Investigating the Synergistic Bactericidal Effects of Cold Plasma and Ultraviolet Radiation on Pseudomonas fragi
Abstract
1. Introduction
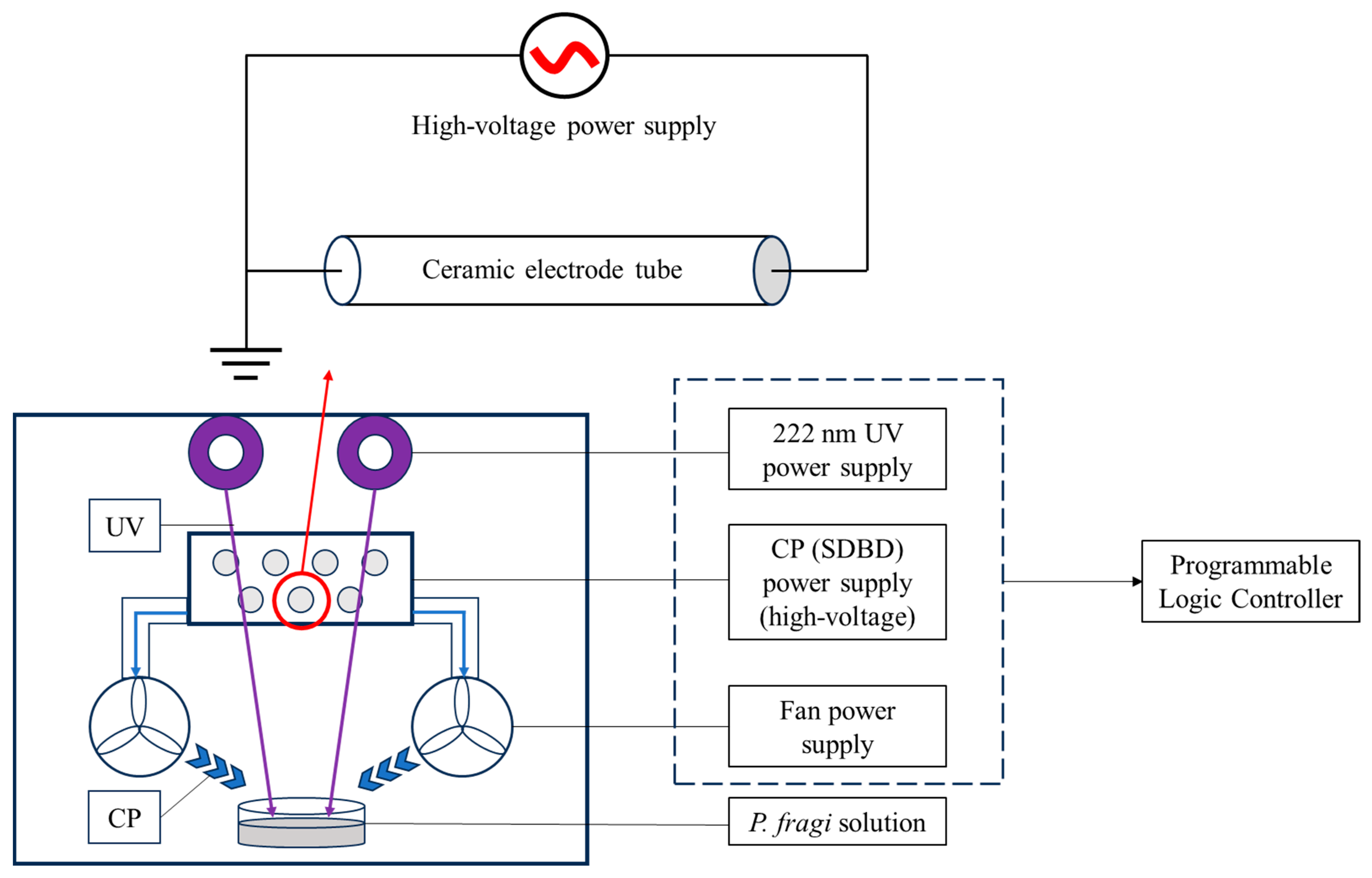
2. Materials and Methods
2.1. Materials
2.2. Bacterial Culture and Treatment
2.3. Optimization of Sterilization Process of SDBD + UV
2.3.1. Single-Factor Experiment
2.3.2. Response Surface Methodology (RSM)
2.4. Optical Emission Spectroscopy (OES) Qualitative Testing
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Plasma Membrane Integrity
2.6.1. PI Staining
2.6.2. Determination of Bacterial Protein Content
2.6.3. Determination of Protein and Nucleic Acid Leakage
2.6.4. Determination of Potassium Ion Leakage
2.7. Determination of Intracellular Reactive Oxygen Species
2.8. Determination of Lipid Peroxidation of Cell Membranes
2.9. Determination of Superoxide Dismutase (SOD) and Catalase (CAT) Activity
2.10. Statistical Analysis
3. Results and Discussion
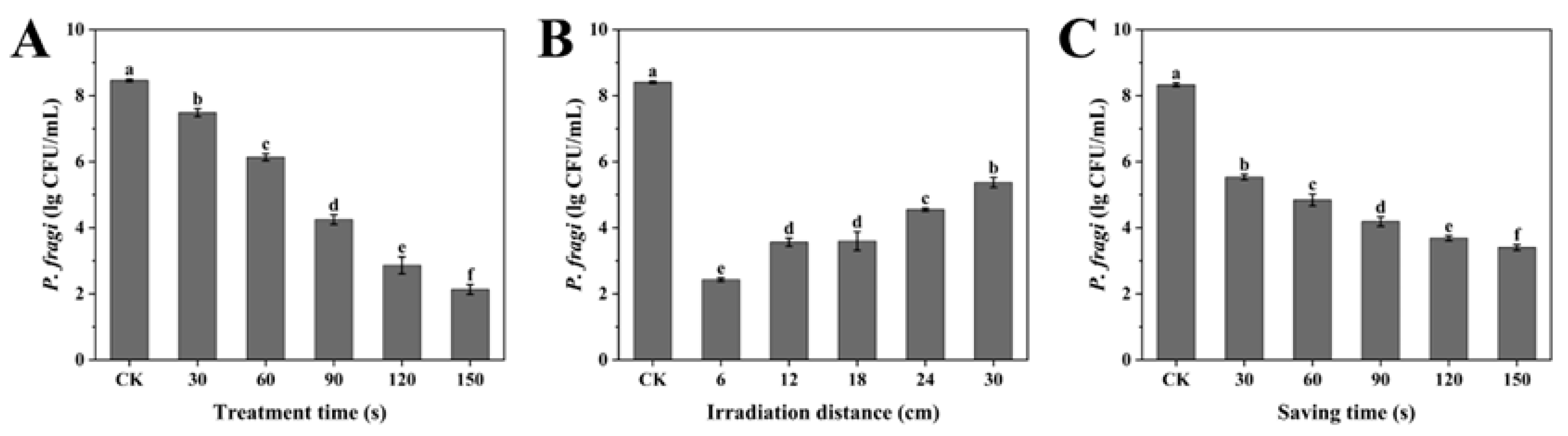
3.1. Single-Factor Test
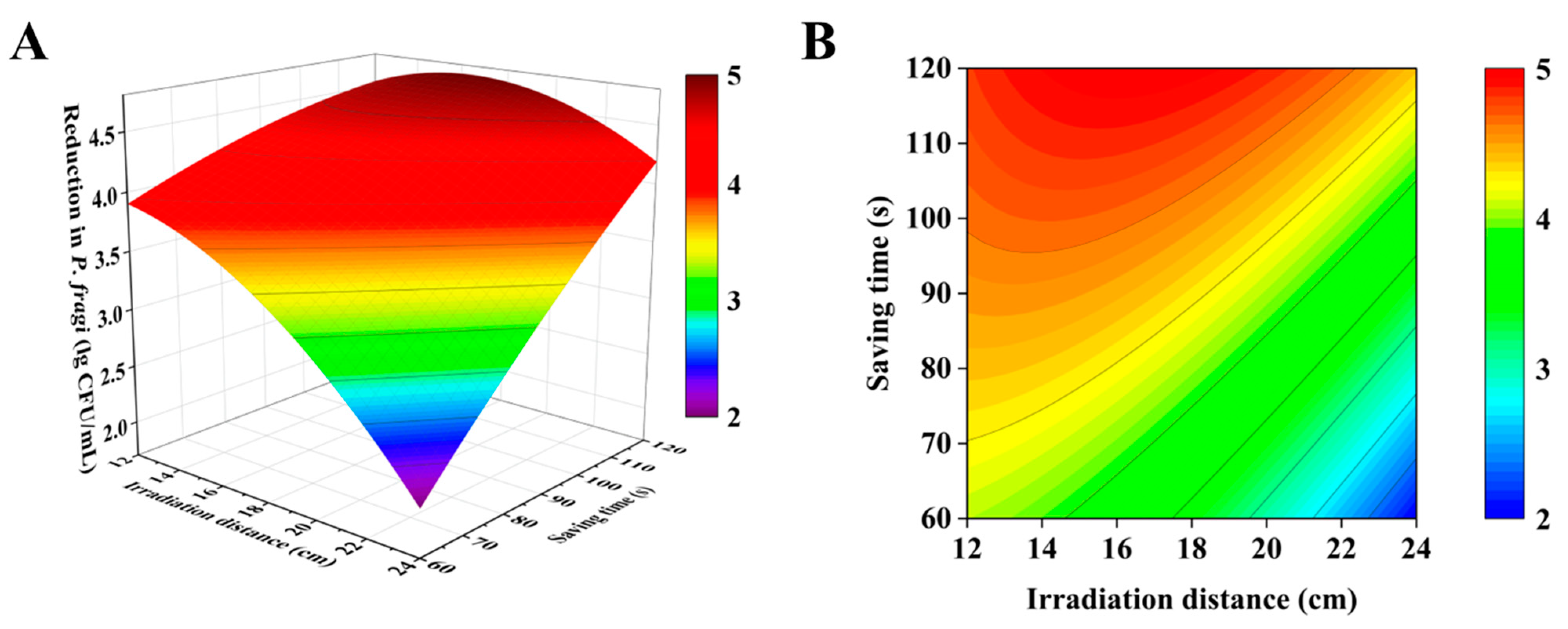
3.2. Response Surface Optimization Test
3.3. Sterilization Test of SDBD + UV Treatment
3.4. OES Qualitative Testing
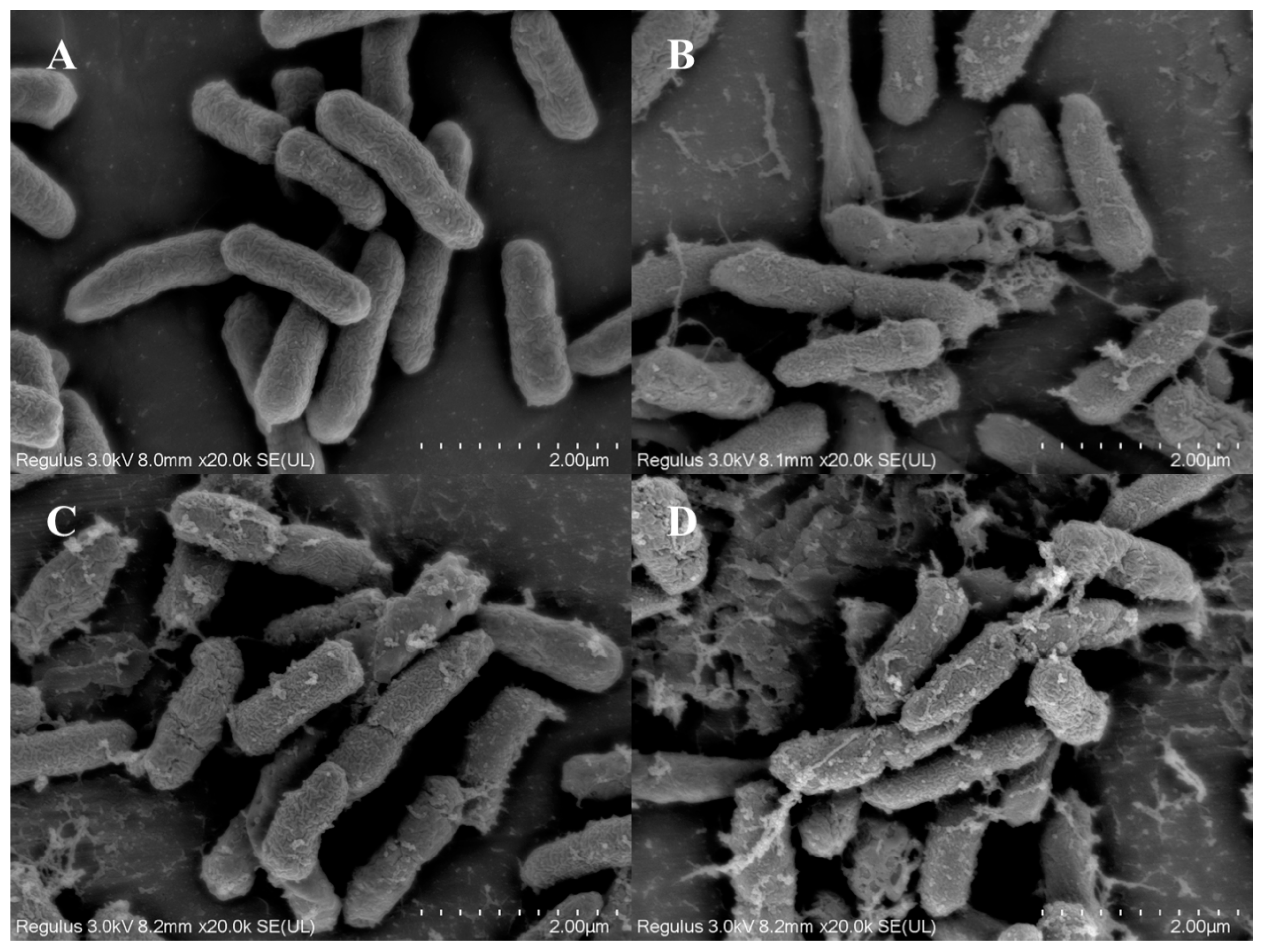
3.5. Observation of Cell Structure
3.6. PI Staining
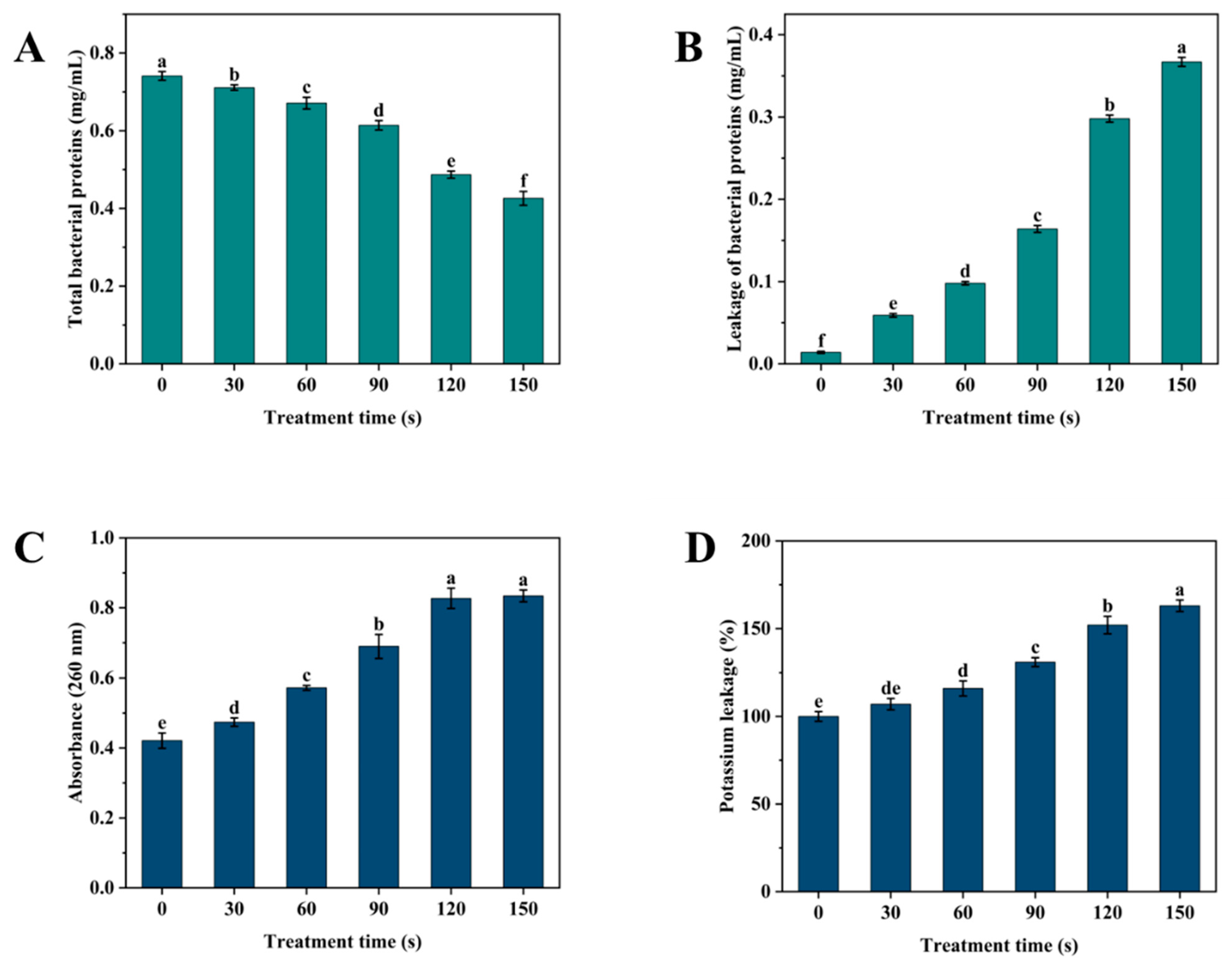
3.7. Determination of Protein Content and Leakage of Bacterial Contents
3.8. Mechanisms of Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Ma, F.; Wu, Y.; Tan, S.; Niu, A.; Qiu, W.; Wang, G. Biosurfactant from Pseudomonas fragi Enhances the Competitive Advantage of Pseudomonas but Reduces the Overall Spoilage Ability of the Microbial Community in Chilled Meat. Food Microbiol. 2023, 115, 104311. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Tang, W.; Ma, F.; Wang, H.; Xu, X.; Qiu, W. AprD Is Important for Extracellular Proteolytic Activity, Physicochemical Properties and Spoilage Potential in Meat-Borne Pseudomonas fragi. Food Control 2021, 124, 107868. [Google Scholar] [CrossRef]
- Huang, J.; Zhai, L.; Wang, J.; Sun, X.; Wang, B.; Wei, Z. An Evaluation of the Sensitivity and Applicability of a Droplet Digital Polymerase Chain Reaction Assay to Simultaneously Detect Pseudomonas aeruginosa and Pseudomonas fragi in Foods. Foods 2024, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, Q.; Ye, T.; Jia, X.; Cao, S.; Fan, T.; Wang, L.; Zeng, X.-A. Recent Developments in the Application of Alternative Sterilisation Technologies to Meat Products: A Review. Int. J. Food Sci. Technol. 2024, 59, 5926–5937. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of Cold Plasma Technology in Ensuring the Safety of Foods and Agricultural Produce: A Review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef] [PubMed]
- Birania, S.; Attkan, A.K.; Kumar, S.; Kumar, N.; Singh, V.K. Cold Plasma in Food Processing and Preservation: A Review. J. Food Process Eng. 2022, 45, e14110. [Google Scholar] [CrossRef]
- Krumpolec, R.; Richter, V.; Zemanek, M.; Homola, T. Multi-Hollow Surface Dielectric Barrier Discharge for Plasma Treatment of Patterned Silicon Surfaces. Surf. Interfaces 2019, 16, 181–187. [Google Scholar] [CrossRef]
- Leonov, S.B.; Adamovich, I.V.; Soloviev, V.R. Dynamics of Near-Surface Electric Discharges and Mechanisms of Their Interaction with the Airflow. Plasma Sources Sci. Technol. 2016, 25, 063001. [Google Scholar] [CrossRef]
- Li, D.; Liu, D.X.; Nie, Q.Y.; Li, H.P.; Chen, H.L.; Kong, M.G. Array of Surface-Confined Glow Discharges in Atmospheric Pressure Helium: Modes and Dynamics. Appl. Phys. Lett. 2014, 104, 204101. [Google Scholar] [CrossRef]
- Portugal, S.; Choudhury, B.; Cardenas, D. Advances on Aerodynamic Actuation Induced by Surface Dielectric Barrier Discharges. Front. Phys. 2022, 10, 923103. [Google Scholar] [CrossRef]
- Giannoglou, M.; Dimitrakellis, P.; Efthimiadou, A.; Gogolides, Ε.; Katsaros, G. Comparative Study on the Effect of Cold Atmospheric Plasma, Ozonation, Pulsed Electromagnetic Fields and High-Pressure Technologies on Sea Bream Fillet Quality Indices and Shelf Life. Food Eng. Rev. 2021, 13, 175–184. [Google Scholar] [CrossRef]
- Pourbagher, R.; Abbaspour-Fard, M.H.; Sohbatzadeh, F.; Rohani, A. In Vivo Antibacterial Effect of Non-Thermal Atmospheric Plasma on Pseudomonas Tolaasii, a Causative Agent of Agaricus Bisporus Blotch Disease. Food Control 2021, 130, 108319. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Cheng, J.-H.; Sun, D.-W. Inactivation of Listeria monocytogenes at Various Growth Temperatures by Ultrasound Pretreatment and Cold Plasma. LWT 2020, 118, 108635. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, B.; Zhang, H.; Lai, A.C. Inactivation of Foodborne Pathogenic and Spoilage Bacteria by Single and Dual Wavelength UV-LEDs: Synergistic Effect and Pulsed Operation. Food Control 2021, 125, 107999. [Google Scholar] [CrossRef]
- Claus, H.; Cooksey, C.C. Reflectance Measurements of Building Materials in the Far UVC (222 Nm) Wavelength Range. In Proceedings of the UV and Higher Energy Photonics: From Materials to Applications, San Diego, CA, USA, 21–25 August 2022; Cho, Y.H., Lerondel, G., Taguchi, A., Eds.; SPIE-International Society for Optical Engineering: Bellingham, WA, USA, 2022; Volume 12201, p. 1220106. [Google Scholar]
- Welch, D.; Buonanno, M.; Grilj, V.; Shuryak, I.; Crickmore, C.; Bigelow, A.W.; Randers-Pehrson, G.; Johnson, G.W.; Brenner, D.J. Far-UVC Light: A New Tool to Control the Spread of Airborne-Mediated Microbial Diseases. Sci. Rep. 2021, 11, 18122. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, W.; Zhao, L.; Qian, J.; Li, S.; Ye, Z.; Zhang, J.; Wang, J. Cold Plasma-222 Nm UV: A New Cold Sterilizing Method for Food Contact Surfaces. Food Control 2023, 152, 109870. [Google Scholar] [CrossRef]
- Herron, J.T.; Green, D.S. Chemical Kinetics Database and Predictive Schemes for Nonthermal Humid Air Plasma Chemistry. Part II. Neutral Species Reactions. Plasma Chem. Plasma Process. 2001, 21, 459–481. [Google Scholar] [CrossRef]
- Radziute, L.; Gaigalas, G.; Kato, D.; Rynkun, P.; Tanaka, M. Extended Calculations of Energy Levels and Transition Rates for Singly Ionized Lanthanide Elements. I. Pr-Gd. Astrophys. J. Suppl. Ser. 2020, 248, 17. [Google Scholar] [CrossRef]
- Huang, D.; Xiao, J.; Zhu, X.; Xu, B.; Wang, Y.; Wang, Y.; Wang, M. Scavenging of Reactive Oxygen Species Effectively Reduces Pseudomonas aeruginosa Biofilms through Disrupting Policing. Environ. Res. 2023, 220, 115182. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, D.; Zheng, S.; Li, Z.; Xu, W. Antimicrobial Behavior and Mechanism of Clove Oil Nanoemulsion. J. Food Sci. Technol. Mysore 2022, 59, 1939–1947. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef]
- Sklodowska, M.; Mielczarz, K.; Chojak-Kozniewska, J.; Naliwajski, M.; Zyzniewska, M.; Goralczyk-Binkowska, A. Antioxidant Response of Cucumber Leaf Tissues Treated with Prohexadione-Ca to Infection with Pseudomonas syringae Pv. Lachrymans. Sci. Hortic. 2021, 289, 110452. [Google Scholar] [CrossRef]
- Mustapha, F.A.; Jai, J.; Nik Raikhan, N.H.; Sharif, Z.I.M.; Yusof, N.M. Response Surface Methodology Analysis towards Biodegradability and Antimicrobial Activity of Biopolymer Film Containing Turmeric Oil against Aspergillus niger. Food Control 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Long, C.; Lin, B. Effects of Material Degradation on Electrical and Optical Characteristics of Surface Dielectric Barrier Discharge. J. Appl. Phys. 2018, 124, 183301. [Google Scholar] [CrossRef]
- Lee, H.; Oh, C.; Hahn, J.W. Calibration and Uncertainty Analysis of an Optical Emission Spectrometer Measuring the Absolute Spectral Radiant Exitance of UV Signatures. Propellants Explos. Pyrotech. 2012, 37, 116–121. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, G.; Chen, S.; Guo, X.; Chen, L.; Qiao, Y.; Shi, L.; Wu, W.; Wang, L. Explore the Interaction Between Lotus Seedpod Procyanidins and Myofibrillar Protein to Inhibit the Adhesion of Pseudomonas fragi. Food Bioprocess Technol. 2024; preview. [Google Scholar] [CrossRef]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical Aspects of Using Bacterial Cell Viability Assays with the Fluorophores SYTO9 and Propidium Iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Liu, G.; Ren, G.; Zhao, L.; Cheng, L.; Wang, C.; Sun, B. Antibacterial Activity and Mechanism of Bifidocin A against Listeria monocytogenes. Food Control 2017, 73, 854–861. [Google Scholar] [CrossRef]
- Kang, J.-W.; Kang, D.-H. The Synergistic Bactericidal Mechanism of Simultaneous Treatment with a 222-Nanometer Krypton-Chlorine Excilamp and a 254-Nanometer Low-Pressure Mercury Lamp. Appl. Environ. Microbiol. 2019, 85, e01952-18. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Shi, C.; Guo, H.; Ni, R.; Qu, J.; Tang, J.; Liu, S. Effect of Oxidative Stress from Nanoscale TiO2 Particles on a Physarum Polycephalum Macroplasmodium under Dark Conditions. Environ. Sci. Pollut. Res. 2017, 24, 17241–17249. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Yang, S.; Wu, G.Y. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, F.; Xiu, Z. Oxidative Stress Induced in Saccharomyces cerevisiae Exposed to Dielectric Barrier Discharge Plasma in Air at Atmospheric Pressure. IEEE Trans. Plasma Sci. 2010, 38, 1885–1891. [Google Scholar] [CrossRef]




| Factor | Low Level (−1) | Medium Level (0) | High Level (1) |
|---|---|---|---|
| Treatment time (s) | 60 | 90 | 120 |
| Irradiation distance (cm) | 12 | 18 | 24 |
| Saving time (s) | 60 | 90 | 120 |
| Experiments | Coded Levels | Reduction in P. Fragi (lg CFU/mL) | ||
|---|---|---|---|---|
| A | B | C | ||
| Treatment Time (s) | Irradiation Distance (cm) | Saving Time (s) | ||
| 1 | 60 (−1) | 12 (−1) | 90 (0) | 2.52 |
| 2 | 120 (1) | 12 (−1) | 90 (0) | 5.56 |
| 3 | 60 (−1) | 24 (1) | 90 (0) | 1.51 |
| 4 | 120 (1) | 24 (1) | 90 (0) | 4.62 |
| 5 | 60 (−1) | 18 (0) | 60 (−1) | 1.53 |
| 6 | 120 (1) | 18 (0) | 60 (−1) | 4.64 |
| 7 | 60 (−1) | 18 (0) | 120 (1) | 2.87 |
| 8 | 120 (1) | 18 (0) | 120 (1) | 6.14 |
| 9 | 90 (0) | 12 (−1) | 60 (−1) | 3.98 |
| 10 | 90 (0) | 24 (1) | 60 (−1) | 2.07 |
| 11 | 90 (0) | 12 (−1) | 120 (1) | 4.57 |
| 12 | 90 (0) | 24 (1) | 120 (1) | 4.11 |
| 13 | 90 (0) | 18 (0) | 90 (0) | 4.11 |
| 14 | 90 (0) | 18 (0) | 90 (0) | 4.2 |
| 15 | 90 (0) | 18 (0) | 90 (0) | 4.32 |
| 16 | 90 (0) | 18 (0) | 90 (0) | 3.86 |
| 17 | 90 (0) | 18 (0) | 90 (0) | 4.01 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 26.98 | 9 | 3 | 132.51 | <0.0001 |
| A-A | 19.63 | 1 | 19.63 | 867.41 | <0.0001 |
| B-B | 2.33 | 1 | 2.33 | 103.11 | <0.0001 |
| C-C | 3.74 | 1 | 3.74 | 165.31 | <0.0001 |
| AB | 1.225 × 10−3 | 1 | 1.225 × 10−3 | 0.054 | 0.8227 |
| AC | 6.4 × 10−3 | 1 | 6.4 × 10−3 | 0.28 | 0.6113 |
| BC | 0.53 | 1 | 0.53 | 23.23 | 0.0019 |
| A2 | 0.2 | 1 | 0.2 | 8.8 | 0.0209 |
| B2 | 0.46 | 1 | 0.46 | 20.27 | 0.0028 |
| C2 | 0.032 | 1 | 0.032 | 1.42 | 0.2715 |
| Residual | 0.16 | 7 | 0.023 | ||
| Lack of Fit | 0.034 | 3 | 0.011 | 0.37 | 0.7818 |
| Pure Error | 0.12 | 4 | 0.031 | ||
| Cor Total | 27.14 | 16 | |||
| R-squared = 0.9848 | Adj R-squared = 0.9652 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Chen, F.; Zhang, J.; Guo, X.; Zhang, J.; Yan, W. Investigating the Synergistic Bactericidal Effects of Cold Plasma and Ultraviolet Radiation on Pseudomonas fragi. Foods 2025, 14, 550. https://doi.org/10.3390/foods14040550
Yuan H, Chen F, Zhang J, Guo X, Zhang J, Yan W. Investigating the Synergistic Bactericidal Effects of Cold Plasma and Ultraviolet Radiation on Pseudomonas fragi. Foods. 2025; 14(4):550. https://doi.org/10.3390/foods14040550
Chicago/Turabian StyleYuan, Haidu, Fei Chen, Jiajia Zhang, Xinglei Guo, Jianhao Zhang, and Wenjing Yan. 2025. "Investigating the Synergistic Bactericidal Effects of Cold Plasma and Ultraviolet Radiation on Pseudomonas fragi" Foods 14, no. 4: 550. https://doi.org/10.3390/foods14040550
APA StyleYuan, H., Chen, F., Zhang, J., Guo, X., Zhang, J., & Yan, W. (2025). Investigating the Synergistic Bactericidal Effects of Cold Plasma and Ultraviolet Radiation on Pseudomonas fragi. Foods, 14(4), 550. https://doi.org/10.3390/foods14040550





