Calcium and Boron Foliar Fertilizer to Relieve Cracking of ‘Liuyuezao’ Pummelos
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials and Treatments
2.2. Mineral Elements
2.3. Fruit Phenotype and Sugar-Acid Content
2.4. Mechanical Properties of Peel
2.5. Cell Wall Polysaccharide and Cell Wall Enzyme Activity
2.6. Real-Time Fluorescence Quantitative PCR Analysis of Cell Wall-Degrading Enzyme Gene Expression
2.7. Statistical Analyses
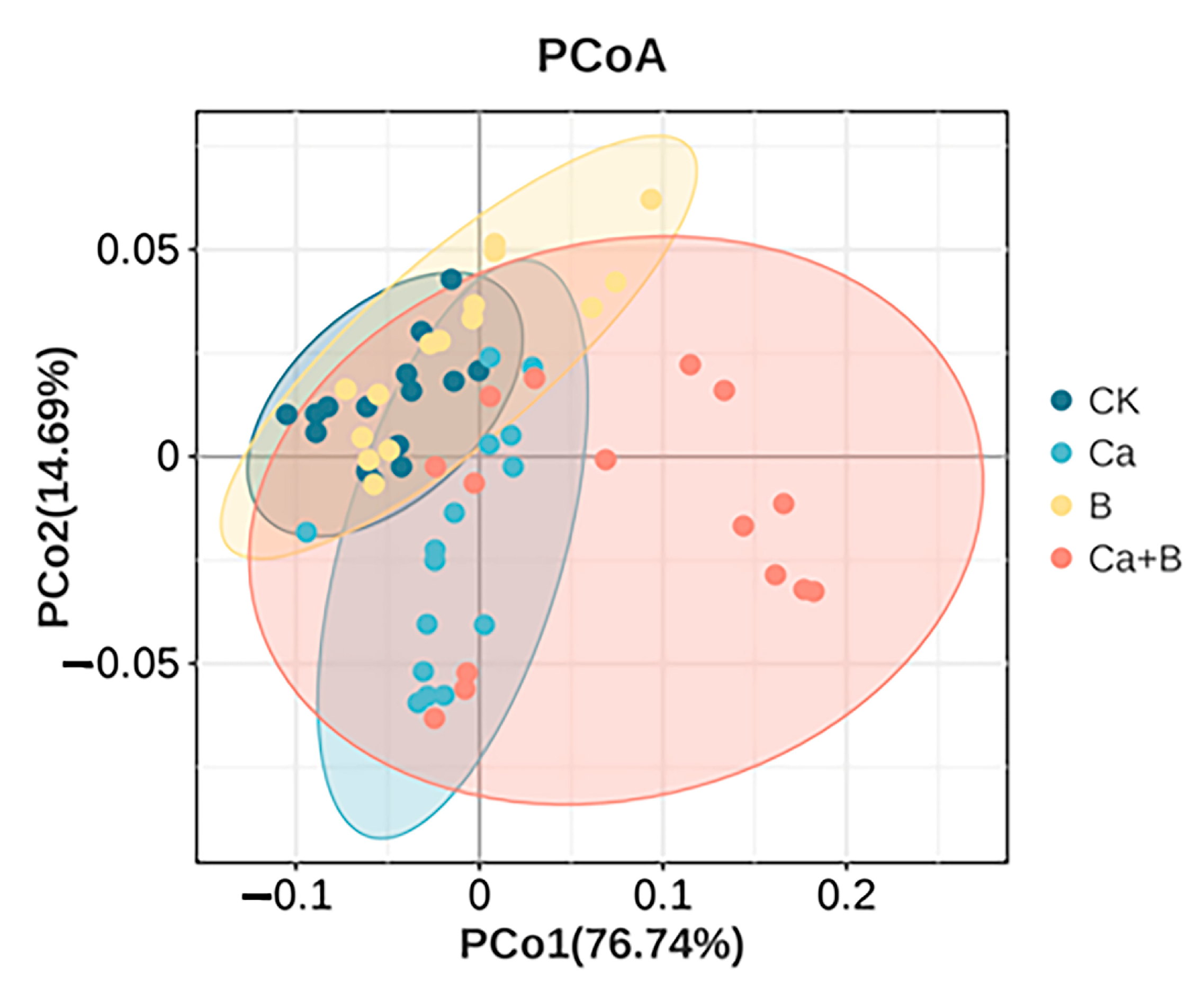
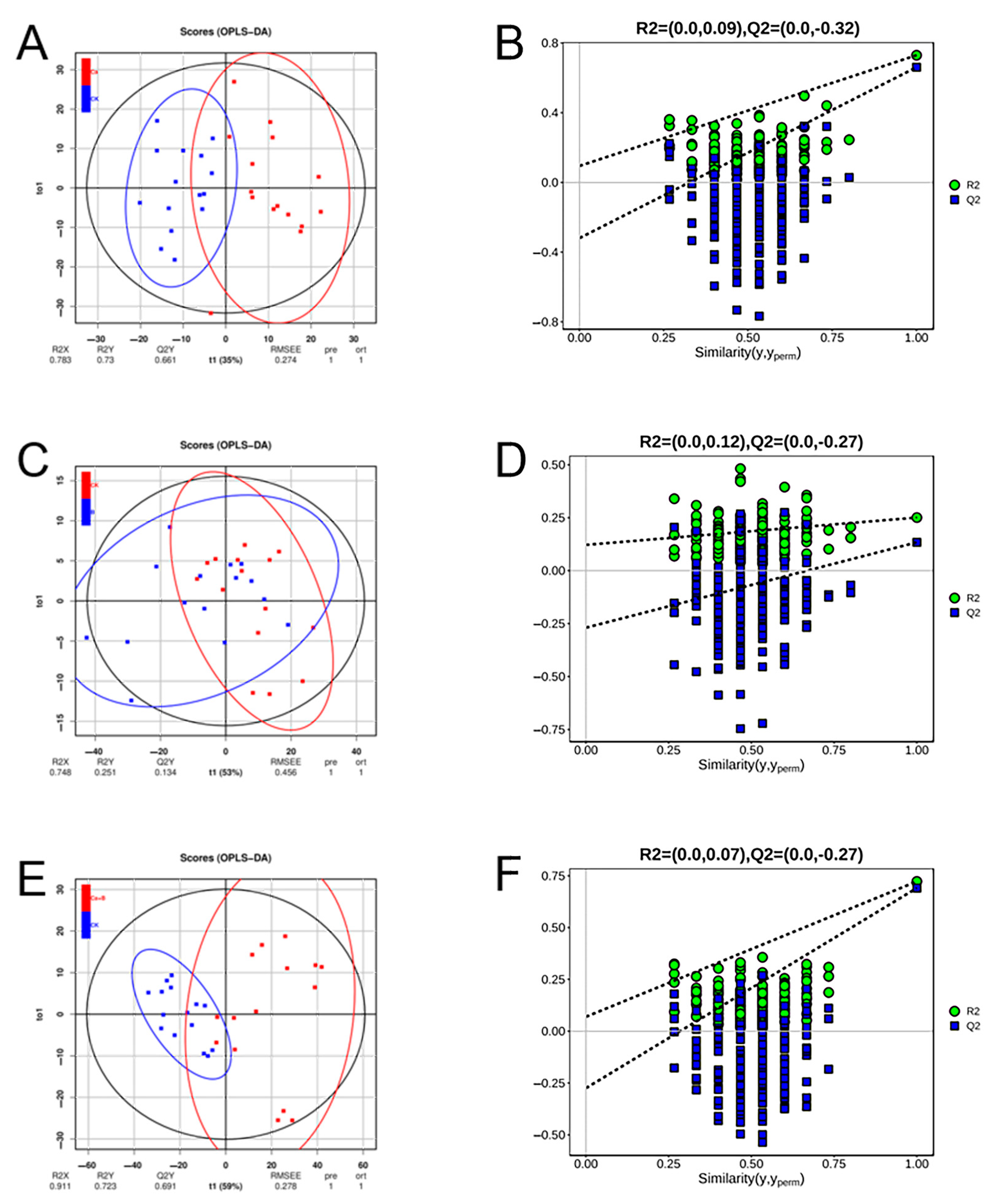
3. Results
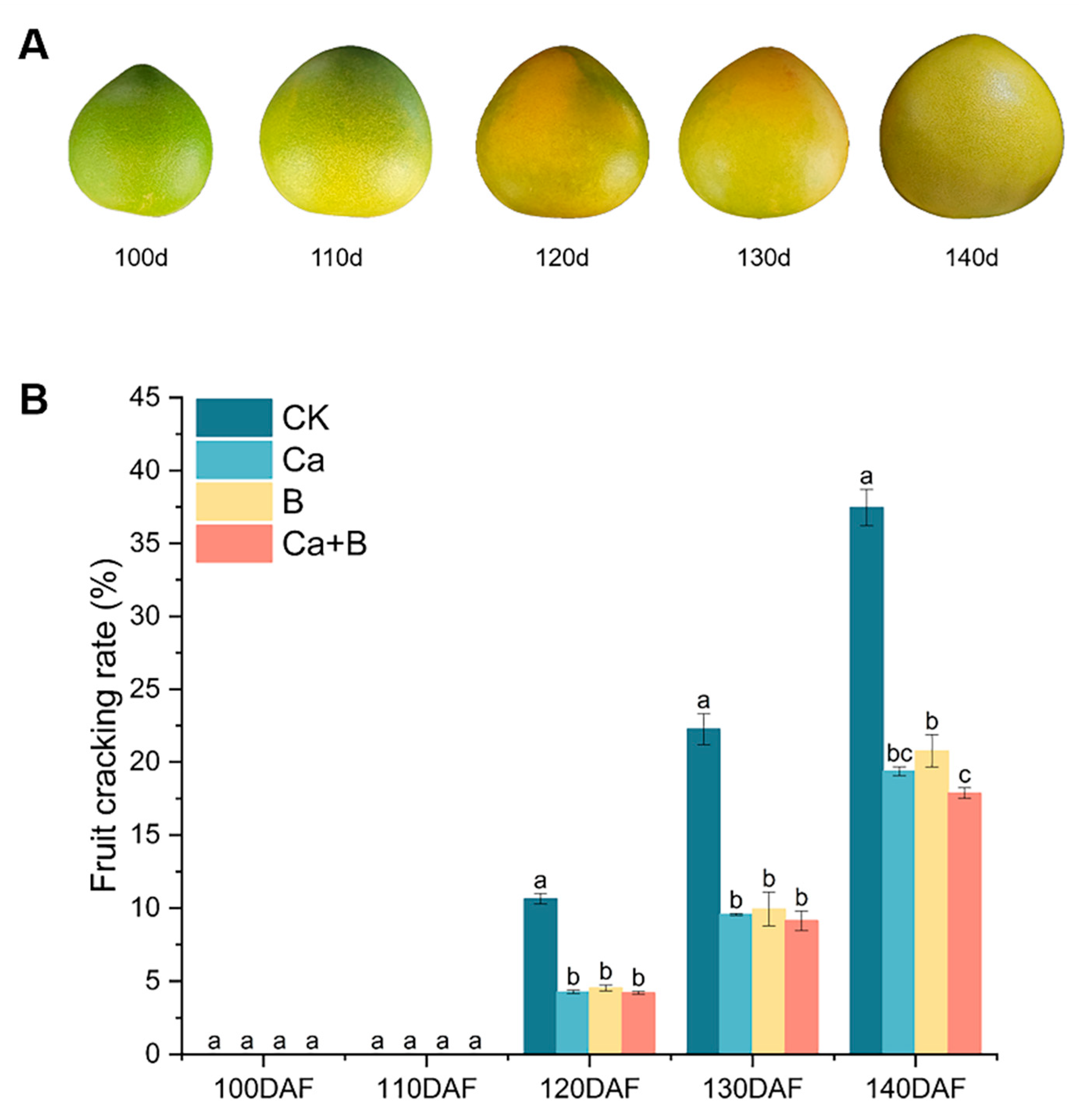
3.1. Changes in Rate of Cracking of Pummelo ‘Liuyuezao’ Fruits
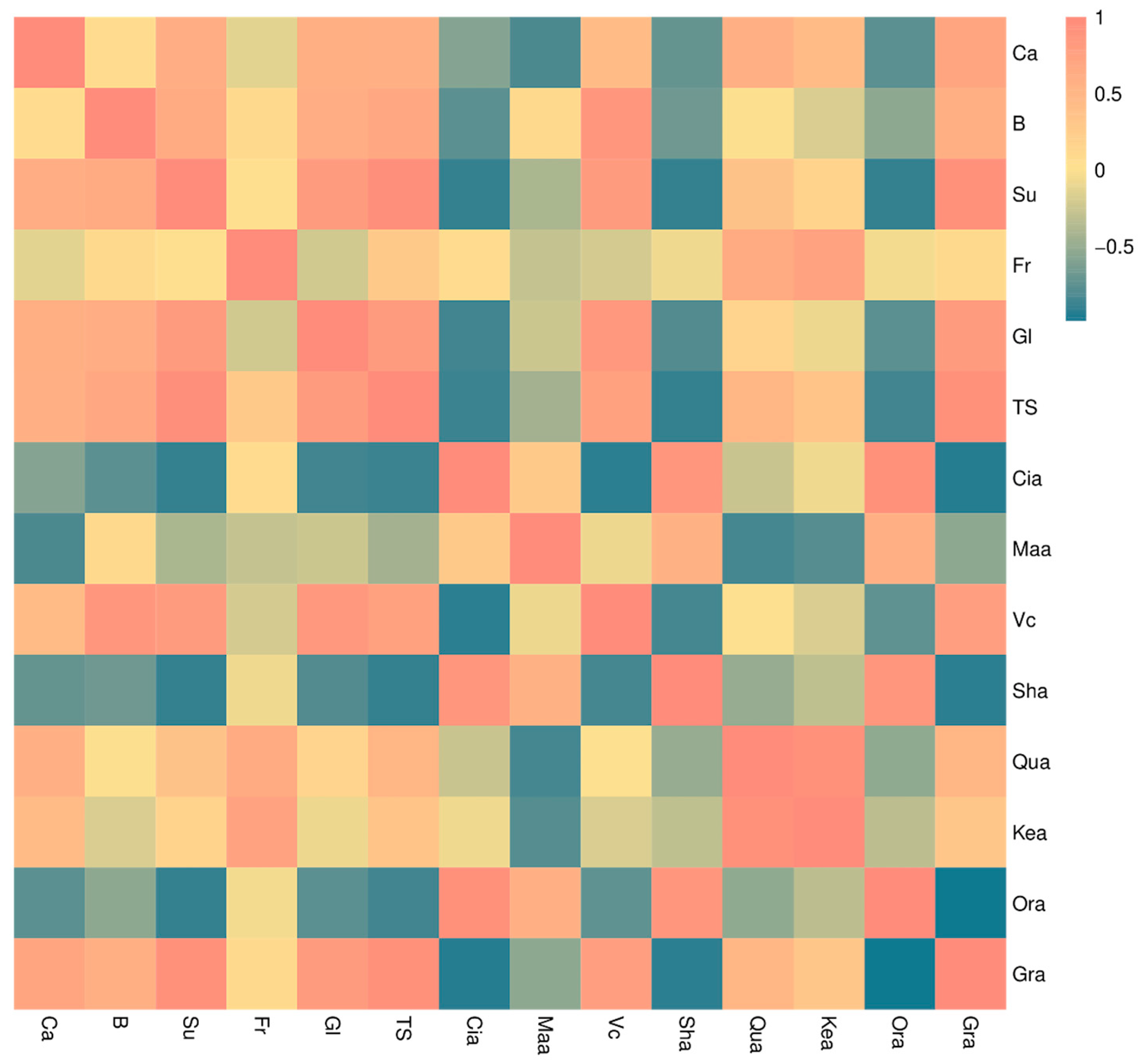
3.2. Mineral Content of ‘Liuyuezao’ Pummelo Pericarp
3.3. Fruit Phenotypes and Sugar–Acid Content of ‘Liuyuezao’ Pummelos
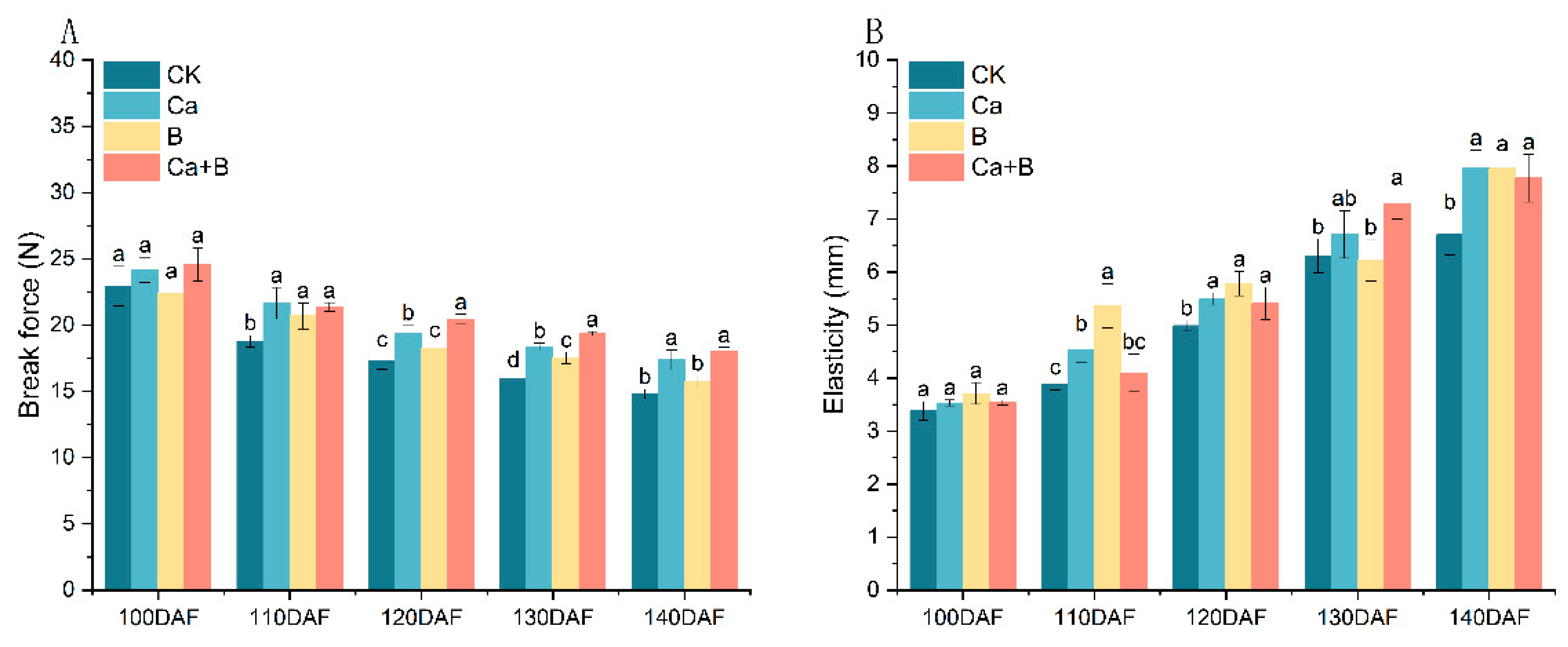
3.4. Changes in Skin Break Force and Elasticity of ‘Liuyuezao’ Pummelos
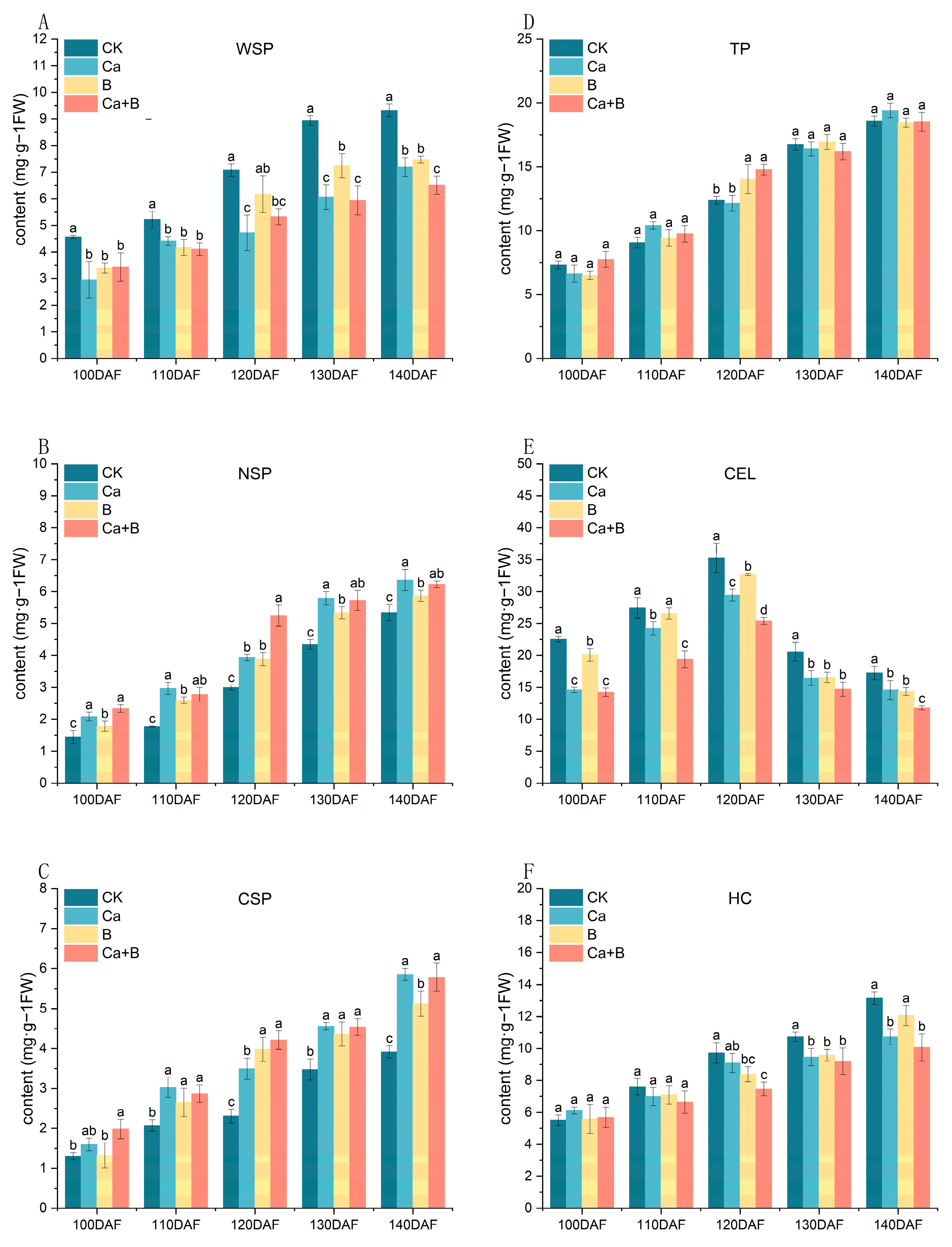
3.5. Changes in Polysaccharide Content of Pericarp Cell Wall of ‘Liuyuezao’ Pummelos
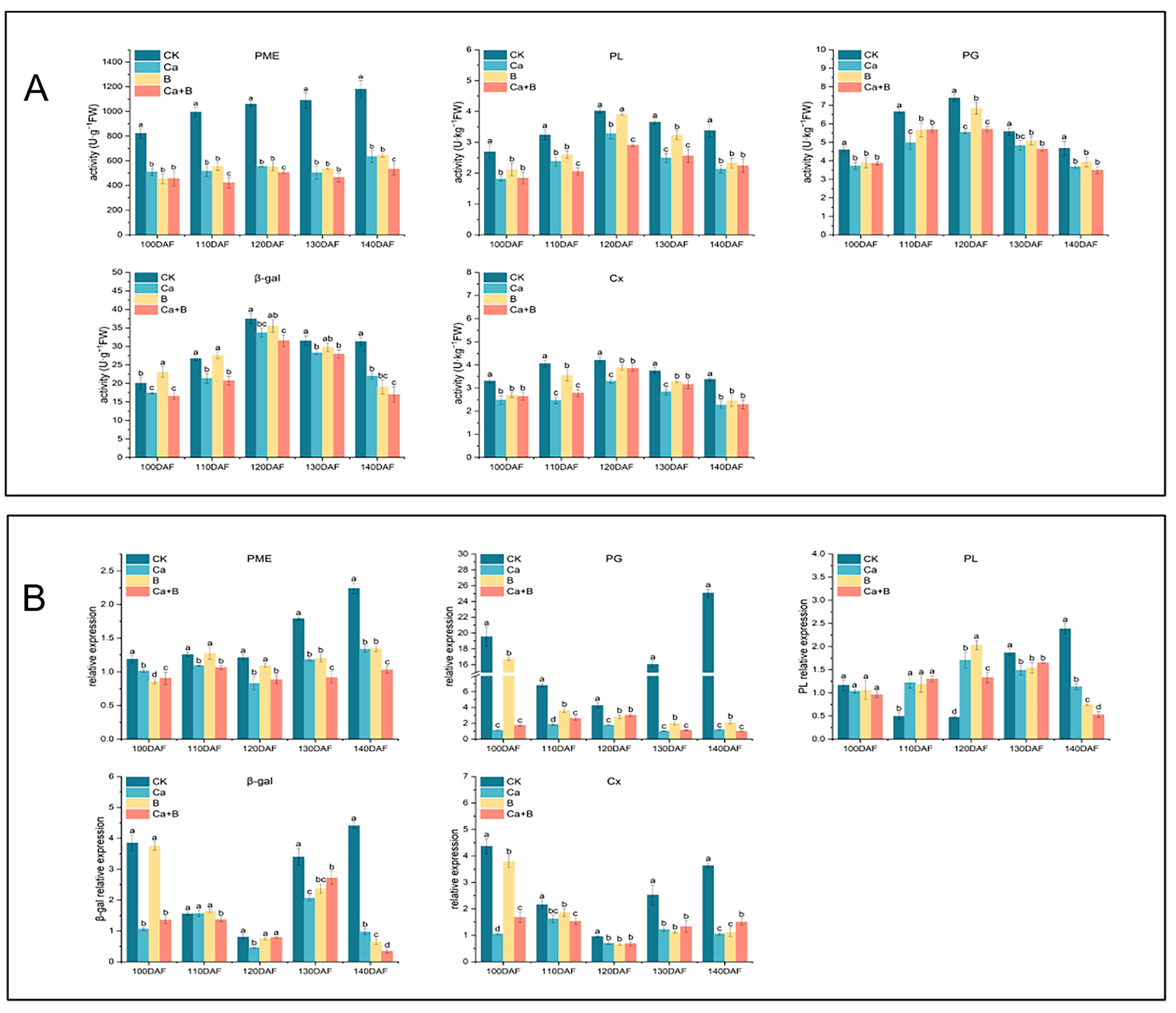
3.6. Expression of Cell Wall Enzymes and Genes Related to Cell Wall Metabolism in Pericarp of ‘Liuyuezao’ Pummelos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.Y.; Li, X.T.; Xu, C.Y.; Lian, R.; Pan, T.F.; Guo, Z.X.; Yu, Y.; She, W.Q. Gene expression in the sugar metabolism between ‘Guanxi’ pummelo and its early-ripening mutant ‘Liuyuezao’. Sci. Hortic. 2022, 305, 111435. [Google Scholar] [CrossRef]
- Xu, S.R.; Chen, Y.Q.; Pan, D.M.; Pan, H.L. Chloroplast Genome Sequence and Characteristics Analysis of Citrus maxima Liuyuezao. Chin. J. Trop. Crops 2021, 42, 1223–1230. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Hassanpouraghdam, M.B.; Paliyath, G.; Farmani, B. The language of calcium in postharvest life of fruits, vegetables and flowers. Sci. Hortic. 2012, 144, 102–115. [Google Scholar] [CrossRef]
- Wang, T.; Tan, L.P.; Chen, Z.F.; Yang, Y.T.; Yuan, Y.; Zheng, Z.D.; Deng, L.J.; Zhang, M.F.; Sun, G.C.; He, S.Y.; et al. Mitigating citrus fruit cracking: The efficacy of chelated calcium or silicon foliar fertilizers in ‘Okitsu no. 58’ citrus fruit. Front. Recent Dev. Plant Sci. 2024, 15, 1402945. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, X.Y.; Qin, M.L.; Wang, W.L.; He, X.; Zhou, W.H. Effect of CaCl2 Sprays in Different Fruit Development Stages on Grape Berry Cracking. Front. Recent Dev. Plant Sci. 2022, 13, 870959. [Google Scholar] [CrossRef]
- Shi, D.C.; Wang, J.; Hu, R.B.; Zhou, G.K.; O’Neill, M.A.; Kong, Y.Z. Boron-bridged RG-II and calcium are required to maintain the pectin network of the Arabidopsis seed mucilage ultrastructure. Plant Mol. Biol. 2017, 94, 267–280. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Z.; Zhang, C.; Hu, E.; Zhou, R.; Jiang, F.L. The composition of pericarp, cell aging, and changes in water absorption in two tomato genotypes: Mechanism, factors, and potential role in fruit cracking. Acta Physiol. Plant 2016, 38, 215. [Google Scholar] [CrossRef]
- Ghanbarpour, E.; Rezaei, M.; Lawson, S. Reduction of Cracking in Pomegranate Fruit After Foliar Application of Humic Acid, Calcium-boron and Kaolin During Water Stress. Erwerbs-Obstbau 2019, 61, 29–37. [Google Scholar] [CrossRef]
- Xu, W.N.; Wang, P.J.; Yuan, L.Q.; Chen, X.; Hu, X.H. Effects of Application Methods of Boron on Tomato Growth, Fruit Quality and Flavor. Horticulturae 2021, 7, 223. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Liu, H.P.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Synergistic roles of leaf boron and calcium during the growing season in affecting sugar and starch accumulation in ripening apple fruit. Acta Physiol. Plant 2013, 35, 2483–2492. [Google Scholar] [CrossRef]
- Islam, M.Z.; Mele, M.A.; Baek, J.P.; Kang, H.M. Cherry tomato qualities affected by foliar spraying with boron and calcium. Hortic. Environ. Biotechnol. 2016, 57, 46–52. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, G.S.; Tian, H.Q.; Fu, D.Q. Fruit cracking: A review. Sheng Wu Gong Cheng Xue Bao 2021, 37, 2737–2752. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Zhou, Q.R.; Huang, J.K.; Ren, H.Q.; Chen, Y.R.; Zhang, J.C.; Du, X.; Zhang, Y.P.; Wang, P. Physiological and molecular mechanism of Ca+GA_3 treatment alleviating fruit cracking incidence in ponkan. J. Fruit Sci. 2024, 41, 1563–1576. [Google Scholar] [CrossRef]
- Huang, X.M.; Wang, H.C.; Lu, X.J.; Yuan, W.Q.; Lu, J.M.; Li, J.G.; Huang, H.B. Cell wall modifications in the pericarp of litchi (Litchi chinensis Sonn.) cultivars that differ in their resistance to cracking. J. Hortic. Sci. Biotechnol. 2006, 81, 231–237. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.T.; Bai, M.; Xu, Y.S.; Fan, S.G.; Yang, G.S. Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.) ‘Xiangfei’. PeerJ 2020, 8, e9896. [Google Scholar] [CrossRef]
- Deng, J.; Shi, Z.J.; Wang, L.C.; Wu, H.B.; Liu, H.M. Effects of calcium treatments on cell wall material metabolism and related enzyme activities and gene expression in grapefruit(Citrus paradisi Macf.). J. Plant Nutr. Fertitizer 2016, 22, 450–458. [Google Scholar] [CrossRef]
- Shao, S.X.; Xu, M.T.; Liao, X.S.; Luo, Q.; Lin, Y.P.; Wang, P.J.; Fang, D.Y.; Huang, Y.B.; Jin, S.; Ye, N.X. Production regions discrimination of Huangguanyin oolong tea by using the content of chemical components and rare earth elements. Food Res. Int. 2023, 165, 112522. [Google Scholar] [CrossRef] [PubMed]
- ValizadehKaji, B.; Mohammaei, M. Foliar Application of Biostimulants and Potassium Silicate Enhances Yield and Fruit Quality of Mandarin Cv. ‘Page’. Appl. Fruit Sci. 2025, 67, 8. [Google Scholar] [CrossRef]
- Zsofi, Z.; Villango, S.; Palfi, Z.; Toth, E.; Balo, B. Texture characteristics of the grape berry skin and seed (Vitis vinifera L. cv. Kékfrankos) under postveraison water deficit. Sci. Hortic. 2014, 172, 176–182. [Google Scholar] [CrossRef]
- Vicente, A.R.; Powell, A.; Greve, L.C.; Labavitch, J.M. Cell wall disassembly events in boysenberry (Rubus idaeus L. × Rubus ursinus Cham. & Schldl.) fruit development. Funct. Plant Biol. 2007, 34, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Li, Y.F.; Yan, L.P.; Xie, J. Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem. 2011, 124, 569–575. [Google Scholar] [CrossRef]
- Rahmani, F.; Amraee, L. Modified CTAB protocol for RNA extraction from Lemon balm (Melissa officinalis L.). Acta Agric. Slov. 2020, 115, 53–57. [Google Scholar] [CrossRef]
- Mu, H.Y.; Chen, J.Z.; Huang, W.J.; Huang, G.; Deng, M.Y.; Hong, S.M.; Ai, P.; Gao, C.; Zhou, H.K. OmicShare tools: A zero-code interactive online platform for biological data analysis and visualization. Imeta 2024, 3, e228. [Google Scholar] [CrossRef]
- Koutinas, N.; Sotiropoulos, T.; Petridis, A.; Almaliotis, D.; Deligeorgis, E.; Therios, I.; Voulgarakis, N. Effects of Preharvest Calcium Foliar Sprays on Several Fruit Quality Attributes and Nutritional Status of the Kiwifruit Cultivar Tsechelidis. Hortscience 2010, 45, 984–987. [Google Scholar] [CrossRef]
- Yu, J.Q.; Ji, F.Y.; Yang, X.K.; Cheng, Y.; Gao, H.S.; Sheng, L.X. A genome-wide investigation of the mechanism underlying the effect of exogenous boron application on sugar content and overall quality of “Benihoppe” strawberries. Plant Physiol. Biochem. 2024, 216, 109116. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Konstankiewicz, K. Calcium effect on mechanical properties of model cell walls and apple tissue. J. Food Eng. 2011, 102, 217–223. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.Z. Citrus Fruit-Cracking: Causes and Occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Jona, R.; Goren, R.; Marmora, M. Effect of gibberellin on cell-wall components of creasing peel in mature ‘Valencia’ orange. Sci. Hortic. 1989, 39, 105–115. [Google Scholar] [CrossRef]
- Yamauchi, T.; Hara, T.; Sonoda, Y. Distribution of Calcium and Boron in the Pectin Fraction of Tomato Leaf Cell Wall. Plant Cell Physiol. 1986, 27, 729–732. [Google Scholar] [CrossRef]
- Wen, M.X.; Shi, X.J. Influence of Calcium on Fruit Cracking of Jincheng Orange and Its Physiological Mechanism. Sci. Agric. Sin. 2012, 45, 1127–1134. [Google Scholar] [CrossRef]
- Chen, J.Q.; Liu, L.Z.; Chen, J.Z.; Zhang, H.L. Effects of various calcium treatments on fruit cracking and cell wall enzyme activities in navel orange. J. South China Agric. Univ. 2014, 35, 29–32. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Preharvest calcium applications inhibit some cell wall-modifying enzyme activities and delay cell wall disassembly at commercial harvest of ‘Fuji Kiku-8’ apples. Postharvest Biol. Technol. 2011, 62, 161–167. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.F. Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.J.; Tang, Y.; Xia, H.; Zhang, X.C.; Yue, M.L.; Qiu, X.; Xu, K.; Wang, Z. Transcriptomic insights into citrus segment membrane’s cell wall components relating to fruit sensory texture. BMC Genom. 2018, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.B.; Labavitch, J.M. Ethylene and ripening-regulated expression and function of fruit cell wall modifying proteins. Plant Sci. 2008, 175, 130–136. [Google Scholar] [CrossRef]
- Belge, B.; Goulao, L.F.; Comabella, E.; Graell, J.; Lara, I. Refrigerated storage and calcium dips of ripe ‘Celeste’ sweet cherry fruit: Combined effects on cell wall metabolism. Sci. Hortic. 2017, 219, 182–190. [Google Scholar] [CrossRef]
- Shen, Y.H.; Lu, B.G.; Feng, L. Isolation of ripening-related genes from ethylene/1-MCP treated papaya through RNA-seq. BMC Genom. 2017, 18, 671. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, K.; Lin, H.; Luo, Q.; Li, T.; Wu, H.; Wang, B.; Guo, Z.; Pan, T.; She, W. Calcium and Boron Foliar Fertilizer to Relieve Cracking of ‘Liuyuezao’ Pummelos. Foods 2025, 14, 595. https://doi.org/10.3390/foods14040595
Du K, Lin H, Luo Q, Li T, Wu H, Wang B, Guo Z, Pan T, She W. Calcium and Boron Foliar Fertilizer to Relieve Cracking of ‘Liuyuezao’ Pummelos. Foods. 2025; 14(4):595. https://doi.org/10.3390/foods14040595
Chicago/Turabian StyleDu, Kaiyang, Han Lin, Qin Luo, Tao Li, Hongyu Wu, Bin Wang, Zhixiong Guo, Tengfei Pan, and Wenqin She. 2025. "Calcium and Boron Foliar Fertilizer to Relieve Cracking of ‘Liuyuezao’ Pummelos" Foods 14, no. 4: 595. https://doi.org/10.3390/foods14040595
APA StyleDu, K., Lin, H., Luo, Q., Li, T., Wu, H., Wang, B., Guo, Z., Pan, T., & She, W. (2025). Calcium and Boron Foliar Fertilizer to Relieve Cracking of ‘Liuyuezao’ Pummelos. Foods, 14(4), 595. https://doi.org/10.3390/foods14040595




