In Vitro Assessment of the Prebiotic Effects of Poria Cocos Polysaccharides Using Fecal Microbiota from Normal-Weight and Obese Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Children’s Research Subjects
2.2. Laboratory Measurements
2.3. Materials and Equipment
2.4. Monosaccharide Analysis of PCP
2.5. Collection of Fecal Samples and In Vitro Fermentation
2.6. In Vitro Culture of Single Strains
2.7. DNA Extraction,16S rRNA Gene Sequencing
2.8. Targeted Metabolomic Profiling
2.9. Statistical Analysis of Data
3. Results
3.1. Demographic and Clinical Characteristics of Subjects
3.2. Monosaccharide Composition of Poria Cocos Polysaccharides
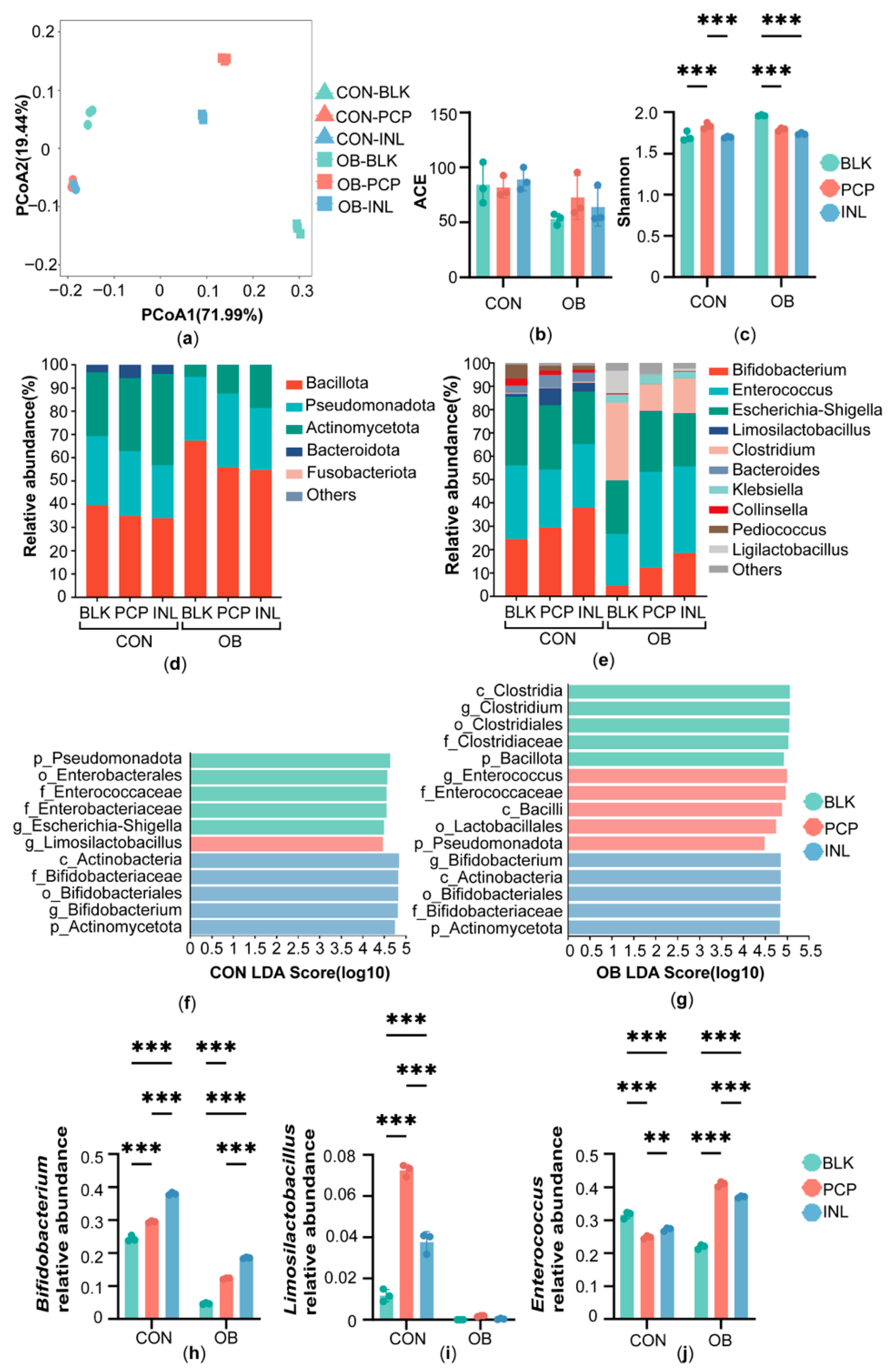
3.3. Effect of PCP on the Gut Microbiota of Normal-Weight and Obese Children
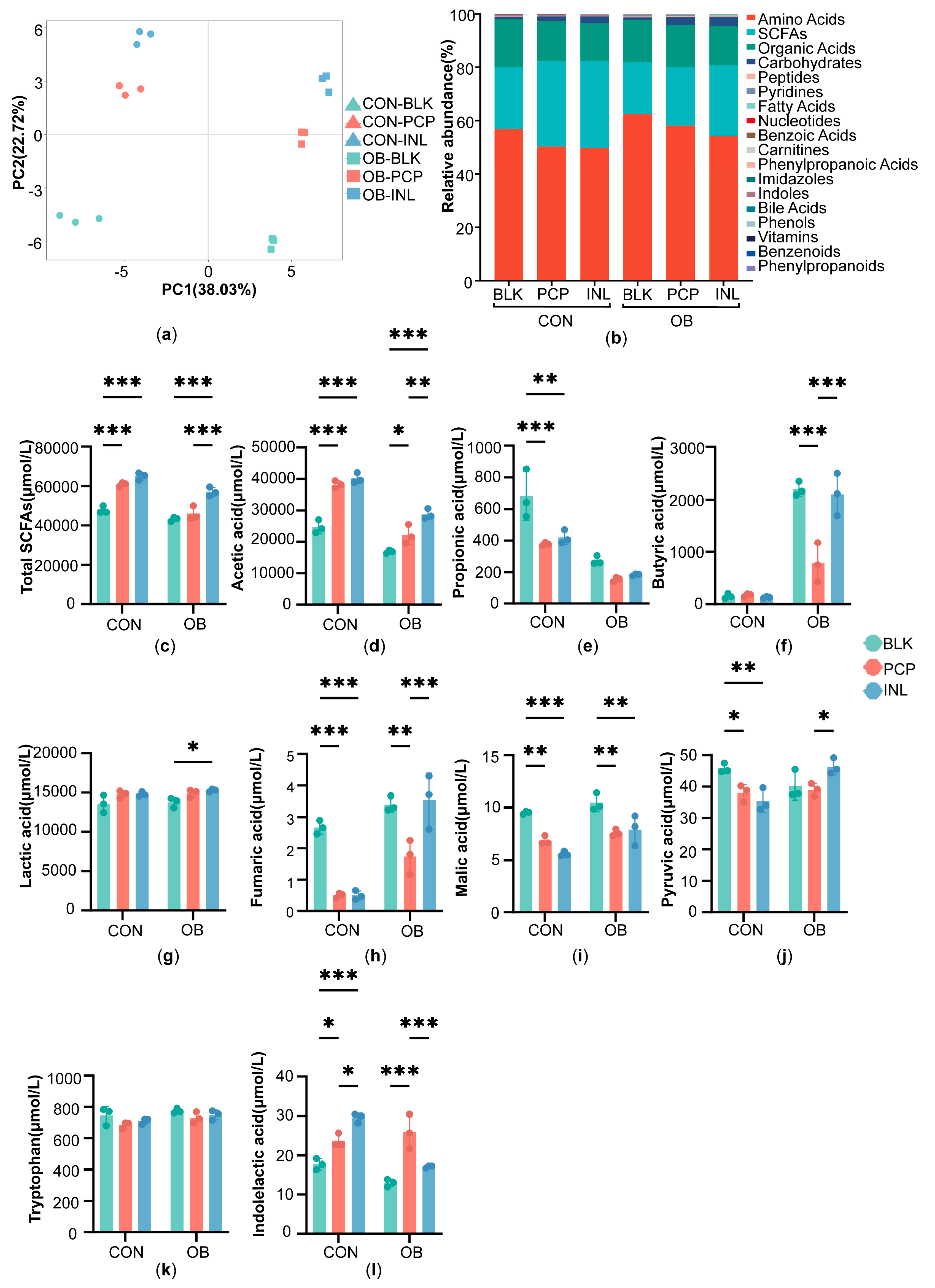
3.4. Impact of PCP on Targeted Metabolites
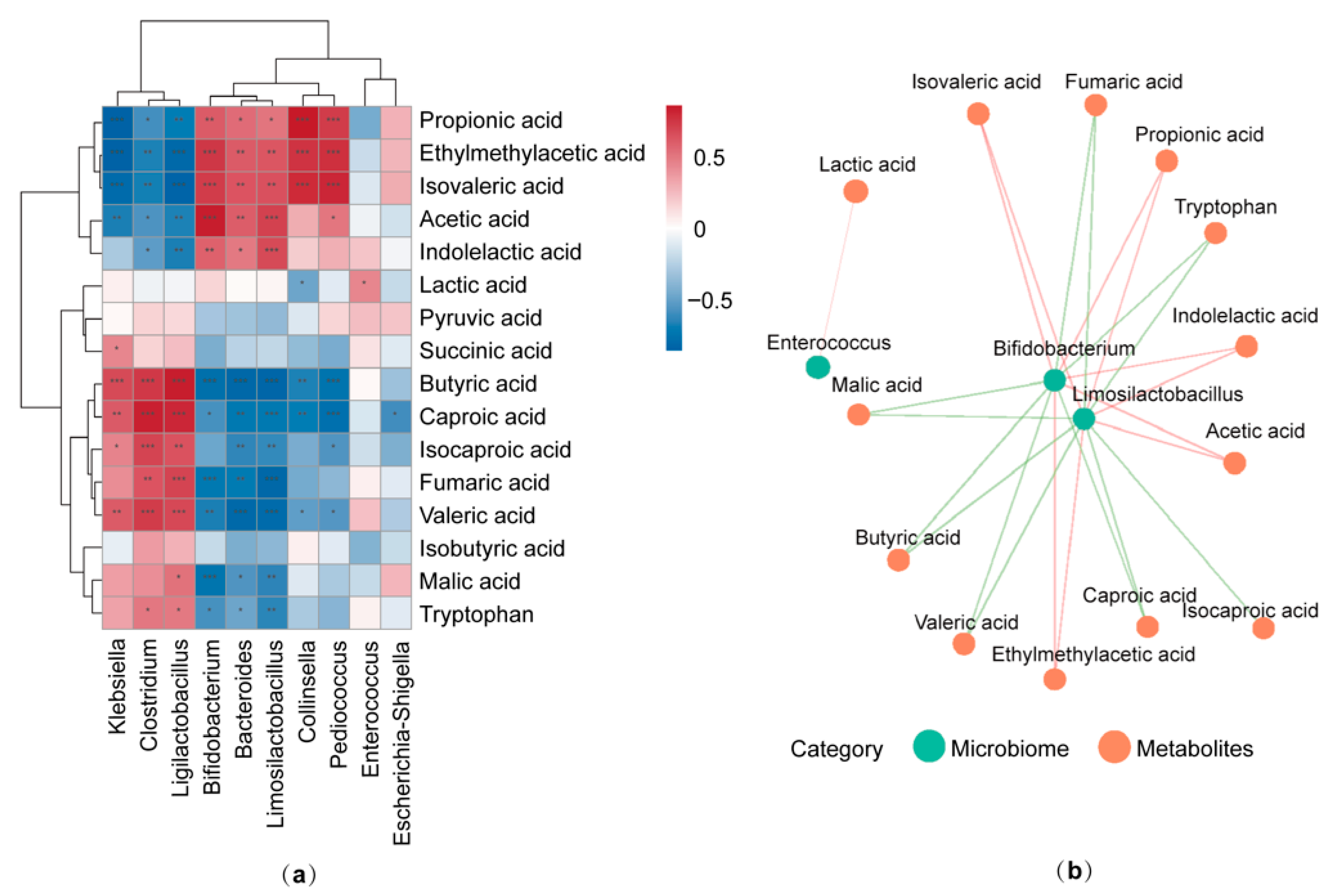
3.5. Correlation Between Gut Microbiota Composition and Targeted Metabolites
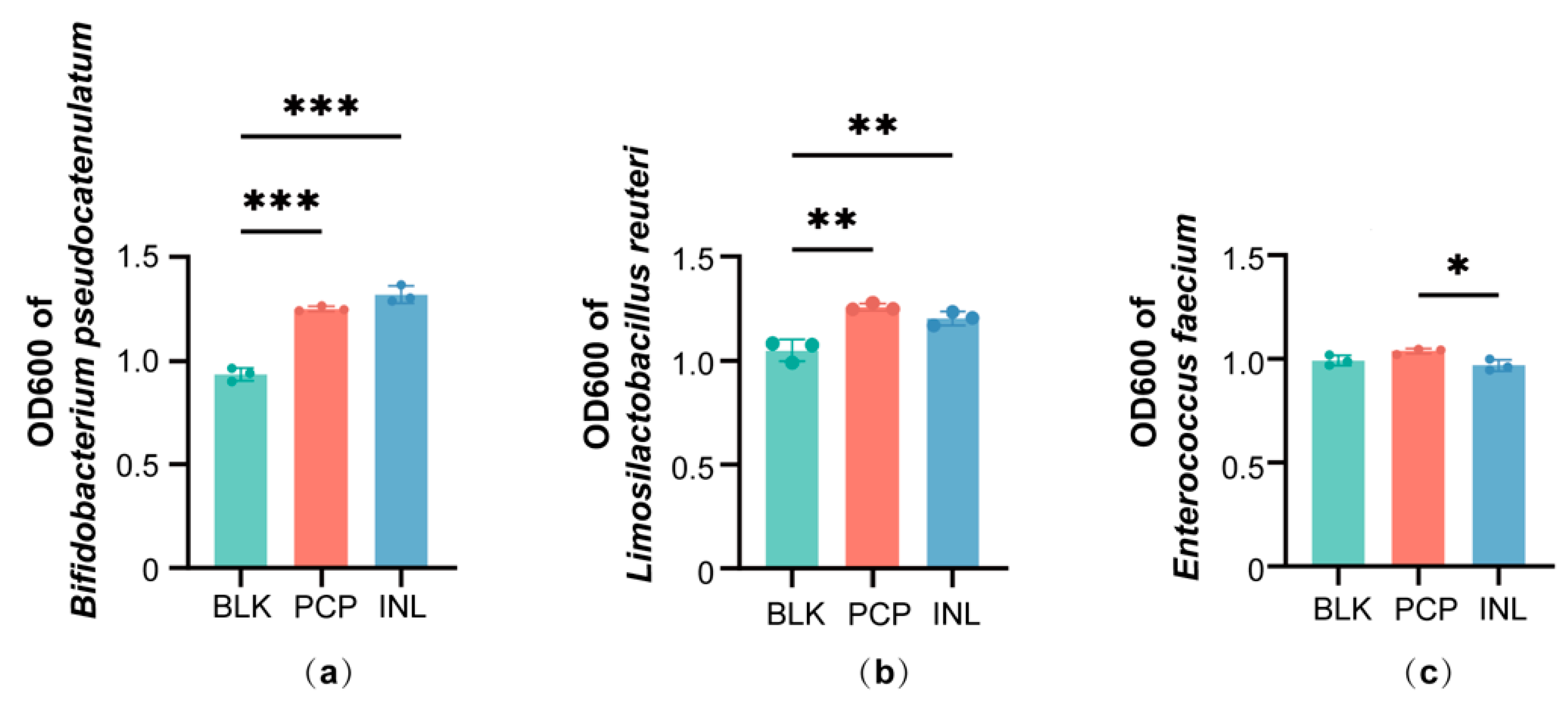
3.6. Effects of PCP in Single-Strain Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Abundance-based Coverage Estimator |
| AhR | Aryl hydrocarbon receptor |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| FBG | Fasting blood glucose |
| FINS | Fasting insulin |
| HDL-C | High-density lipoprotein cholesterol |
| HOMA-β | Homeostasis model assessment of β-cell function |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| INL | Inulin |
| LDL-C | Low-density lipoprotein cholesterol |
| LEfSe | Linear Discriminant Analysis Effect Size |
| OTUs | Operational taxonomic units |
| PCoA | Principal coordinate analysis |
| PCP | Poria cocos polysaccharides |
| PLS-DA | Partial least squares discriminant analysis |
| SCFAs | Short-chain fatty acids |
| SCR | Serum creatinine |
| SD | Standard deviation |
| TC | Total cholesterol |
| TFA | Trifluoroacetic acid |
| TG | Triglycerides |
| UA | Uric acid |
| WGOC | Working Group on Obesity in China |
References
- Zhao, M.; Guan, Z.; Tang, N.; Cheng, Y. The Differences between the Water- and Alkaline-Soluble Poria Cocos Polysaccharide: A Review. Int. J. Biol. Macromol. 2023, 235, 123925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Yang, Y.; Wei, L.; Wang, C.; Xu, J.; Song, J.; Liu, S.; Bai, J.; Suo, H. Regulatory Effects of Poria Cocos Polysaccharides on Gut Microbiota and Metabolites: Evaluation of Prebiotic Potential. NPJ Sci. Food 2025, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Liu, L.; Zhao, J.; Zhang, Y.; Chen, L. Antioxidant Activity and Metabolic Profile Changes in Poria Cocos Fermented by Breast-Milk-Derived Limosilactobacillus Reuteri HM-R28. Front. Nutr. 2025, 12, 1625875. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria Cocos Polysaccharide Improves Intestinal Barrier Function and Maintains Intestinal Homeostasis in Mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef]
- Ng, C.Y.J.; Lai, N.P.Y.; Ng, W.M.; Siah, K.T.H.; Gan, R.-Y.; Zhong, L.L.D. Chemical Structures, Extraction and Analysis Technologies, and Bioactivities of Edible Fungal Polysaccharides from Poria Cocos: An Updated Review. Int. J. Biol. Macromol. 2024, 261, 129555. [Google Scholar] [CrossRef]
- Deng, L.; Huang, G. Preparation, Structure and Application of Polysaccharides from Poria Cocos. RSC Adv. 2024, 14, 31008–31020. [Google Scholar] [CrossRef]
- Wan, J.; Wang, F.; Xiao, Y.; Cheng, Y.; Zheng, S.; Jiang, Q.; Tan, B.; Li, X.; Chen, J.; Liao, S. Poria Cocos Polysaccharide Alleviates Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice by Modulating Intestinal Inflammatory Responses and Microbial Dysbiosis. Int. J. Biol. Macromol. 2024, 283, 137450. [Google Scholar] [CrossRef]
- Sun, M.; Yao, L.; Yu, Q.; Duan, Y.; Huang, J.; Lyu, T.; Yu, N.; Peng, D.; Chen, W.; Wang, Y.; et al. Screening of Poria Cocos Polysaccharide with Immunomodulatory Activity and Its Activation Effects on TLR4/MD2/NF-κB Pathway. Int. J. Biol. Macromol. 2024, 273, 132931. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Zuo, W.-F.; Pang, Q.; Yao, L.-P.; Zhang, Y.; Peng, C.; Huang, W.; Han, B. Gut Microbiota: A Magical Multifunctional Target Regulated by Medicine Food Homology Species. J. Adv. Res. 2023, 52, 151–170. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, Y.; Liu, M.; Zhang, X.; Guo, S.; Su, S.; Pan, Y.; Qiu, Z.; Zhang, X.; Duan, J.; et al. Chemical Structures of Lycii Fructus Polysaccharides Tailored the Gut Microbiota Composition of Aged Caenorhabditis elegans. Food Hydrocolloid. 2025, 169, 111608. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Zhang, R.; Chen, Y.; Wang, F.; Zhang, M. Gut Microbial Fermentation Promotes the Intestinal Anti-Inflammatory Activity of Chinese Yam Polysaccharides. Food Chem. 2023, 402, 134003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Song, Z.; Tang, S.; Liu, Z.; Pang, M.; Zhang, D.; Wu, X.; Yu, X.; Wang, P.; et al. Enhanced Bioactivity of Honeysuckle-Cassia Seeds Extracts through Lactobacillus Acidophilus and Bacillus Subtilis Co-Fermentation: Impact on Alcoholic Liver Disease and Gut Microbiota. Food Chem. 2025, 486, 144463. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Hou, C.-L.; Rao, X.-H.; Zhang, A.-Q.; Xiao, G.-M.; Wang, L.-Y.; Jin, K.-N.; Sun, P.-L.; Chen, L.-C. In Vitro Fermentation Characteristics of Polysaccharides from Coix Seed and Its Effects on the Gut Microbiota. Int. J. Biol. Macromol. 2024, 262, 129994. [Google Scholar] [CrossRef]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and Adolescent Obesity. Nat. Rev. Dis. Primers 2023, 9, 24. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Q.; Hu, R.; Yang, X.; Fang, C.; Yao, L.; Lv, J.; Wang, L.; Shi, M.; Zhang, W.; et al. Effects of Prevotella Copri on Insulin, Gut Microbiota and Bile Acids. Gut Microbes 2024, 16, 2340487. [Google Scholar] [CrossRef]
- Larruy-García, A.; Mahmood, L.; Miguel-Berges, M.L.; Masip, G.; Seral-Cortés, M.; De Miguel-Etayo, P.; Moreno, L.A. Diet Quality Scores, Obesity and Metabolic Syndrome in Children and Adolescents: A Systematic Review and Meta-Analysis. Curr. Obes. Rep. 2024, 13, 755–788. [Google Scholar] [CrossRef]
- Saeed, N.K.; Al-Beltagi, M.; Bediwy, A.S.; El-Sawaf, Y.; Toema, O. Gut Microbiota in Various Childhood Disorders: Implication and Indications. World J. Gastroenterol. 2022, 28, 1875–1901. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; van der Wal, P.; Kiefte-De Jong, J.C.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.P.; Jaddoe, V.W.V.; et al. Diversity, Compositional and Functional Differences between Gut Microbiota of Children and Adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef]
- Li, H.; Ji, C.-Y.; Zong, X.-N.; Zhang, Y.-Q. Body Mass Index Growth Curves for Chinese Children and Adolescents Aged 0 to 18 Years. Zhonghua Er Ke Za Zhi 2009, 47, 493–498. [Google Scholar] [PubMed]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.; Brison, Y.; Remaud-Simeon, M.; Monsan, P.; Gibson, G.R.; Rastall, R.A. In Vitro Fermentation of Linear and α-1,2-Branched Dextrans by the Human Fecal Microbiota. Appl. Environ. Microbiol. 2011, 77, 5307–5315. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Y.; Yue, S.-R.; Lu, A.-P.; Zhang, L.; Ji, G.; Liu, B.-C.; Wang, R.-R. The Improvement of Nonalcoholic Steatohepatitis by Poria Cocos Polysaccharides Associated with Gut Microbiota and NF-κB/CCL3/CCR1 Axis. Phytomedicine 2022, 103, 154208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ruan, D.; Shashkov, A.S.; Kilcoyne, M.; Savage, A.V.; Zhang, L. Chemical Components and Molecular Mass of Six Polysaccharides Isolated from the Sclerotium of Poria Cocos. Carbohyd. Res. 2004, 339, 327–334. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Lee, A.H.; Rodriguez Jimenez, D.M.; Meisel, M. Limosilactobacillus Reuteri—A Probiotic Gut Commensal with Contextual Impact on Immunity. Gut Microbes 2025, 17, 2451088. [Google Scholar] [CrossRef]
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. (Eds.) Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Anania, C.; Di Marino, V.P.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.M.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium Animalis Subsp. Lactis BB12 and Enterococcus Faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Ning, X.; Qin, Y.; Wang, Y.; Yu, Z.; Dong, R.; Zhang, Y.; Sun, S. Expansion of Escherichia-Shigella in Gut Is Associated with the Onset and Response to Immunosuppressive Therapy of IgA Nephropathy. J. Am. Soc. Nephrol. 2022, 33, 2276–2292. [Google Scholar] [CrossRef]
- Shetty, S.A.; Boeren, S.; Bui, T.P.N.; Smidt, H.; de Vos, W.M. Unravelling Lactate-Acetate and Sugar Conversion into Butyrate by Intestinal Anaerobutyricum and Anaerostipes Species by Comparative Proteogenomics. Environ. Microbiol. 2020, 22, 4863–4875. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Patloka, O.; Komprda, T.; Franke, G. Review of the Relationships Between Human Gut Microbiome, Diet, and Obesity. Nutrients 2024, 16, 3996. [Google Scholar] [CrossRef]
- Kim, H.; Oh, N.; Kwon, M.; Kwon, O.-H.; Ku, S.; Seo, J.; Roh, S. Exopolysaccharide of Enterococcus Faecium L15 Promotes the Osteogenic Differentiation of Human Dental Pulp Stem Cells via P38 MAPK Pathway. Stem Cell Res. Ther. 2022, 13, 446. [Google Scholar] [CrossRef]
- Mi, J.; He, T.; Hu, X.; Wang, Z.; Wang, T.; Qi, X.; Li, K.; Gao, L.; Liu, C.; Zhang, Y.; et al. Enterococcus Faecium C171: Modulating the Immune Response to Acute Lethal Viral Challenge. Int. J. Antimicrob. Agents 2023, 62, 106969. [Google Scholar] [CrossRef]
- Gu, F.; Borewicz, K.; Richter, B.; van der Zaal, P.H.; Smidt, H.; Buwalda, P.L.; Schols, H.A. In Vitro Fermentation Behavior of Isomalto/Malto-Polysaccharides Using Human Fecal Inoculum Indicates Prebiotic Potential. Mol. Nutr. Food Res. 2018, 62, e1800232. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Odamaki, T.; Xiao, J. Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants. Microorganisms 2019, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Colorectal Tumorigenesis via Epigenetic Regulation of CD8+ T Cell Immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Kuang, Z.; Wang, L. The Role of Microbial Indole Metabolites in Tumor. Gut Microbes 2024, 16, 2409209. [Google Scholar] [CrossRef]
- Wang, A.; Guan, C.; Wang, T.; Mu, G.; Tuo, Y. Lactiplantibacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Intestinal Barrier Integrity through the AhR/Nrf2/NF-κB Axis. J. Agric. Food. Chem. 2024, 72, 9236–9246. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Rivera, J.E.; Lan, R.; Ferruzzi, M.G.; Børsheim, E.; Tas, E.; Diaz, E.C. Associations Between Serum Gut-Derived Tryptophan Metabolites and Cardiovascular Health Markers in Adolescents with Obesity. Nutrients 2025, 17, 2430. [Google Scholar] [CrossRef]
- Bircher, L.; Geirnaert, A.; Hammes, F.; Lacroix, C.; Schwab, C. Effect of Cryopreservation and Lyophilization on Viability and Growth of Strict Anaerobic Human Gut Microbes. Microb. Biotechnol. 2018, 11, 721–733. [Google Scholar] [CrossRef]
- Isenring, J.; Bircher, L.; Geirnaert, A.; Lacroix, C. In Vitro Human Gut Microbiota Fermentation Models: Opportunities, Challenges, and Pitfalls. Microbiome Res. Rep. 2023, 2, 2. [Google Scholar] [CrossRef]
- Selak, M.; Rivière, A.; Moens, F.; Van den Abbeele, P.; Geirnaert, A.; Rogelj, I.; Leroy, F.; De Vuyst, L. Inulin-Type Fructan Fermentation by Bifidobacteria Depends on the Strain Rather than the Species and Region in the Human Intestine. Appl. Microbiol. Biotechnol. 2016, 100, 4097–4107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, D.-Y.; Liu, X.-Q.; Luo, Y.; Chen, X.; Zhang, W.-N.; Yang, X.; Luo, F.-H.; Wang, R.-R. In Vitro Assessment of the Prebiotic Effects of Poria Cocos Polysaccharides Using Fecal Microbiota from Normal-Weight and Obese Children. Foods 2025, 14, 4077. https://doi.org/10.3390/foods14234077
Qiu D-Y, Liu X-Q, Luo Y, Chen X, Zhang W-N, Yang X, Luo F-H, Wang R-R. In Vitro Assessment of the Prebiotic Effects of Poria Cocos Polysaccharides Using Fecal Microbiota from Normal-Weight and Obese Children. Foods. 2025; 14(23):4077. https://doi.org/10.3390/foods14234077
Chicago/Turabian StyleQiu, Dan-Yi, Xiao-Qin Liu, Yue Luo, Xin Chen, Wen-Na Zhang, Xin Yang, Fei-Hong Luo, and Rui-Rui Wang. 2025. "In Vitro Assessment of the Prebiotic Effects of Poria Cocos Polysaccharides Using Fecal Microbiota from Normal-Weight and Obese Children" Foods 14, no. 23: 4077. https://doi.org/10.3390/foods14234077
APA StyleQiu, D.-Y., Liu, X.-Q., Luo, Y., Chen, X., Zhang, W.-N., Yang, X., Luo, F.-H., & Wang, R.-R. (2025). In Vitro Assessment of the Prebiotic Effects of Poria Cocos Polysaccharides Using Fecal Microbiota from Normal-Weight and Obese Children. Foods, 14(23), 4077. https://doi.org/10.3390/foods14234077




