heterologous ic-ELISA for the Detection of Multiple Pyrethroids in Water, Milk, Celery, and Leek. Foods 2025, 14, 768. https://doi.org/10.3390/foods14050768
Hou S, Zhang D, Xu Z, Shen Y, Wang Y. A Broad-Spectrum Monoclonal Antibody-Based Heterologous ic-ELISA for the Detection of Multiple Pyrethroids in Water, Milk, Celery, and Leek. Foods. 2025; 14(5):768. https://doi.org/10.3390/foods14050768
Chicago/Turabian StyleHou, Sulin, Dandan Zhang, Zhenyu Xu, Yun Shen, and Yulian Wang. 2025. "A Broad-Spectrum Monoclonal Antibody-Based Heterologous ic-ELISA for the Detection of Multiple Pyrethroids in Water, Milk, Celery, and Leek" Foods 14, no. 5: 768. https://doi.org/10.3390/foods14050768
APA StyleHou, S., Zhang, D., Xu, Z., Shen, Y., & Wang, Y. (2025). A Broad-Spectrum Monoclonal Antibody-Based Heterologous ic-ELISA for the Detection of Multiple Pyrethroids in Water, Milk, Celery, and Leek. Foods, 14(5), 768. https://doi.org/10.3390/foods14050768





