Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Patterns of Important Foodborne Pathogens Isolated from Retail Chicken Meat and Associated Environments in India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Inclusion, Exclusion Criteria and Quality Check of the Reviewed Literatures
2.3. Data Extraction
2.4. Meta-Analysis
3. Results
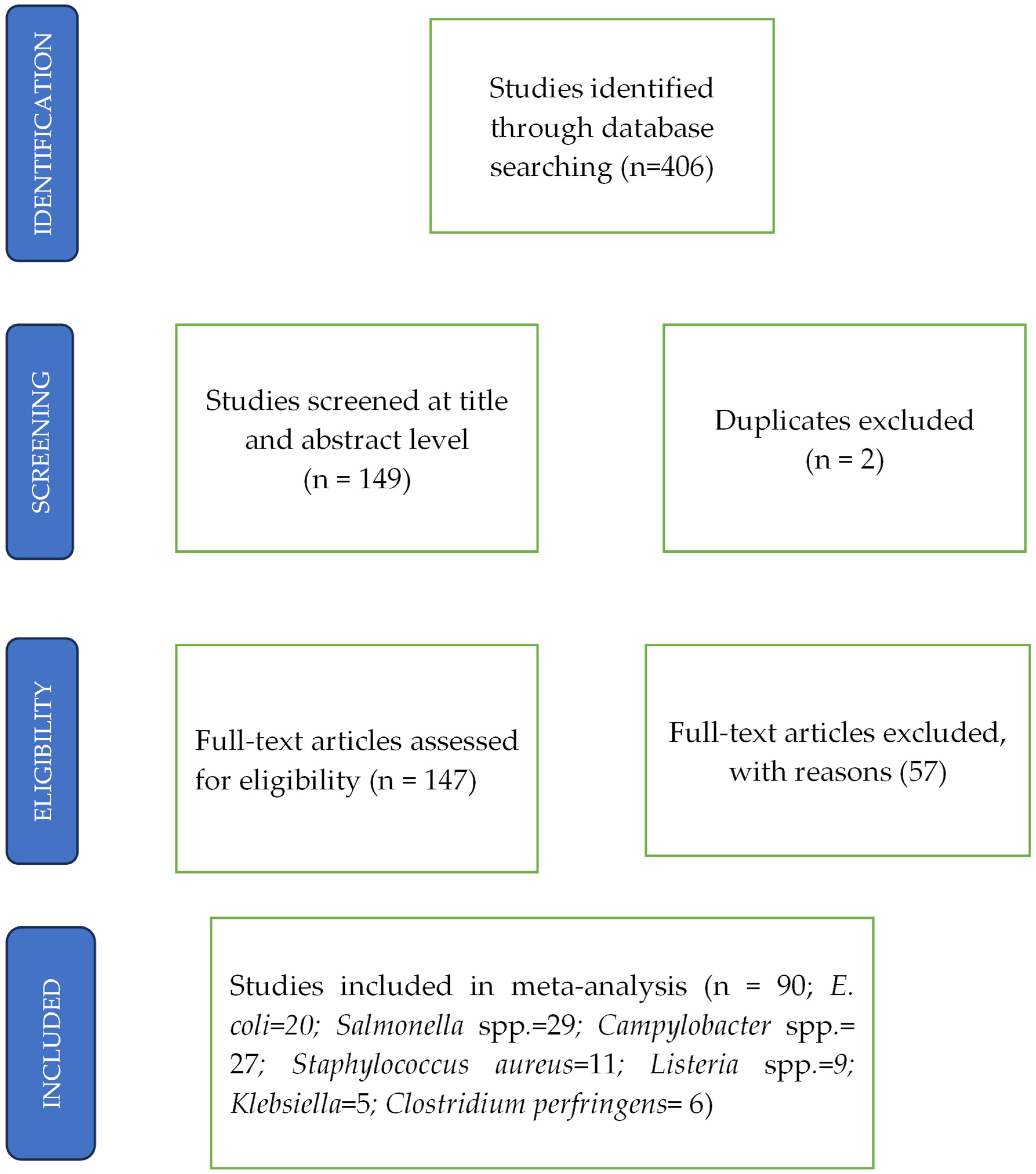
3.1. Search Results and Study Characteristics
3.2. Descriptive Analysis of All Included Studies
3.3. Prevalence of Pathogens in Poultry Meat
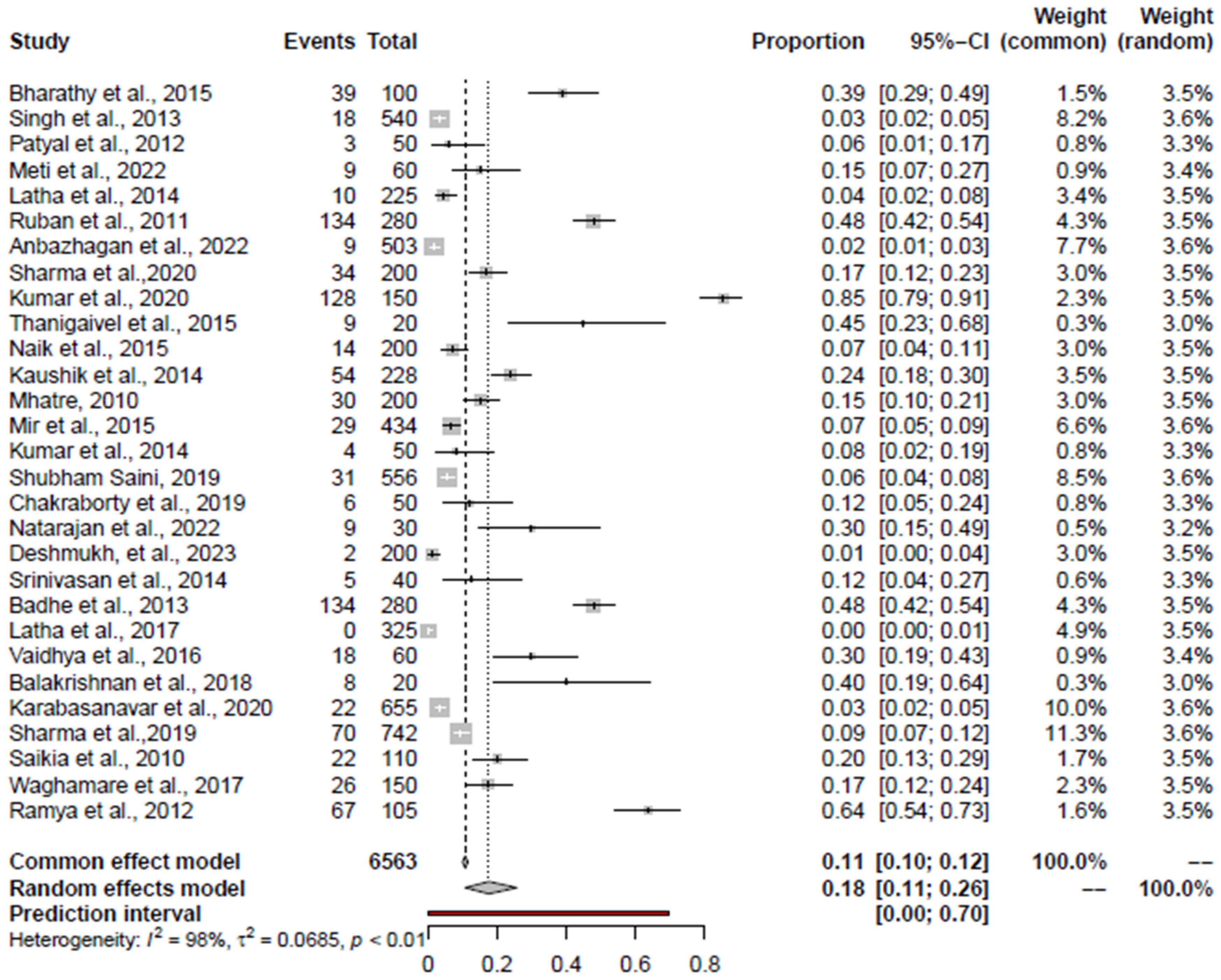
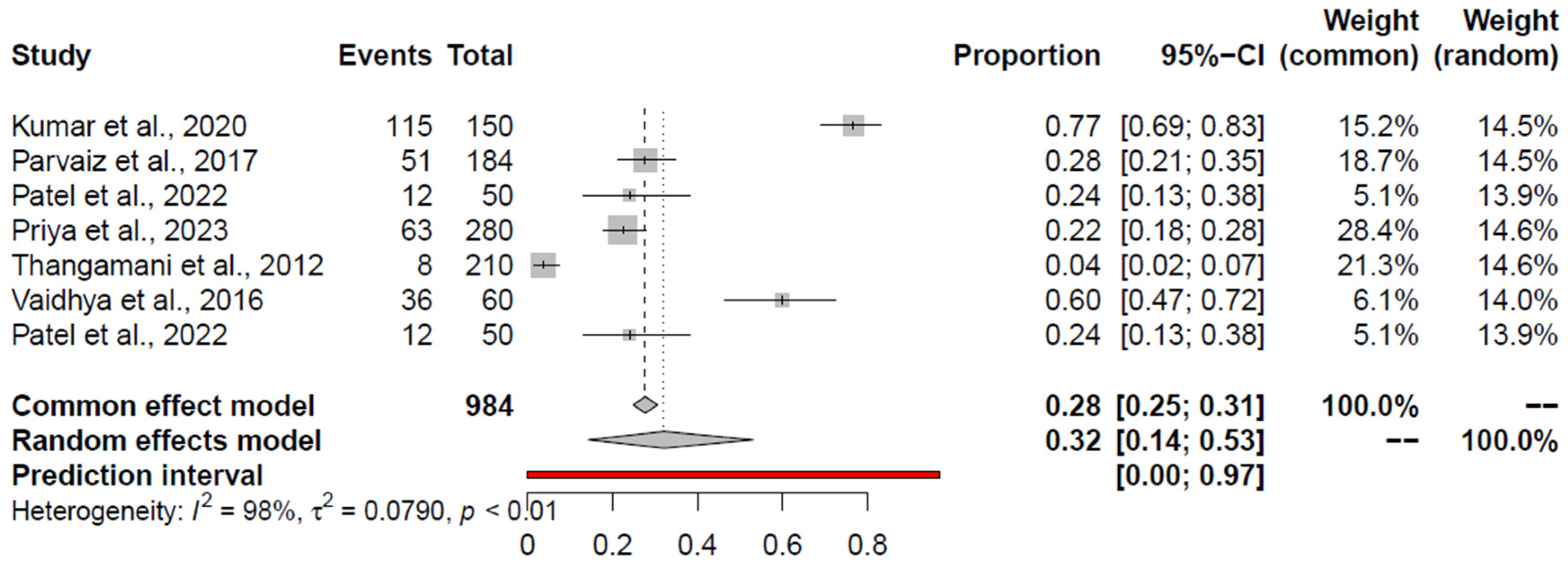
3.4. Pooled Prevalence of Salmonella spp.
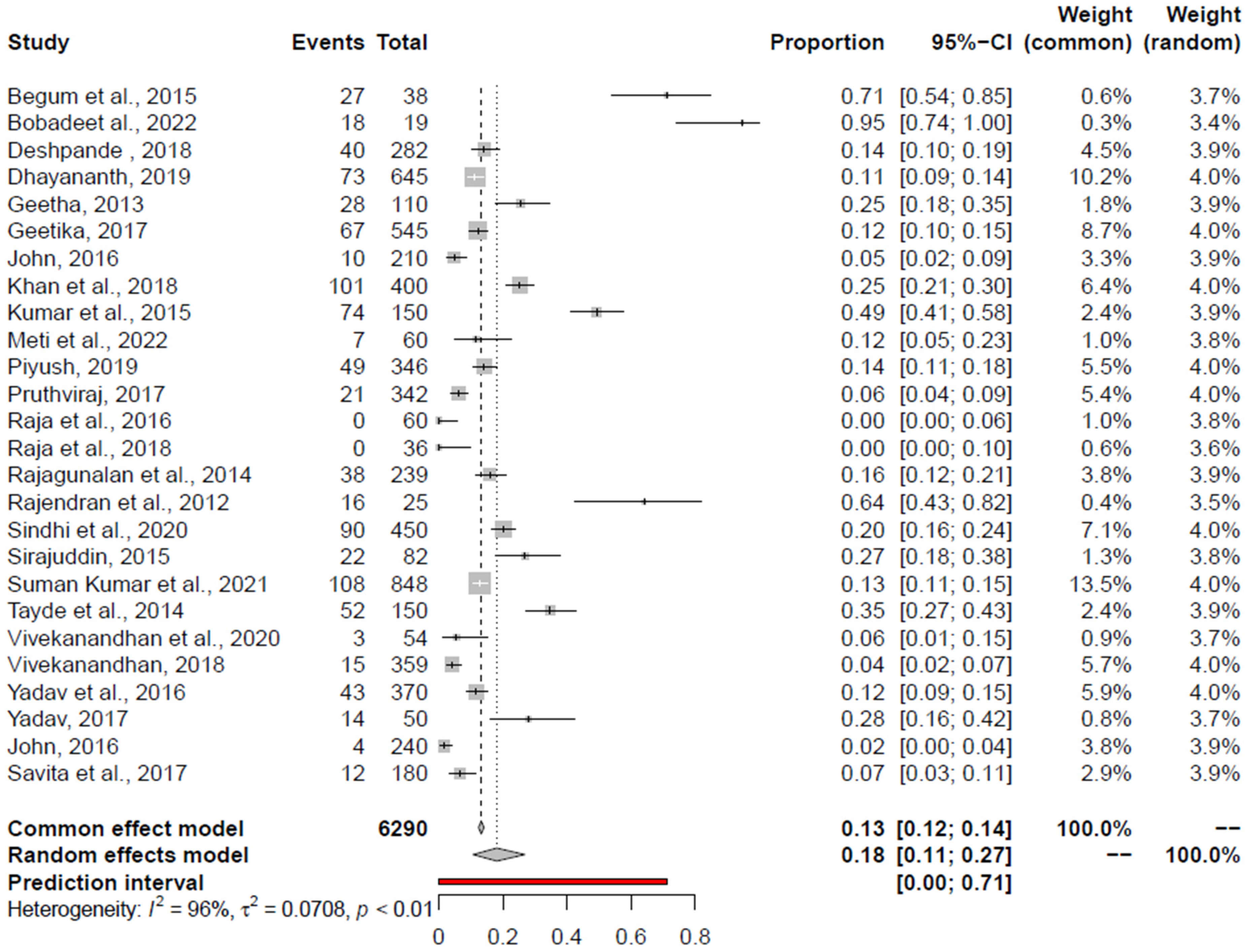
3.5. Pooled Prevalence of Campylobacter spp.
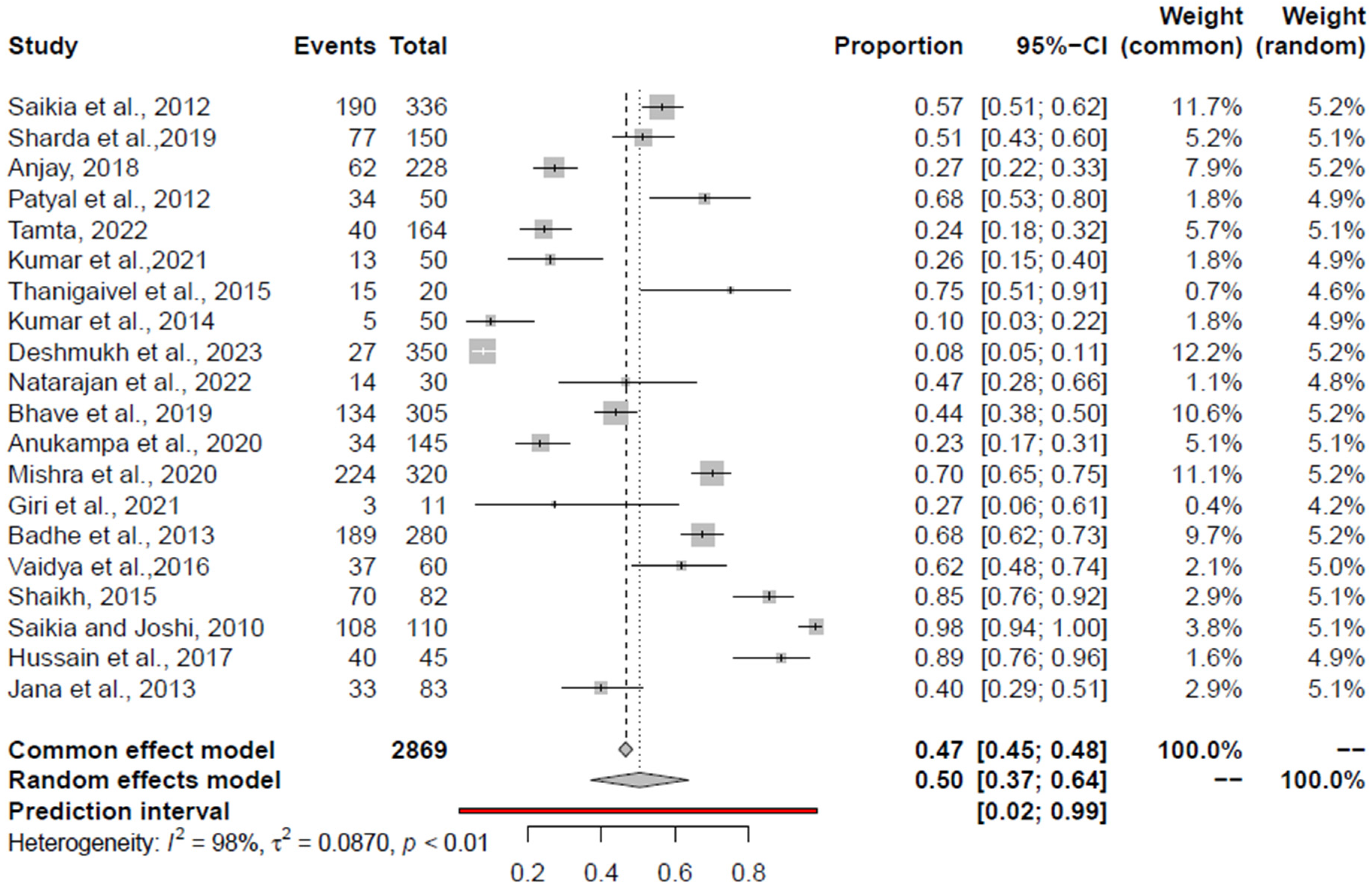
3.6. Pooled Prevalence of E. coli
3.7. Pooled Prevalence of C. perfringens
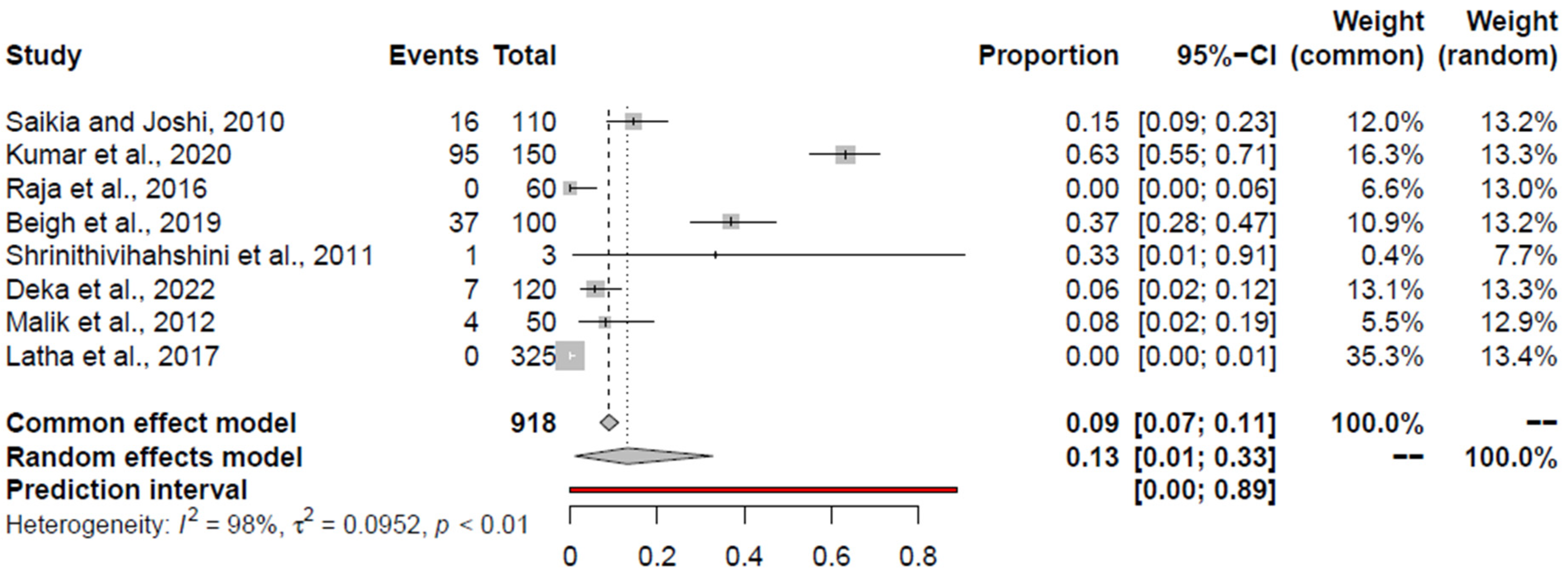
3.8. Pooled Prevalence of Listeria spp.
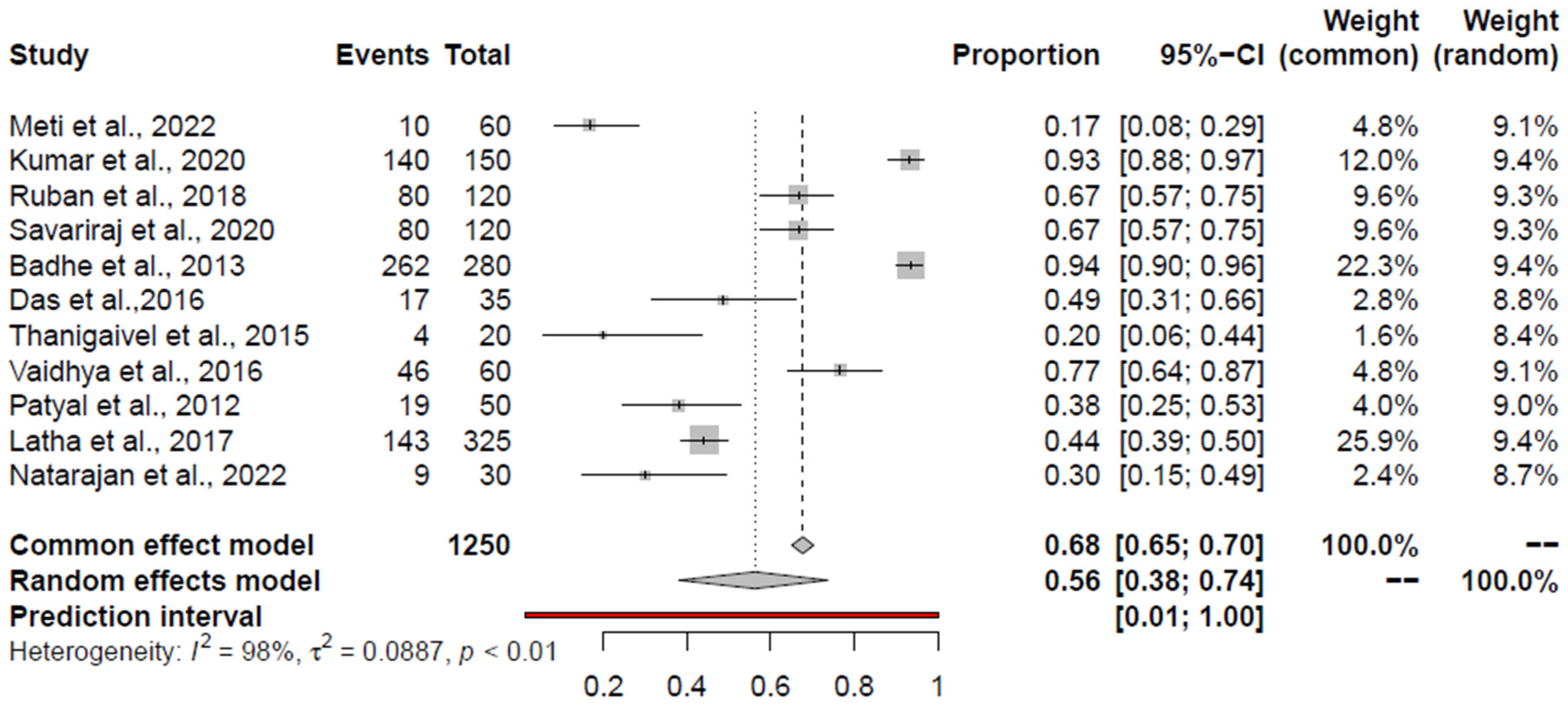
3.9. Pooled Prevalence of S. aureus and K. pneumoniae
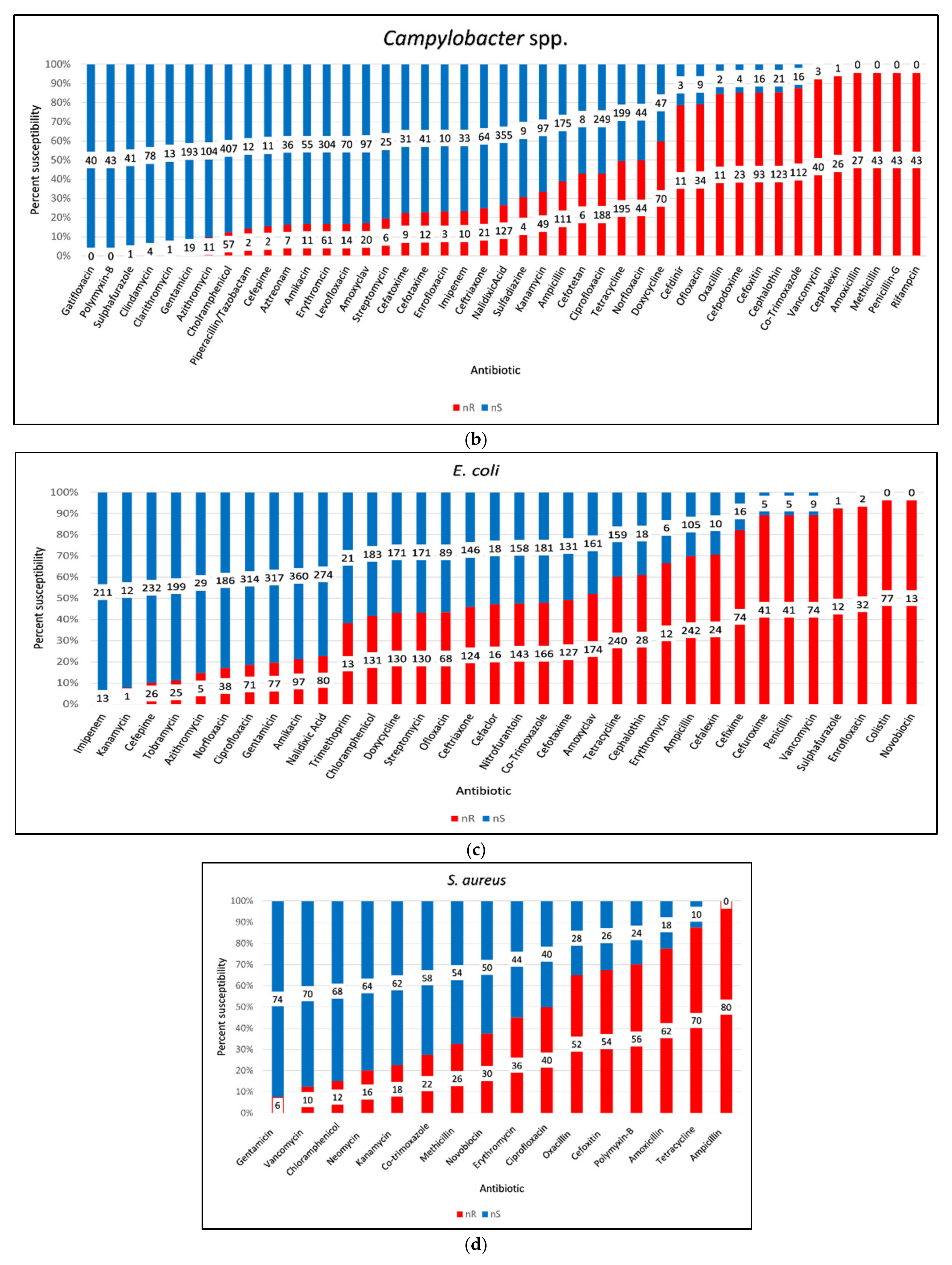
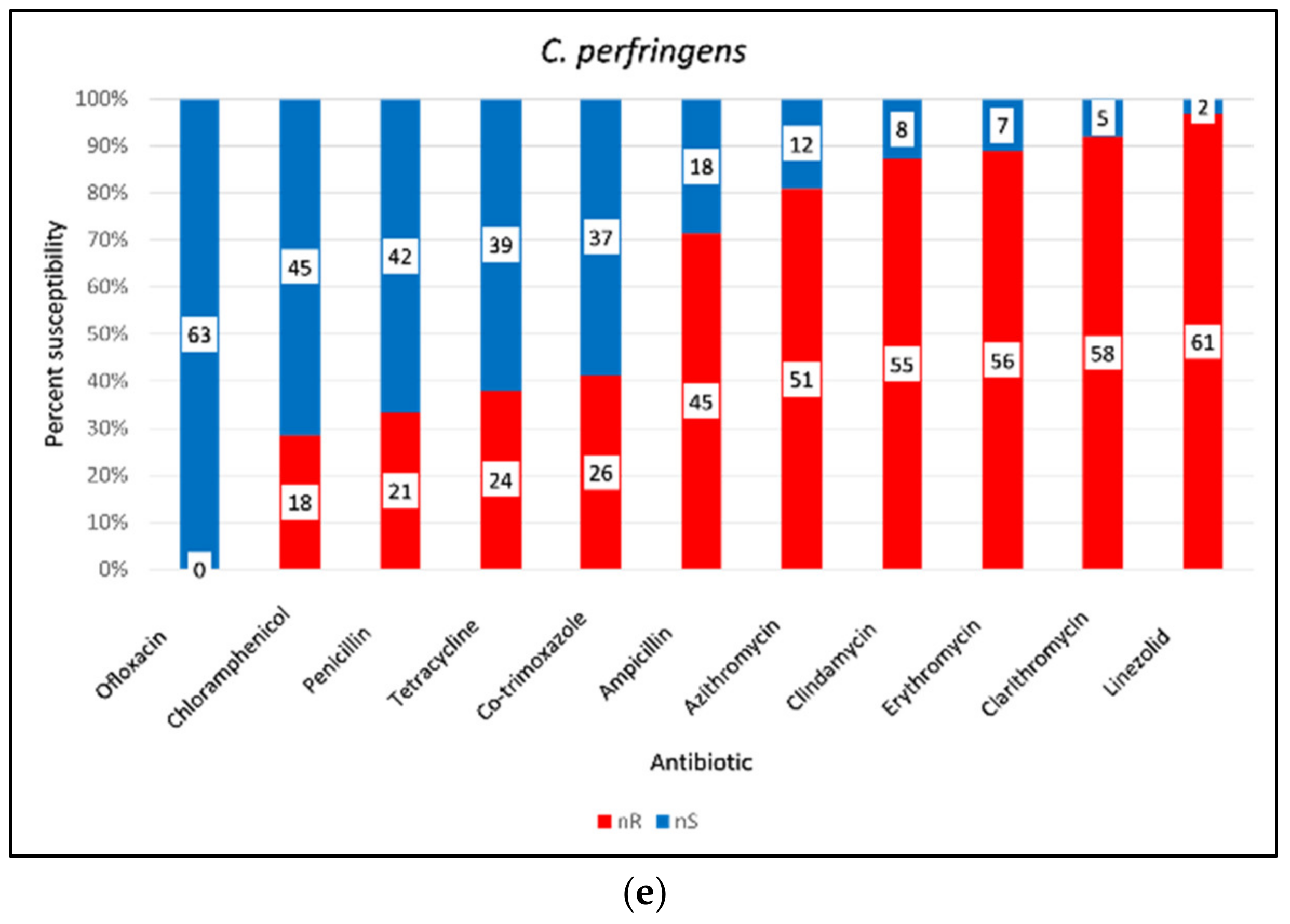
3.10. AMR of Bacterial Pathogens from Retail Chicken Meat and Its Associated Environments
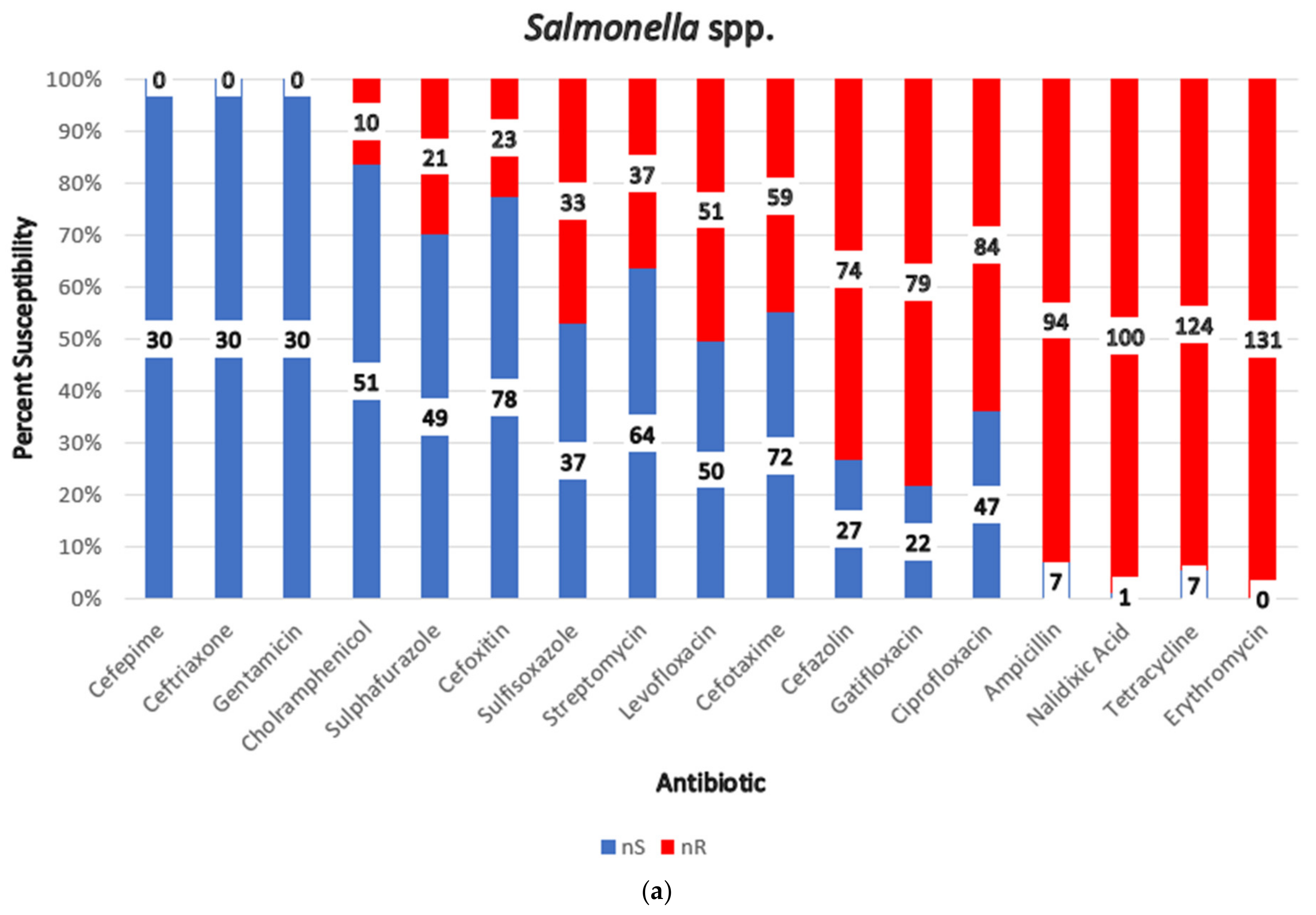
3.11. Antimicrobial Resistance (AMR) of Gram-Negative Bacteria
3.12. Antimicrobial Resistance (AMR) of Gram-Positive Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scudiero, L.; Tak, M.; Alarcón, P.; Shankar, B. Understanding Household and Food System Determinants of Chicken and Egg Consumption in India. Food Sec. 2023, 15, 1231–1254. [Google Scholar] [CrossRef]
- Basford, S.E. Putting Their Eggs in India’s Basket: What Vertical Integration, Church’s Chicken, and Globalization Mean to Increasing Chicken Consumption in India. Purs.-J. Undergrad. Res. Univ. Tenn. 2011, 3, 7. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 27 September 2024).
- Economic Survey. Available online: https://www.indiabudget.gov.in/economicsurvey/ (accessed on 27 September 2024).
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Manual, A. Dietary Guidelines for Indians. Nat. Inst. Nutr. 2011, 2, 89–117. [Google Scholar]
- Mani, R. Livestock and Products Annual—India; USDA Foreign Agricultural Service: Washington, DC, USA, 2021.
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kristkova, Z.S.; Grace, D.; Kuiper, M. The Economics of Food Safety in India: A Rapid Assessment; Wageningen University & Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Grace, D. Burden of Foodborne Disease in Low-Income and Middle-Income Countries and Opportunities for Scaling Food Safety Interventions. Food Sec. 2023, 15, 1475–1488. [Google Scholar] [CrossRef]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.; Angulo, F.; Gibb, H.; Kirk, M.; Lake, R. Attribution of Global Foodborne Disease to Specific Foods: Findings from a World Health Organization Structured Expert Elicitation. PLoS ONE 2017, 12, e0183641. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef]
- Gonçalves-Tenório, A.; Silva, B.N.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Rasschaert, G.; Houf, K.; Godard, C.; Wildemauwe, C.; Pastuszczak-Frak, M.; De Zutter, L. Contamination of Carcasses with Salmonella during Poultry Slaughter. J. Food Prot. 2008, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.; Gould, L.H.; Hall, A.J.; Herman, K.; Vieira, A.R.; Walsh, K.A.; Williams, I.T. Surveillance for Foodborne Disease Outbreaks—United States, 1998–2008. Morb. Mortal. Wkly. Rep. 2013, 62, 1–34. [Google Scholar]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.; Engberg, J. Antimicrobial Resistance of Thermophilic Campylobacter. Vet. Res. 2001, 32, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, D.A.; Korolik, V. Antibiotic Resistance and Resistance Mechanisms in Campylobacter Jejuni and Campylobacter Coli. FEMS Microbiol. Lett. 2007, 277, 123–132. [Google Scholar] [CrossRef]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial Drug Resistance in Escherichia coli from Humans and Food Animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K. Extended-Spectrum β-Lactamase Genes of Escherichia coli in Chicken Meat and Humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-Resistant Escherichia coli from Retail Poultry Meat with Different Antibiotic Use Claims. BMC Microbiol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Petinaki, E.; Spiliopoulou, I. Methicillin-Resistant Staphylococcus aureus among Companion and Food-Chain Animals: Impact of Human Contacts. Clin. Microbiol. Infect. 2012, 18, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.G.; Kuan, C.H.; Loo, Y.Y.; Chang, W.S.; Lye, Y.L.; Soopna, P.; Tang, J.Y.H.; Nakaguchi, Y.; Nishibuchi, M.; Afsah-Hejri, L. Listeria Monocytogenes in Retailed Raw Chicken Meat in Malaysia. Poult. Sci. 2012, 91, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of Foodborne Disease Outbreaks Caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic Resistance in Enterobacteriaceae: Mechanisms and Clinical Implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pu, S.; Jia, X.; Xu, X.; Yang, S.; Shi, J.; Sun, S.; Zhang, L. Multidrug Resistance Mechanisms of Carbapenem Resistant Klebsiella pneumoniae Strains Isolated in Chongqing, China. Ann. Lab. Med. 2017, 37, 398–407. [Google Scholar] [CrossRef][Green Version]
- Foodborne Antimicrobial Resistance; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2022; ISBN 978-92-5-135734-7.
- OECD. Stemming the Superbug Tide: Just A Few Dollars More; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- IHME. Available online: https://www.healthdata.org/sites/default/files/2023-09/India.pdf (accessed on 23 July 2024).
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food-Producing Animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing Heterogeneity in Meta-Analysis: Q Statistic or I2 Index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Harris, R.J.; Deeks, J.J.; Altman, D.G.; Bradburn, M.J.; Harbord, R.M.; Sterne, J.A.C. Metan: Fixed- and Random-Effects Meta-Analysis. Stata J. 2008, 8, 3–28. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch. Public. Health 2014, 72, 39. [Google Scholar] [CrossRef] [PubMed]
- Baujat, B.; Mahé, C.; Pignon, J.; Hill, C. A Graphical Method for Exploring Heterogeneity in Meta-analyses: Application to a Meta-analysis of 65 Trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Method. 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Sangeetha, A.; Dhanalakshmi, M. Prevalence of Salmonella in Chicken Meat and Its Slaughtering Place from Local Markets in Orathanadu, Thanjavur District, Tamil Nadu. J. Entomol. Zool. Stud. 2018, 6, 2468–2471. [Google Scholar]
- Karabasanavar, N.S.; Benakabhat Madhavaprasad, C.; Agalagandi Gopalakrishna, S.; Hiremath, J.; Shivanagowda Patil, G.; Barbuddhe, S.B. Prevalence of Salmonella Serotypes S. Enteritidis and S. Typhimurium in Poultry and Poultry Products. J. Food Saf. 2020, 40, e12852. [Google Scholar] [CrossRef]
- Ruban, S.W.; Fairoze, N. Effect of Processing Conditions on Microbiological Quality of Market Poultry Meats in Bangalore, India. J. Anim. Vet. Adv. 2011, 10, 188–191. [Google Scholar]
- Anbazhagan, P.V.; Thavitiki, P.R.; Varra, M.; Annamalai, L.; Putturu, R.; Lakkineni, V.R.; Pesingi, P.K. Evaluation of Efflux Pump Activity of Multidrug-Resistant Salmonella Typhimurium Isolated from Poultry Wet Markets in India. Infect. Drug Resist. 2019, 12, 1081–1088. [Google Scholar] [CrossRef]
- Chakraborty, S.; Roychoudhury, P.; Samanta, I.; Subudhi, P.K.; Das, M.; De, A.; Bandyopadhayay, S.; Joardar, S.N.; Mandal, M.; Qureshi, A. Molecular Detection of Biofilm, Virulence and Antimicrobial Resistance Associated Genes of Salmonella Serovars Isolated from Pig and Chicken of Mizoram, India. Indian J. Anim. Res. 2020, 54, 608–613. [Google Scholar] [CrossRef]
- Meti, M.; Daimary, B.; Rao, V.A. Detection of food-borne pathogens in chicken meat sold in retail outlets of chennai city. Asian J. Microbiol. Biotechnol. Environ. Sci. 2022, 24, 137–143. [Google Scholar] [CrossRef]
- Bharathy, S.; Swetha, C.S.; Sudhanthirakodi, S. A Prospective Study on Antibiogram Pattern for Salmonella Isolated from Poultry Origin and Milk Samples of Local Chicken Retailers and Local Vendors in Tirupathi, India. Int. J. Agric. Sci. Vet. Med. 2015, 3, 11–16. [Google Scholar]
- Sharma, J.; Kumar, D.; Hussain, S.; Pathak, A.; Shukla, M.; Kumar, V.P.; Anisha, P.N.; Rautela, R.; Upadhyay, A.K.; Singh, S.P. Prevalence, Antimicrobial Resistance and Virulence Genes Characterization of Nontyphoidal Salmonella Isolated from Retail Chicken Meat Shops in Northern India. Food Control 2019, 102, 104–111. [Google Scholar] [CrossRef]
- Saini, S. Molecular Characterization of Non-Typhoidal Salmonella Serovars Isolated from Commercial Broiler Farms and Retail Chicken Meat Shops with Reference to Virulence and Antimicrobial Resistance. Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar, India, 2019. [Google Scholar]
- Renu Singh, R.S.; Yadav, A.S.; Tripathi, V.; Singh, R.P. Antimicrobial Resistance Profile of Salmonella Present in Poultry and Poultry Environment in North India. Food Control 2013, 33, 545–548. [Google Scholar] [CrossRef]
- Saikia, P.; Joshi, S.R. Retail Market Poultry Meats of North-East India—A Microbiological Survey for Pathogenic Contaminants. Res. J. Microbiol. 2010, 5, 36–43. [Google Scholar] [CrossRef][Green Version]
- Mhatre, S.D. Isolation, Identification and Molecular Characterization of Salmonella Isolates of Poultry Meat. Ph.D. Thesis, Anand Agricultural University, Anand, India, 2010. [Google Scholar]
- Irfan, M.A.; Sudhir, K.K.; Sunil, M. Isolation, Serotype Diversity and Antibiogram of Salmonella Enterica Isolated from Different Species of Poultry in India. Asian Pac. J. Trop. Biomed. 2015, 553–559. [Google Scholar]
- Kumar, P.V.; Singh, S.; Krishnaiah, N.; Shashi Kumar, M.; Kala Kumar, B. Incidence of Food Borne Pathogens of Chicken Sold in and around Greater Hyderabad Municipal Corporation. Pharma Innov. J. 2020, 9, 214–217. [Google Scholar]
- Srinivasan, P.; Balasubramaniam, G.A.; Gopala, T.R.; Murthy, K.; Saravanan, S.; Balachandran, P. Prevalence and Pathology of Salmonellosis in Commercial Layer Chicken from Namakkal, India. Pak. Vet. J. 2014, 34, 324–328. [Google Scholar]
- Sharma, V.; Sharma, S.; Verma, A.; Dahiya, D.K.; Karnani, M. Feed Safety Evaluation for Prevalence of Zoonotic Salmonella spp. in Animal Feed. Indian J. Anim. Sci. 2020, 90, 17–21. [Google Scholar] [CrossRef]
- Badhe, S.R.; Fairoze, N.; Sudarshan, S. Prevalence of Food Borne Pathogens in Market Samples of Chicken Meat in Bangalore, India. Indian J. Anim. Res. 2013, 47, 262–264. [Google Scholar]
- Kumar, P.; Rao, J.; Haribabu, Y. Microbiological Quality of Meat Collected from Municipal Slaughter Houses and Retail Meat Shops from Hyderabad Karnataka Region, India. APCBEE Procedia 2014, 8, 364–369. [Google Scholar] [CrossRef]
- Thanigaivel, G.; Anandhan, A.S. Isolation and Characterization of Microorganisms from Raw Meat Obtained from Different Market Places in and around Chennai. J. Pharm. Chem. Biol. Sci. 2015, 3, 295–301. [Google Scholar]
- Vaidya, D.N.; Ghugare, P.S.; Kutty, M. Prevalence of Pathogens in Raw Chicken Sold at Retail Poultry Shops in Pune City, India. J. Glob. Biosci. 2016, 5, 3970–3975. [Google Scholar]
- Patyal, A.; Gangil, R.; Singh, P.K.; Mathur, K.N.; Sudan, V. Bacteriological Quality of Market Chicken Meat in Jaipur City. J. Vet. Public Health 2012, 10, 45–48. [Google Scholar]
- Babar, A.; Suryawanshi, R.; Deshmukh, O.; Kurkure, N.; Patil, V.; Devangare, A.; Kulkarni, R. Occurrence, Characterization and Antimicrobial Resistance Pattern of Pathogenic Salmonella Isolated from Chicken and Chicken Meat Products. Indian J. Vet. Sci. Biotechnol. 2023, 19, 29–33. [Google Scholar]
- Kaushik, P.; Anjay, A.; Kumari, S.; Dayal, S.; Kumar, S. Antimicrobial Resistance and Molecular Characterisation of E. coli from Poultry in Eastern India. Vet. Ital. 2018, 54, 197–204. [Google Scholar] [PubMed]
- Anju, P.; Latha, C.; Sunil, B.; Sethulekshmi, C. Detection of Salmonella and Yersinia spp. in Uncooked Retail Chicken Meat in Kerala by Multiplex PCR. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 1028–1034. [Google Scholar]
- Waghamare, R.N.; Paturkar, A.M.; Zende, R.J.; Vaidya, V.M.; Gandage, R.S.; Aswar, N.B.; Khilari, R.S. Studies on Occurrence of Invasive Salmonella spp. from Unorganised Poultry Farm to Retail Chicken Meat Shops in Mumbai City, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 630–641. [Google Scholar] [CrossRef][Green Version]
- Ramya, P.; Madhavarao, T.; Rao, L.V. Study on the Incidence of Salmonella Enteritidis in Poultry and Meat Samples by Cultural and PCR Methods. Vet. World 2012, 5, 541–545. [Google Scholar] [CrossRef]
- Naik, V.K.; Shakya, S.; Patyal, A.; Gade, N.E. Isolation and Molecular Characterization of Salmonella spp. from Chevon and Chicken Meat Collected from Different Districts of Chhattisgarh, India. Vet. World 2015, 8, 702. [Google Scholar] [CrossRef]
- Latha, C.; Anu, C.J.; Ajaykumar, V.J.; Sunil, B. Prevalence of Listeria monocytogenes, Yersinia enterocolitica, Staphylococcus aureus, and Salmonella enterica Typhimurium in Meat and Meat Products Using Multiplex Polymerase Chain Reaction. Vet. World 2017, 10, 927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natarajan, S.; Nagarajan, B.; DineshKumar, R.; Ravichandran, M.; Muniasamy, P.; Chandrasekar, V. Occurrence of Multi Drug Resistant Bacteria from Raw Chicken Meat of South India Retail Markets. Int. J. Life Sci. Pharma Res. 2022, 12, L105–L111. [Google Scholar] [CrossRef]
- Dhayananth, B. Burden Assessment and Characterization of Thermophilic Campylobacter in Broiler Chickens. Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar, India, 2019. [Google Scholar]
- Yadav, R.; Maherchandani, S. Virulence Characterization of Campylobacter Jejuni Isolated from Poultry in India. Int. J. Livest. Res. 2020, 10, 132–140. [Google Scholar] [CrossRef]
- Yadav, M. Occurrence and Molecular Characterization of Campylobacter Species in Chicken Meat. Ph.D. Thesis, Lala Lajpat Rai University Of Veterinary And Animal Sciences, Hisar, India, 2017. [Google Scholar]
- Pruthviraj, T.N. Molecular Epidemiology of Campylobacter Species from Poultry and Humans. Ph.D. Thesis, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India, 2017. [Google Scholar]
- Rajagunalan, S.; Bisht, G.; Pant, S.; Singh, S.P.; Singh, R.; Dhama, K. Prevalence and Molecular Heterogeneity Analysis of Campylobacter jejuni and Campylobacter coli Isolated from Human, Poultry and Cattle. Pantnagar India Vet. Arh. 2014, 84, 493–504. [Google Scholar]
- Vivekanandhan, R.; Dubal, Z.B.; Muralikrishna, P.; Arun Prince Milton, A.; Kadwalia, A.; Vengatachalam, M.; Priyadharshini, R. Occurrence of Thermophilic Campylobacter spp. in Free-Ranging Chickens. J. Entomol. Zool. Stud. 2020, 8, 534–537. [Google Scholar]
- Deshpande, P.R. Identification and Antimicrobial Resistance of Campylobacter Species from Poultry. Ph.D. Thesis, Maharashtra Animal & Fishery Sciences University, Nagpur, India, 2018. [Google Scholar]
- Suman Kumar, M.; Ramees, T.P.; Dhanze, H.; Gupta, S.; Dubal, Z.B.; Kumar, A. Occurrence and Antimicrobial Resistance of Campylobacter Isolates from Broiler Chicken and Slaughter House Environment in India. Anim. Biotechnol. 2023, 34, 199–207. [Google Scholar] [CrossRef]
- Rajendran, P.; Babji, S.; George, A.T.; Rajan, D.P.; Kang, G.; Ajjampur, S.S. Detection and Species Identification of Campylobacter in Stool Samples of Children and Animals from Vellore, South India. Indian J. Med. Microbiol. 2012, 30, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Tayde, R.S.; Brahmbhatt, M.N. Biotyping of Thermophilic Campylobacter spp. Isolated from Poultry in and around Anand City, Gujarat, India. Vet. World 2014, 7, 321–324. [Google Scholar] [CrossRef]
- Geetha, M. Molecular Identification of Campylobacter jejuni in Chicken Meat by Polymerase Chain Reaction. Indian Vet. J. 2013, 90, 87. [Google Scholar]
- Sindhi, S.; Mathapati, B.; Parmar, V.; Marandi, S.; Sumankumar, M.; Javia, B.; Ghodasara, S.N.; Kathiriya, J.B.; Bhedi, K.R.; Patel, J.S. Prevalence of Campylobacter spp. Isolated from Poultry, Human, and Environment in Junagadh District of Gujarat, India. Int. J. Curr. Microbiol. App Sci. 2020, 9, 3319–3326. [Google Scholar] [CrossRef]
- Garhia, G. Studies on Prevalence, Virulence Genes and Antimicrobial Resistance of Thermophilic Campylobacters Isolated from Poultry Farms of Kumaon Region. Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar, India, 2017. [Google Scholar]
- Bisht, P. Isolation, Characterization and Prevalence of Thermophilic Campylobacters in Poultry Farms and Meat Vendors Using Novel Enrichment Method. Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar, India, 2019. [Google Scholar]
- Vivekanandhan, R. Antimicrobial Resistance Profile of Thermophilic Campylobacter spp. from Different Poultry Settings. Ph.D. Thesis, Indian Veterinary Research Institute, Izatnagar, India, 2018. [Google Scholar]
- Begum, S.; Sekar, M.; Gunaseelan, L.; Gawande, M.; Suganya, G.; Malar, P.A.S.; Karthikeyan, A. Molecular Identification of Campylobacter jejuni and C. coli from Chicken, Calves and Dogs to Determine Its Potential Threat on Human Being. Vet. World 2015, 8, 1420. [Google Scholar] [CrossRef][Green Version]
- Shaikh, S.M. Prevalence, Characterization and Antibiotic Resistance of Campylobacter jejuni and E. coli from Chicken Meat Sold in and Around Parbhani City. Ph.D. Thesis, Maharashtra Animal & Fishery Sciences University, Nagpur, India, 2015. [Google Scholar]
- Raja, S.S.; Apparao, V.; Narendra Babu, R.; Balamurugan, N. Detecting the Occurrence of Campylobacter jejuni in Chicken Meat by PCR in Retail Outlets of Chennai, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 4174–4177. [Google Scholar] [CrossRef]
- Begum, S.; Sekar, M.; Gunaseelan, L. Antimicrobial susceptibility pattern of thermophilic Campylobacter species from chicken, calves and dog. J. Dis. Glob. Health 2015, 3, 44–48. [Google Scholar]
- Yadav, R.; Gahlot, K.; Yadav, J.; Purva, M.; Bhati, T.; Deora, A.; Kumar, P.; Maherchandani, S.; Kashyap, S.K. Prevalence of Thermophilic Campylobacter jejuni Isolated from Cloacal Sample of Poultry. Haryana Vet. 2016, 55, 195–197. [Google Scholar]
- Raja, S.; Rao, V.A.; Babu, R.N. Detection of Emerging Food Pathogens in Chicken Meat Using Multiplex Polymerase Chain Reaction. J. Agric. Sci. 2016, 8, 217. [Google Scholar] [CrossRef][Green Version]
- Joby, E.J. Occurrence of Campylobacter spp. in Chicken Egg Production Chain. Ph.D. Thesis, College of Veterinary and Animal Sciences-Mannuthy, Thrissur, India, 2016. [Google Scholar]
- Savita, R.K.; Latha, C.; Sunil, B.; Sunanda, C. Occurrence of campylobacter species in chicken egg from central Kerala. J. Vet. Anim. Sci. 2018, 49, 53–58. [Google Scholar]
- Bobade, S.; Vijayarani, K.; Tirumurugaan, K.G.; Thangavelu, A.; Vairamuthu, S. Morphological, Biochemical and Genotypic Analysis of Zoonotic Campylobacter jejuni Isolated from Chicken Meat Samples. Indian J. Anim. Res. 2024, 58, 478–483. [Google Scholar] [CrossRef]
- Khan, J.A.; Rathore, R.S.; Abulreesh, H.H.; Qais, F.A.; Ahmad, I. Prevalence and Antibiotic Resistance Profiles of Campylobacter jejuni Isolated from Poultry Meat and Related Samples at Retail Shops in Northern India. Foodborne Pathog. Dis. 2018, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.; Kolhe, R.; Mahadevaswamy, R.; Bhong, C.; Jadhav, S.; Nalband, S.; Gandhale, D.; Muglikar, D. Phylogrouping and Antimicrobial Resistance Analysis of Extraintestinal Pathogenic Escherichia coli Isolated from Poultry Species. Turk. J. Vet. Anim. Sci. 2019, 43, 117–126. [Google Scholar] [CrossRef]
- Hussain, A.; Shaik, S.; Ranjan, A.; Nandanwar, N.; Tiwari, S.K.; Majid, M.; Baddam, R.; Qureshi, I.A.; Semmler, T.; Wieler, L.H. Risk of Transmission of Antimicrobial Resistant Escherichia coli from Commercial Broiler and Free-Range Retail Chicken in India. Front. Microbiol. 2017, 8, 2120. [Google Scholar] [CrossRef]
- Kumar, S.; Anwer, R.; Yadav, M.; Sehrawat, N.; Kumar, V.; Sharma, A.K. Isolation and Characterization of Acinetobacter baumannii from Chicken Meat Samples in North India. Asian J. Biol. Life Sci. 2021, 10, 462–468. [Google Scholar] [CrossRef]
- Singh, A.; Chhabra, D.; Sharda, R.; Shukla, S.; Audarya, S.D.; Sikrodia, R.; Gangil, R.; Singh, N. Antibiotic Resistance in E. coli Isolated from Poultry. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 89–94. [Google Scholar] [CrossRef]
- Senapati, I.A.; Mishra, R.; Kundu, A.K.; Mishra, B.P.; Rath, P.K. Prevalence and Characterization of Escherichia coli from Poultry Meat in Bhubaneswar. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 2047–2055. [Google Scholar] [CrossRef]
- Saikia, P.; Joshi, S.R. A Study on the Occurrence of Non-O157 Shiga Toxin Producing Escherichia coli Isolates in Retail Chicken Meats Marketed in North-East India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 337–342. [Google Scholar] [CrossRef]
- Jana, A.; Mondal, A. Serotyping, Pathogenicity and Antibiogram of Escherichia coli Isolated from Raw Poultry Meat in West Bengal, India. Vet. Ital. 2013, 49, 361–365. [Google Scholar]
- Giri, S.; Kudva, V.; Shetty, K.; Shetty, V. Prevalence and Characterization of Extended-Spectrum β-Lactamase-Producing Antibiotic-Resistant Escherichia coli and Klebsiella pneumoniae in Ready-to-Eat Street Foods. Antibiotics 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Tamta, S. Epidemiological Analysis of Herbal and Conventional Antimicrobial Drug Resistance Patterns of E. coli and K. pneumoniae Isolates from Foods of Animal Origin. Ph.D. Thesis, Indian Veterinary Research Institute, Izatnagar, India, 2022. [Google Scholar]
- Anukampa, S.K.; Vivekanandhan, R.; Bhoomika, B.A.M.; Abass, G.; Singh, S.; Dosar, S.; OR, V.K.; Dubal, Z.B. Presence of Escherichia coli and Klebsiella pneumonia in Foods of Animal Origin Sold at. J. Vet. Public Health 2020, 18, 151–156. [Google Scholar]
- Deshmukh, O.; Suryawanshi, R.; Kurkure, N.; Kaore, M.; Badar, S.; Shinde, O.; Awandkar, S.; Gaikwad, N. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from poultry and poultry products. Indian J. Ani. Sci. 2023, 93, 431–436. [Google Scholar] [CrossRef]
- Ruban, S.W.; Babu, R.N.; Abraham, R.J.; Senthilkumar, T.M.A.; Kumaraswamy, P.; Porteen, K.; Vemala, G. Prevalence and Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Retail Chicken Meat in Chennai, India. J. Anim. Res. 2018, 8, 423–427. [Google Scholar] [CrossRef]
- Ruban, S.W.; Ravindran, N.B.; Kannan, P.; Rao, V.A. Occurrence and Enterotoxin Gene Profiles of Staphylococcus aureus Isolated from Retail Chicken Meat. Food Sci. Technol. Int. 2021, 27, 619–625. [Google Scholar] [CrossRef]
- Das, P.; Mazumder, P.B. Prevalence of Staphylococcus in Raw Meat Samples in Southern Assam, India. J. Agric. Vet. Sci. 2016, 9, 23–29. [Google Scholar]
- Malla, B.A.; Malik, S.V.S.; Dixit, B.; Tamta, S. Screening of Poultry Meat and Caecal Samples for Listeria Species Using Isolation, Bio-Chemical Characterization and Latex Agglutination Assay. J. Vet. Public Health 2019, 17, 110–114. [Google Scholar]
- Beigh, Q.; Shakya, S.; Patyal, A.; Ali, S.L.; Bhonsle, D. Isolation, Identification and Antibiotic Susceptibility Profiling of Listeria spp. from Raw Chicken Meat in Durg District of Chhattisgarh, India. J. Anim. Res. 2019, 9, 543–549. [Google Scholar] [CrossRef]
- Shrinithivihahshini, N.; Mariyaselvam, S.; Duraisamy, M.; Rengaraj, C. Occurrence of Listeria Monocytogenes in Food and Ready to Eat Food Products Available in Tiruchirappalli, Tamil Nadu, India. World J. Life Sci. Med. Res. 2011, 1, 70–75. [Google Scholar]
- Deka, A.; Hazarika, R.A.; Barua, A.G.; Saikia, G.K.; Borah, P.; Shakuntala, I.; Roychoudhury, P.; Bora, D.P. Prevalence of Listeria Monocytogenes in Foods of Animal Origin: Study from Assam, a North-Eastern State of India. Eur. J. Vet. Med. 2022, 2, 20–25. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, J.K.; Neelesh Sharma, N.S. Prevalence of Listeria monocytogenes in Different Types of Meat. Vet. Pract. 2012, 13, 204–205. [Google Scholar]
- Thangamani, A.; Subramanian, S. Prevalence of Clostridium perfringens in the Chicken Meat Rendered at Retail Outlets of Namakkal, Tamilnadu. J. Adv. Vet. Res. 2012, 2, 157–159. [Google Scholar]
- Dar, P.S.; Wani, S.A.; Wani, A.H.; Hussain, I.; Maqbool, R.; Ganaie, M.Y.; Kashoo, Z.A.; Qureshi, S. Isolation, Identification and Molecular Characterization of Clostridium perfringens from Poultry in Kashmir Valley, India. J. Entomol. Zool. Stud. 2017, 5, 409–414. [Google Scholar]
- Patel, N.M. Prevalence and Antimicrobial Resistance Clostridium perfringens from Various Meat Samples in Anand, Gujarat. Int. J. Agric. Sci. 2022, 14, 11840–11843. [Google Scholar]
- Priya, G.B.; Srinivas, K.; Shilla, H.; Milton, A.A.P. High Prevalence of Multidrug-Resistant, Biofilm-Forming Virulent Clostridium perfringens in Broiler Chicken Retail Points in Northeast India. Foods 2023, 12, 4185. [Google Scholar] [CrossRef]
- Tewari, R.; Mitra, S.; Venugopal, N.; Das, S.; Ganaie, F.; Sen, A.; Shome, R.; Rahman, H.; Shome, B.R. Phenotypic and Molecular Characterization of Extended Spectrum β-Lactamase, Ampc β-Lactamase and Metallo β-Lactamase Producing Klebsiella spp. from Farm Animals in India. Indian J. Anim. Res. 2019, 53, 938–943. [Google Scholar]
- Modh, K.S.; Parmar, B.C.; Chaudhary, J.H.; Bhanderi, B.B.; Nayak, J.B.; Thakur, S.; Pargi, Z.B.; Patel, N.M. Molecular Detection of Klebsiella spp. from Poultry Meat. Ind. J. Pure App. Biosci. 2021, 9, 511–518. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Guidance on the Requirements for the Development of Microbiological Criteria. EFS2 2017, 15, e05052. [Google Scholar] [CrossRef]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae Counts on Pig and Ruminant Carcasses along the Slaughterline, Factors Influencing the Counts and Relationship between Visual Faecal Contamination of Carcasses and Counts: A Review. EFS3 2014, 11, EN-634. [Google Scholar] [CrossRef]
- Pitout, J.D. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Olszak, T.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Futoma-Koloch, B.; Gawel, A.; Drulis-Kawa, Z.; Choroszy-Krol, I. Comparative Characteristics and Pathogenic Potential of Escherichia coli Isolates Originating from Poultry Farms, Retail Meat, and Human Urinary Tract Infection. Life 2022, 12, 845. [Google Scholar] [CrossRef]
- Althaus, D.; Zweifel, C.; Stephan, R. Analysis of a Poultry Slaughter Process: Influence of Process Stages on the Microbiological Contamination of Broiler Carcasses. Ital. J. Food Saf. 2017, 6, 7097. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Capek, M.; Stephan, R. Microbiological Contamination of Cattle Carcasses at Different Stages of Slaughter in Two Abattoirs. Meat Sci. 2014, 98, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Generic HACCP Model for Poultry Slaughter. International Meat and Poultry HACCP Alliance. Kansas City, MI, USA, 1996. Available online: https://ehaccp.org/ehaccpdocs/USDA/USDA%20Generic%20HACCP%20Model%20for%20Poultry%20Slaughter.pdf (accessed on 30 August 2024).
- Gunjan; Himanshu; Mukherjee, R.; Vidic, J.; Manzano, M.; Leal, E.; Raj, V.S.; Pandey, R.P.; Chang, C.-M. Comparative Meta-Analysis of Antimicrobial Resistance from Different Food Sources along with One Health Approach in the Egypt and UK. BMC Microbiol. 2023, 23, 291. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.; Ramaili, T.; Lekota, K.E.; Ndou, R.; Mphuti, N.; Bezuidenhout, C.; Thekisoe, O. A Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Profile of Escherichia coli Isolated from Water in Africa (2000–2021). Heliyon 2023, 9, e16123. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Shu, G.; Qiu, J.; Zheng, Y.; Chang, L.; Li, H.; Xu, F.; Zhang, W.; Yin, L.; Fu, H.; Yan, Q. Association between Phenotypes of Antimicrobial Resistance, ESBL Resistance Genes, and Virulence Genes of Salmonella Isolated from Chickens in Sichuan, China. Animals 2023, 13, 2770. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Plasmid-Mediated Antimicrobial Resistance in Salmonella Enterica. Curr. Issues Mol. Biol. 2003, 5, 113–122. [Google Scholar] [CrossRef]
- Moser, A.I.; Kuenzli, E.; Campos-Madueno, E.I.; Büdel, T.; Rattanavong, S.; Vongsouvath, M.; Hatz, C.; Endimiani, A. Antimicrobial-Resistant Escherichia coli Strains and Their Plasmids in People, Poultry, and Chicken Meat in Laos. Front. Microbiol. 2021, 12, 708182. [Google Scholar] [CrossRef]
- Zhang-Barber, L.; Turner, A.K.; Barrow, P.A. Vaccination for Control of Salmonella in Poultry. Vaccine 1999, 17, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and Prebiotics in Animal Feeding for Safe Food Production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.T. A Review of Practical Salmonella Control Measures in Animal Feed. J. Appl. Poult. Res. 2011, 20, 102–113. [Google Scholar] [CrossRef]
- Kotzekidou, P. Factors Influencing Microbial Safety of Ready-to-Eat Foods. In Food Hygiene and Toxicology in Ready-to-Eat Foods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 33–50. [Google Scholar]
- Carron, M.; Alarcon, P.; Karani, M.; Muinde, P.; Akoko, J.; Onono, J.; Fèvre, E.M.; Häsler, B.; Rushton, J. The Broiler Meat System in Nairobi, Kenya: Using a Value Chain Framework to Understand Animal and Product Flows, Governance and Sanitary Risks. Prev. Vet. Med. 2017, 147, 90–99. [Google Scholar] [CrossRef]
- Food Safety and Standards (Food Products Standards and Food Additives) Regulations. 2011. Available online: https://fssai.gov.in/upload/uploadfiles/files/Compendium_Food_Additives_Regulations_13_09_2021.pdf (accessed on 30 August 2024).
- Food Safety and Standards (Licensing and Registration of Food Businesses), Regulations 2011. Available online: https://fssai.gov.in/upload/uploadfiles/files/Compendium_Licensing_Regulations.pdf (accessed on 30 August 2024).
- Gulati, A.; Juneja, R. Poultry Revolution in India: Lessons for Smallholder Production Systems; ZEF Working Paper Series, No. 225; University of Bonn, Center for Development Research (ZEF): Bonn, Germany, 2023. [Google Scholar]
- Kaushik, P.; Kumari, S.; Bharti, S.K.; Dayal, S. Isolation and Prevalence of Salmonella from Chicken Meat and Cattle Milk Collected from Local Markets of Patna, India. Vet. World 2014, 7, 62. [Google Scholar] [CrossRef][Green Version]
- Tayde, R.S. Isolation, Identification and Characterization of Campylobacter Species from Raw Poultry Meat by Conventional and Molecular Method. Ph.D. Thesis, Anand Agricultural University, Anand, India, 2010. [Google Scholar]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies on chicken meat associated pathogens between 2010 and 2023 | Studies before 2010 or after 2023 |
| Studies on foodborne pathogens in India | Studies on foodborne pathogens in countries other than India |
| Studies with clear methodologies and sampling procedures | Studies with insufficient or unclear methodology and incomplete outcome data |
| Conducted within the chicken food value chain, covering production, processing, distribution, and consumption stages | Research conducted outside the chicken food value chain |
| Studies reporting on occurrence, prevalence, and AMR in bacterial pathogens | Studies lacking data on occurrence, prevalence, and AMR |
| Full-text availability of chosen studies | Exclusive focus on non-bacterial pathogens or exploration of pathogens unrelated to chicken meat in the food value chain |
| Articles or theses available in English | Articles without full-text availability or not in English |
| Bacteria | Prevalence Studies | AMR Studies |
|---|---|---|
| Salmonella spp. | 23 (retail chicken meat), 14 (chicken-associated environment) | 3 |
| Campylobacter spp. | 16 (retail chicken meat), 17 (chicken-associated environment) | 10 |
| E. coli | 21 (retail chicken meat), 4 (chicken-associated environment), 3 (meat products) | 6 |
| S. aureus | 11 (retail chicken meat) | 1 |
| L. monocytogenes | 9 (retail chicken meat) | - |
| C. perfringens | 5 (retail chicken meat), 2 (chicken-associated samples) | 1 |
| Klebsiella spp. | 5 (retail chicken meat), 3 (meat products), 2 (chicken-associated samples) | - |
| Pathogen | Sample Type/Category | Percent Pooled Prevalence (95% CI) | Influential Studies | Percent Pooled Prevalence After Removal of the Influential Study (95% CI) | Heterogeneity (I2) | Between-Study Variance (τ²) | Regression Test (p Value) | Rank Correlation Test (p Value) |
|---|---|---|---|---|---|---|---|---|
| Salmonella spp. | RCM + AE | 18 (11; 26) | Kumar et al., 2020 [59] | 15.47 (9.73; 22.27%) | 0.98 | 0.0685 | 0.0046 | 0.0447 |
| RCM | 20 (12; 30) | Kumar et al., 2020 [59] | 17.27 (10.64; 25.04) | 0.98 | 0.0677 | - | 0.1942 | |
| AE | 13 (04; 27) | Ramya et al., 2012 [71] | 7.68 (3.16; 13.65) | 0.96 | 0.0871 | 0.0116 | 0.015 | |
| Campylobacter spp. | RCM + AE | 18 (11; 27) | Bobade et al., 2022 [98] | 15.77 (9.83; 22.76) | 0.96 | 0.0708 | <0.0001 | 0.0001 |
| RCM | 17 (8; 28) | Khan et al., 2018 [99] | 16 (7; 28) | 0.94 | 0.0714 | <0.0001 | 0.0001 | |
| AE | 21 (11; 33) | Rajendran et al., 2012 [83] | 17.14 (9.47; 26.46). | 0.96 | 0.0689 | <0.0001 | 0.0003 | |
| E. coli | RCM + AE | 50 (37; 64) | None | - | 0.98 | 0.0870 | 0.8496 | 0.9745 |
| RCM | 57 (43; 71) | None | - | 0.97 | 0.0949 | 0.1917 | 0.9235 | |
| AE | 28 (11; 49) | Deshmukh et al., 2023 [110] | 40 (34.33; 45.80). | 0.97 | 0.0425 | 0.3006 | 0.75 | |
| CMP | 7 (0; 27) | Giri et al., 2021 [107] Anukampa et al., 2020 [109] | - | 0.83 | 0.0440 | 0.0243 | 1 | |
| C. perfringens | RCM + AE | 35 (10; 65) | Kumar et al., 2020 [59] | 24.87 (11.4; 41.35) | 0.99 | 0.0790 | - | 0.3567 |
| RCM | 32 (14; 53) | Kumar et al., 2020 [59] | 24.69 (5.51; 51.35) | 0.98 | 0.1154 | 0.4866 | 0.8167 | |
| K. pneumoniae | RCM + AE | 21 (7; 38) | Tewari et al., 2019 [122] | 10.48 (7.77; 13.49) | 0.85 | 0.0477 | <0.0001 | 0.0167 |
| RCM | 13 (8; 19) | None | - | 0.46 | 0.0030 | 0.1652 | 0.75 | |
| Listeria spp. | RCM | 13 (1; 33) | Kumar et al., 2020 [59] | 7.03 (0.4; 20.41) | 0.98 | 0.0952 | 0.3851 | 0.1789 |
| S. aureus | RCM | 56 (38; 74) | None | - | 0.98 | 0.0887 | 0.0047 | - |
| Bacteria | Study | No. of Isolates | Significant Findings (Resistance %) | Remarks |
|---|---|---|---|---|
| Salmonella spp. | Sharma et al., 2019 [53] | 70 | Nalidixic acid (98.57%). ampicillin (95.71%), ciprofloxacin (82.86%), gatifloxacin (81.43%) | Every isolate in the study was multidrug-resistant. Over 92% of isolates were resistant to five antibiotic classes. Tetracycline and erythromycin showed universal resistance (100%). |
| Mhatre, 2010 [57] | 30 | 100% sensitivity to cefotaxime, cefepime, ceftriaxone, chloramphenicol, ciprofloxacin, and gentamicin; 100% resistance to erythromycin and tetracycline. | ||
| Saini, 2019 [54] | 31 | Ampicillin (87.09%), ciprofloxacin (83.87%), tetracycline (77.42%), cefotaxime (74.19%), gatifloxacin (70.97%) | Erythromycin and nalidixic acid 100% resistance | |
| Campylobacter spp. | Khan et al., 2018 [99] | 101 | Co-trimoxazole (84.1%), cephalothin (81.1%), tetracycline (59.4%) | 97% overall resistance, 94% multidrug resistance |
| Suman Kumar et al., 2021 [82] | 103 | Tetracycline (64.1%), doxycycline (54.4%), ampicillin (46.3%), nalidixic acid (42.7%) | 54.37% multidrug resistance, common resistance in chicken meat | |
| Pruthviraj, 2017 [78] | 23 | Amikacin (26.08%), tetracycline (17.39%) | Majority sensitive to most drugs, 4.34% resistance to erythromycin | |
| Deshpande, 2018 [81] | 31 | Tetracycline (87.09%), ciprofloxacin (70.96%), nalidixic acid (38.70%) | ||
| Yadav, 2017 [77] | 14 | Nalidixic acid (100%), ampicillin (85.72%), ciprofloxacin (42.86%) | Varying resistance patterns observed | |
| Yadav et al., 2016 [94] | 43 | Polymyxin-B (100%), chloramphenicol (97.67%), gentamicin (95.35%) | Complete resistance to penicillin-G, methicillin, rifampicin | |
| Dhayananth, 2019 [75] | 40 | Cefoxitin (95%), ciprofloxacin (80%), nalidixic acid (25%) | Various resistance patterns observed | |
| Garhia, 2017 [87] | 42 | Cefoxitin (97.61%), ciprofloxacin (64.28%), nalidixic acid (33.33%) | Majority resistant to cefoxitin | |
| Vivekanandhan, 2018 [89] | 13 | Oxacillin, tetracycline, cefpodoxime (84.61% each), ciprofloxacin (69.23%) | Resistance levels varied among antibiotics | |
| Begum et al., 2015 [93] | 27 | Amoxicillin, co-trimoxazole (100%), cephalexin (96.29%) | Majority sensitive to gentamicin, intermediate ciprofloxacin | |
| E. coli | Singh et al., 2019 [103] | 77 | Ampicillin, colistin, nitrofurantoin (100%), cefixime (80.52%), co-trimoxazole (72.7%) | Widespread drug resistance observed; 87% sensitivity to amikacin, 100% sensitivity to chloramphenicol |
| Senapati et al., 2020 [104] | 224 | Oxytetracycline (64.73%), chloramphenicol (58.48%), ampicillin/cloxacillin (57.14%), ciprofloxacin (77.68%) | Diverse resistance patterns, significant susceptibility to cefepime and imipenem (about 94%) | |
| Kaushik et al., 2018 [68] | 62 | Cefuroxime, penicillin (89.1% each), ampicillin (80.43%), vancomycin (74.1%), ciprofloxacin (76%) | Diverse resistance patterns, 87% susceptibility to amikacin and gentamicin, 93% to ciprofloxacin | |
| Jana and Mondal, 2013 [106] | 13 | Novobiocin (100%), cefixime, sulphafurazole and vancomycin (92%), tetracycline (84.6%) | Complete sensitivity to chloramphenicol and amikacin | |
| Deshmukh et al., 2023 [110] | 34 | Enrofloxacin (94.11%), tetracycline, lincomycin (85.29% each), cephalexin (70.58%), cefixime (47.06%) | Varied resistance patterns, high sensitivity to gentamicin | |
| Garhia, 2017 [87] | 42 | Cefoxitin (97.61%), ciprofloxacin (64.28%), nalidixic acid (33.33%) | Majority resistant to cefoxitin | |
| Vivekanandhan, 2018 [89] | 13 | Oxacillin, tetracycline, cefpodoxime (84.61% each), ciprofloxacin (69.23%) | Resistance levels varied among antibiotics | |
| Begum et al., 2015 [93] | 27 | Amoxicillin, co-trimoxazole (100%), cephalexin (96.29%) | Majority sensitive to gentamicin | |
| S. aureus | Ruban et al., 2018 [112] | 80 | Ampicillin (100%), tetracycline (87.50%), amoxicillin (77.50%), ciprofloxacin (50%) | Varied resistance patterns, notable susceptibility to gentamicin and vancomycin |
| C. perfringens | Priya et al., 2023 [122] | 63 | Linezolid (96.83%), clarithromycin (92.06%), erythromycin (88.89%), clindamycin (87.30%), ampicillin (71.43%) | Multidrug resistance prevalent; 100% susceptibility to ofloxacin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, H.; Kumar, M.S.; Dubal, Z.B.; Bhilegaonkar, K.N.; Nguyen-Viet, H.; Grace, D.; Thapliyal, S.; Sanjumon, E.S.; Sneha, E.N.P.; Premkumar, D.; et al. Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Patterns of Important Foodborne Pathogens Isolated from Retail Chicken Meat and Associated Environments in India. Foods 2025, 14, 555. https://doi.org/10.3390/foods14040555
Ayoub H, Kumar MS, Dubal ZB, Bhilegaonkar KN, Nguyen-Viet H, Grace D, Thapliyal S, Sanjumon ES, Sneha ENP, Premkumar D, et al. Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Patterns of Important Foodborne Pathogens Isolated from Retail Chicken Meat and Associated Environments in India. Foods. 2025; 14(4):555. https://doi.org/10.3390/foods14040555
Chicago/Turabian StyleAyoub, Haris, Murthy Suman Kumar, Zunjar Baburao Dubal, Kiran Narayan Bhilegaonkar, Hung Nguyen-Viet, Delia Grace, Sakshi Thapliyal, Ekkoruparambil Sethurajan Sanjumon, Elisetty Naga Pavana Sneha, Dharavath Premkumar, and et al. 2025. "Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Patterns of Important Foodborne Pathogens Isolated from Retail Chicken Meat and Associated Environments in India" Foods 14, no. 4: 555. https://doi.org/10.3390/foods14040555
APA StyleAyoub, H., Kumar, M. S., Dubal, Z. B., Bhilegaonkar, K. N., Nguyen-Viet, H., Grace, D., Thapliyal, S., Sanjumon, E. S., Sneha, E. N. P., Premkumar, D., Rajendran, V. K. O., & Deka, R. P. (2025). Systematic Review and Meta-Analysis on Prevalence and Antimicrobial Resistance Patterns of Important Foodborne Pathogens Isolated from Retail Chicken Meat and Associated Environments in India. Foods, 14(4), 555. https://doi.org/10.3390/foods14040555








