A New Insight into the Weight Gain Method to Monitor and Evaluate Lipid Peroxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fatty Acid Composition
2.3. Initial Quality Indicators of the Oils
2.3.1. Peroxide Value (PV)
2.3.2. Conjugated Diene Value (CDV)
2.3.3. Carbonyl Value (CV)
2.3.4. Acid Value (AV)
2.4. Total Tocopherols Content
2.5. Total Phenolics Content
2.6. Peroxidation
2.7. Kinetic Data Analyses
2.7.1. PV
2.7.2. Weight Gain
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the Oils
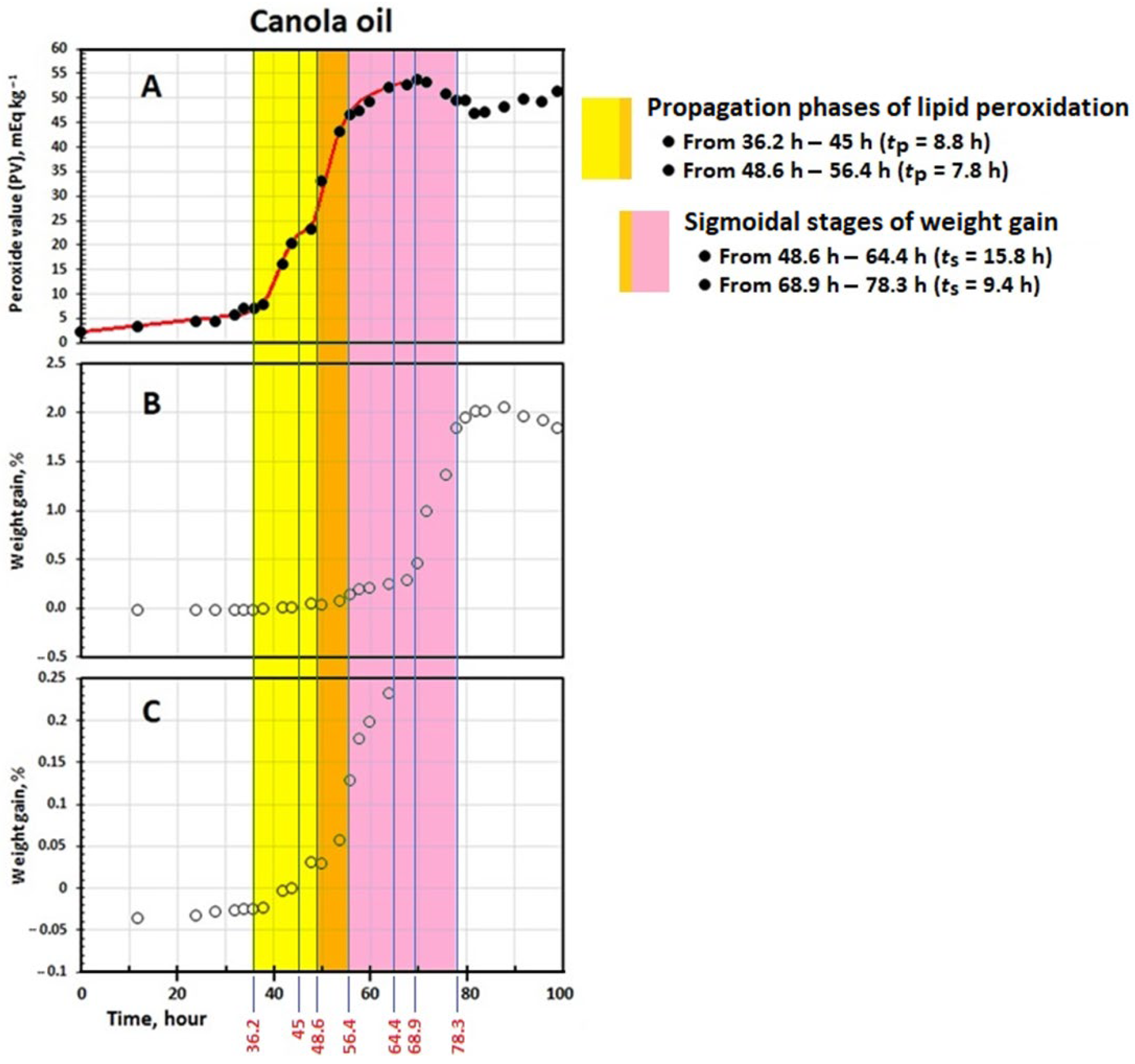
3.2. Kinetics of Change in PV
3.3. Kinetics of Change in Weight
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wsowicz, E.; Gramza, A.; Hes, M.; Jelen, H.H.; Korczak, J.; Matecka, M.; Mildner-Szkudlarz, S.; Rudzinska, M.; Samotyja, U.; Zawirska-Wojtasiak, R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004, 13, 87–100. [Google Scholar]
- Schaich, K.M. Challenges in elucidating lipid oxidation mechanisms: When, where, and how do products arise? In Lipid Oxidation: Challenges in Food Systems; Logan, A., Nienaber, U., Pan, X., Eds.; AOCS Press: Urbana, IL, USA, 2013; pp. 1–53. [Google Scholar]
- Jiang, S.; Xie, Y.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the oxidative stability of edible oil by electron spin resonance spectroscopy. Food Chem. 2020, 309, 125714. [Google Scholar] [CrossRef]
- Farhoosh, R. A reconsidered approach providing kinetic parameters and rate constants to analyze the oxidative stability of bulk lipid systems. Food Chem. 2020, 327, 127088. [Google Scholar] [CrossRef]
- Sarfaraz Khabbaz, E.; Jaldani, S.; Farhoosh, R. Unusual multiphase peroxidation of sunflower oil: A kinetic study. LWT Food Sci. Technol. 2023, 184, 114981. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Yanishlieva, N. Kinetic analysis of lipid oxidation data. In Analysis of Lipid Oxidation; Kamal-Eldin, A., Pokorny, J., Eds.; AOCS Press: Urbana, IL, USA, 2005; pp. 234–263. [Google Scholar]
- Pinchuk, I.; Lichtenberg, D. Analysis of the kinetics of lipid peroxidation in terms of characteristic time-points. Chem. Phys. Lipids 2014, 178, 63–76. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Niklova, I.; Schmidt, S.; Habalova, K.; Sekretar, S. Effect of evening primrose extracts on oxidative stability of sunflower and rapeseed oils. Eur. J. Lipid Sci. Technol. 2001, 103, 299–306. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, U.N. Methods for measuring oxidative rancidity in fats and oils. In Food Lipids, Chemistry, Nutrition, and Biotechnology; Akoh, C.C., Min, D.B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 387–408. [Google Scholar]
- Matikainen, J.; Laantera, M.; Kaltia, S. Determination of degree of oxidation of methyl linoleate and linolenate by weighing method. J. Am. Oil Chem. Soc. 2003, 80, 591–593. [Google Scholar] [CrossRef]
- Tumosa, C.S.; Mecklenburg, M.F. Weight changes on oxidation of drying and semi-drying oils. Collect. Forum 2003, 18, 116–123. [Google Scholar]
- Farhoosh, R. Quantitative criteria characterizing the time change pattern of total lipid-peroxidation carbonyls. Sci. Rep. 2022, 12, 22345. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Mendez, E.; Sanhueza, J.; Speisky, H.; Valenzuela, A. Validation of the Rancimat test for the assessment of the relative stability of fish oils. J. Am. Oil Chem. Soc. 1996, 73, 1033–1037. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Pegg, R.B. Measurement of Primary Lipid Oxidation Products, Current Protocols in Food Analytical Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Privett, O.S.; Blank, M.L. The initial stages of autoxidation. J. Am. Oil Chem. Soc. 1962, 39, 465–469. [Google Scholar] [CrossRef]
- Endo, Y.; Li, C.M.; Tagiri-Endo, M.; Fujimoto, K. A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2-propanol as a solvent. J. Am. Oil Chem. Soc. 2001, 78, 1021–1024. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Urbana, IL, USA, 1993. [Google Scholar]
- Wong, M.; Timms, R.; Goh, E. Colorimetric determination of total tocopherols in palm oil, olein and stearin. J. Am. Oil Chem. Soc. 1988, 65, 258–261. [Google Scholar] [CrossRef]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Farhoosh, R. New insights into the kinetic and thermodynamic evaluations of lipid peroxidation. Food Chem. 2022, 375, 131659. [Google Scholar] [CrossRef]
- Przybylski, R.; Mag, T.; Eskin, N.A.M.; McDonald, B.E. Canola oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 61–123. [Google Scholar]
- Moreau, R.A. Corn oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 149–173. [Google Scholar]
- Pattee, H.E. Peanut oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 431–465. [Google Scholar]
- Hsieh, R.J.; Kinsella, J.E. Oxidation of polyunsaturated fatty acids: Mechanisms, products, and inhibition with emphasis on fish. Adv. Food Nutr. Res. 1989, 33, 233–341. [Google Scholar]
- Kiralan, M.; Ketenoglu, O.; Sezer Kiralan, S. Trans fatty acids—Occurrence, technical aspects, and worldwide regulations. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier BV: Amsterdam, The Netherlands, 2021; Volume 70, pp. 313–343. [Google Scholar]
- Codex Alimentarius. Codex Standard for Named Vegetable Oils; (CODEX-STAN 210, Amended 2003–2005); FAO: Rome, Italy, 2013. [Google Scholar]
- Jaldani, S.; Sarfaraz Khabbaz, E.; Jooyandeh, M.; Farhoosh, R. Kinetics of simultaneous change in the concentration of total lipid hydroperoxides and total conjugated dienes during peroxidation of canola, sunflower, and olive oils. Food Chem. 2024, 435, 137605. [Google Scholar] [CrossRef]
- White, P.J. Conjugated diene, anisidine value, and carbonyl value analyses. In Methods to Assess Quality and Stability of Oils and Fat-Containing Foods; Warner, K., Eskin, M., Eds.; AOCS Publishing: Urbana, IL, USA, 1995; pp. 159–179. [Google Scholar]
- Yanishlieva, N.V.; Marinova, E.M. Kinetic evaluation of the antioxidant activity in lipid oxidation. In Lipid Oxidation Pathways; Kamal-Eldin, A., Ed.; AOCS Press: Urbana, IL, USA, 2003; pp. 85–110. [Google Scholar]
- Crapiste, G.H.; Brevedan, M.I.V.; Carelli, A.A. Oxidation of sunflower oil during storage. J. Am. Oil Chem. Soc. 1999, 76, 1437–1443. [Google Scholar] [CrossRef]

| Oil Sample | |||
|---|---|---|---|
| Canola | Corn | Peanut | |
| Major fatty acids (%w/v) | |||
| C16:0 | 4.90 ± 0.1 c | 11.0 ± 0.2 a | 9.64 ± 0.08 b |
| C18:0 | 2.03 ± 0.01 c | 2.40 ± 0.01 b | 3.65 ± 0.00 a |
| C18:1 cis-∆9 | 61.1 ± 0.1 b | 31.3 ± 0.0 c | 62.6 ± 0.2 a |
| C18:1 trans | 0.11 ± 0.00 a | 0.03 ± 0.01 b | - |
| C18:2 cis-∆9,12 | 20.9 ± 0.0 b | 52.3 ± 0.2 a | 21.0 ± 0.1 b |
| C18:2 trans | 0.10 ± 0.00 b | 0.65 ± 0.04 a | 0.02 ± 0.00 c |
| C18:3 cis-∆9,12,15 | 7.42 ± 0.02 a | 0.81 ± 0.00 b | 0.12 ± 0.01 c |
| C18:3 trans | 0.20 ± 0.00 a | 0.08 ± 0.01 b | - |
| Calculated oxidizability (Cox) value | 4.47 ± 0.00 b | 5.96 ± 0.02 a | 2.82 ± 0.01 c |
| Polyene index | 3.60 ± 0.03 a | 3.74 ± 0.06 a | 1.41 ± 0.00 b |
| Peroxide value (PV, mEq kg−1) | 2.03 ± 0.05 b | 0.96 ± 0.04 c | 3.52 ± 0.11 a |
| Conjugated diene value (CDV, µmol g−1) | 13.0 ± 0.5 b | 14.5 ± 0.2 a | 6.70 ± 0.08 c |
| Carbonyl value (CV, µmol g−1) | 0.28 ± 0.04 b | 0.74 ± 0.05 a | 0.32 ± 0.05 b |
| Acid value (AV, mg g−1) | 0.47 ± 0.09 b | 0.25 ± 0.04 c | 0.78 ± 0.04 a |
| Total tocopherols content (mg kg−1) | 600 ± 12 a | 592 ± 8 a | 210 ± 5 b |
| Total phenolics content (mg kg−1) | 261 ± 1 a | 205 ± 2 b | 57 ± 2 c |
| Additives † | |||
| tert-Butylhydroquinone (TBHQ, mg kg−1) | 75 | 75 | - |
| Citric acid (mg kg−1) | 50 | 100 | - |
| Canola Oil | Corn Oil | Peanut Oil | ||
|---|---|---|---|---|
| Step 1 | Step 2 | |||
| Initiation phase | ||||
| IP (h) | 36.2 ± 0.1 b | 48.6 ± 0.3 a | 25.4 ± 0.1 c | 13.1 ± 0.4 d |
| kIP (mEq kg−1 h−1) | 0.09 ± 0.01 d | 1.34 ± 0.40 a | 0.16 ± 0.01 c | 0.36 ± 0.03 b |
| Oi (kg mEq−1 h2) | 412 ± 44 a | 38.8 ± 1.3 c | 160 ± 8 b | 36.1 ± 3.1 c |
| PVIP (mEq kg−1) | 5.21 ± 0.32 c | 26.4 ± 2.2 a | 4.72 ± 0.18 c | 8.28 ± 0.37 b |
| Propagation phase | ||||
| tp (h) | 8.79 ± 0.40 c | 7.85 ± 0.85 c | 14.3 ± 0.8 b | 28.2 ± 0.2 a |
| tmax (h) | 45.0 ± 0.4 b | 56.4 ± 0.6 a | 39.6 ± 0.8 c | 41.4 ± 0.4 c |
| kc (h−1) | 0.36 ± 0.02 a | 0.25 ± 0.02 b | 0.26 ± 0.02 b | 0.12 ± 0.00 c |
| kd (kg mEq−1 h−1) | 147 ± 6 (×10−4) a | 48.3 ± 4.1 (×10−4) b | 48.0 ± 3.4 (×10−4) b | 20.7 ± 0.5 (×10−4) c |
| PVmax (mEq kg−1) | 24.3 ± 0.7 c | 52.8 ± 1.7 b | 53.5 ± 0.8 b | 58.8 ± 1.1 a |
| rmax (mEq kg−1 h−1) | 2.17 ± 0.11 b | 3.35 ± 0.12 a | 3.43 ± 0.15 a | 1.79 ± 0.03 c |
| rn (h−1) | 894 ± 34 (×10−4) a | 636 ± 37 (×10−4) b | 641 ± 36 (×10−4) b | 304 ± 3 (×10−4) c |
| Canola Oil | Corn Oil | Peanut Oil | ||
|---|---|---|---|---|
| Step 1 | Step 2 | |||
| IPw (h) | 48.6 ± 0.1 b | 68.9 ± 0.2 a | 36.8 ± 0.9 c | 37.7 ± 0.8 c |
| ts (h) | 15.8 ± 1.1 a | 9.37 ± 0.35 b | 8.60 ± 0.36 b | 5.25 ± 0.83 c |
| tmaxw (h) | 64.4 ± 1.1 b | 78.3 ± 0.5 a | 45.4 ± 2.6 c | 42.9 ± 0.1 c |
| Wmax (%) | 0.28 ± 0.01 c | 1.98 ± 0.06 a | 0.17 ± 0.02 d | 1.31 ± 0.01 b |
| rIP (% h−1) | 475 ± 55 (×10−6) d | 5836 ± 263 (×10−6) a | 724 ± 30 (×10−6) c | 4716 ± 123 (×10−6) b |
| rmaxw (% h−1) | 0.0191 ± 0.0005 c | 0.1861 ± 0.0054 b | 0.0202 ± 0.0058 c | 0.2456 ± 0.0098 a |
| Oiw (% h2) | 10.3 ± 1.3 (×104) a | 1.2 ± 0.2 (×104) c | 5.1 ± 0.3 (×104) b | 0.8 ± 0.0 (×104) d |
| rnw (h−1) | 0.0680 ± 0.0052 d | 0.0940 ± 0.0050 c | 0.1198 ± 0.0461 b | 0.1875 ± 0.0543 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashaei, H.; Farhoosh, R. A New Insight into the Weight Gain Method to Monitor and Evaluate Lipid Peroxidation. Foods 2025, 14, 700. https://doi.org/10.3390/foods14040700
Pashaei H, Farhoosh R. A New Insight into the Weight Gain Method to Monitor and Evaluate Lipid Peroxidation. Foods. 2025; 14(4):700. https://doi.org/10.3390/foods14040700
Chicago/Turabian StylePashaei, Haniye, and Reza Farhoosh. 2025. "A New Insight into the Weight Gain Method to Monitor and Evaluate Lipid Peroxidation" Foods 14, no. 4: 700. https://doi.org/10.3390/foods14040700
APA StylePashaei, H., & Farhoosh, R. (2025). A New Insight into the Weight Gain Method to Monitor and Evaluate Lipid Peroxidation. Foods, 14(4), 700. https://doi.org/10.3390/foods14040700







