Current Trends in Gluten-Free Biscuit Formulation Using Rice Flour Enriched with Chestnut Flour and Fruit Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Biscuit Ingredients and Manufacturing Process
2.2. Proximate Composition of Composite Flours, Fruit Powders, and Biscuits
2.3. Physical Properties of Biscuit Formulations
2.4. Macro- and Microelement Content of Composite Flours, Fruit Powders, and Biscuits
2.5. Phytochemical Profile of Composite Flours, Fruit Powders, and Biscuits
2.5.1. Preparation of Alcoholic Extracts from Samples
2.5.2. Assessment of Total Phenolic Content
2.5.3. Assessment of Total Flavonoid Content
2.5.4. Assessment of Antioxidant Capacity
DPPH (1,1-diphenyl-2-picryl-hydrazyl) Assay
FRAP Assay
2.6. Characterization of Flours and Fruit Powders Using Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Small- and Wide-Angle X-Ray Scattering (SAXS/WAXS) of Flours and Fruit Powders
2.8. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Composite Flours, Fruit Powders, and Biscuits
3.2. Physical Properties of Biscuit Formulations
3.3. Macro- and Microelement Profile of Composite Flours, Fruit Powders, and Biscuits
3.4. Phytochemical Profile and Antioxidant Activity of Composite Flours and Fruit Powders
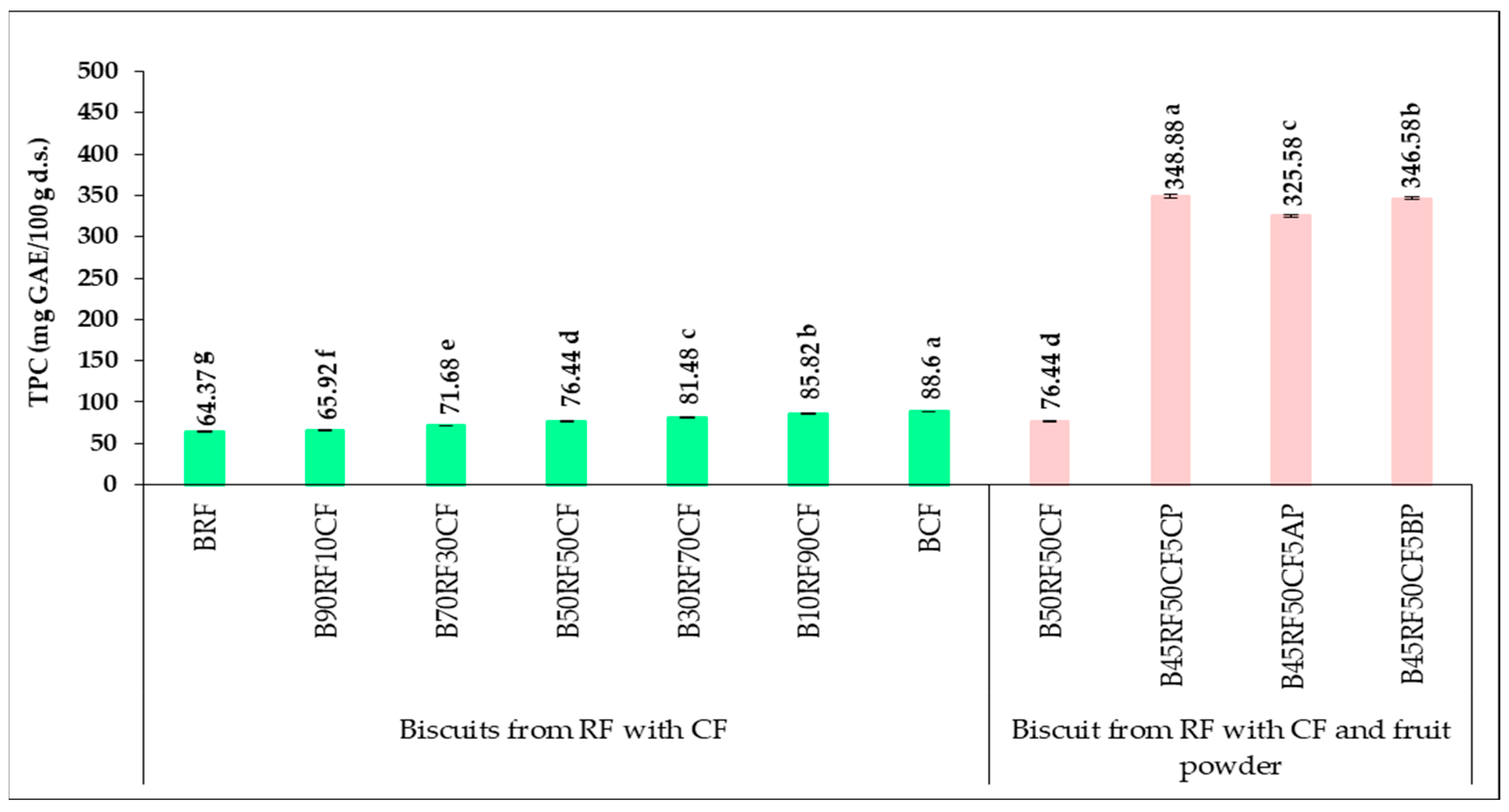
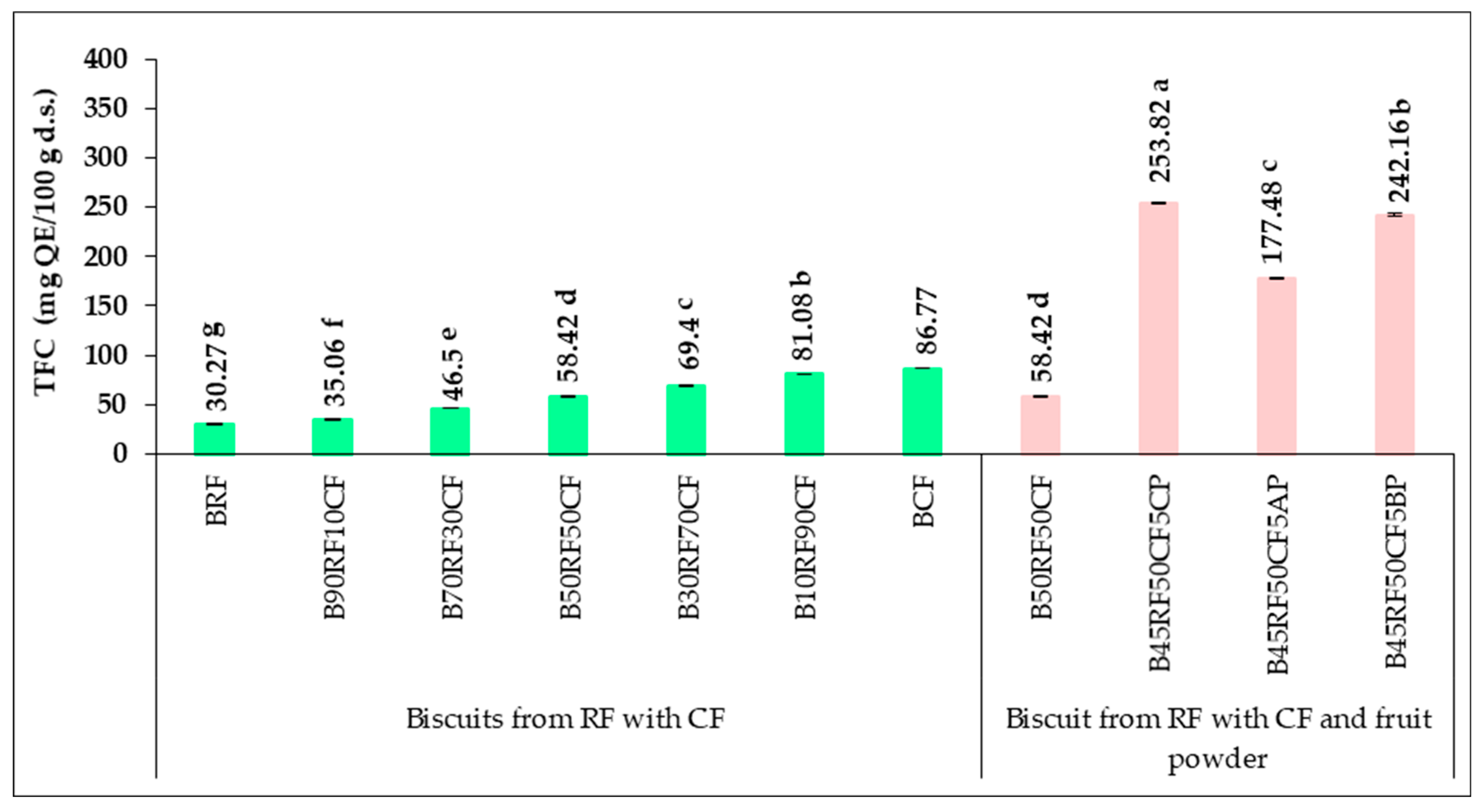
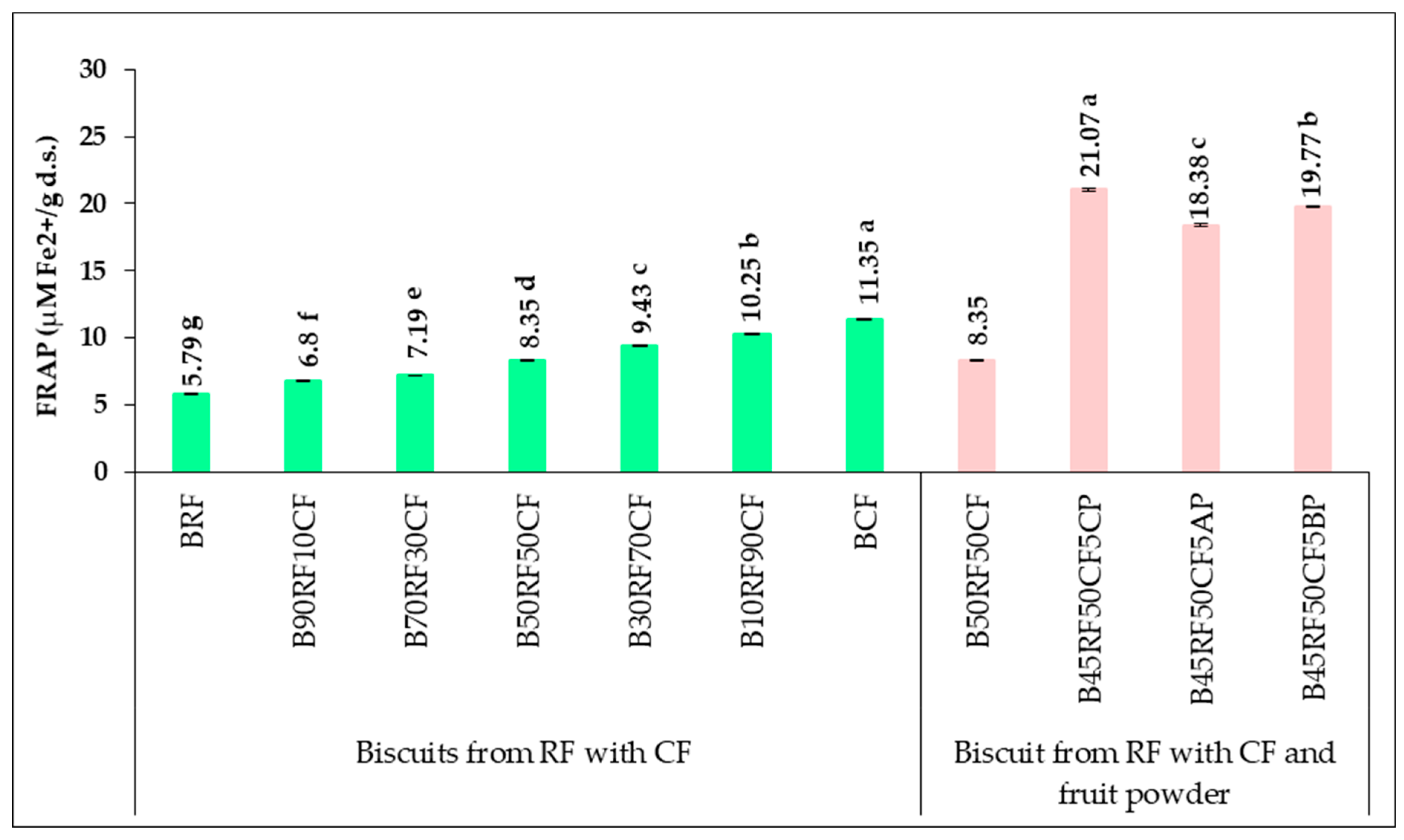
3.5. Phytochemical Profile and Antioxidant Activity of Biscuit Formulations
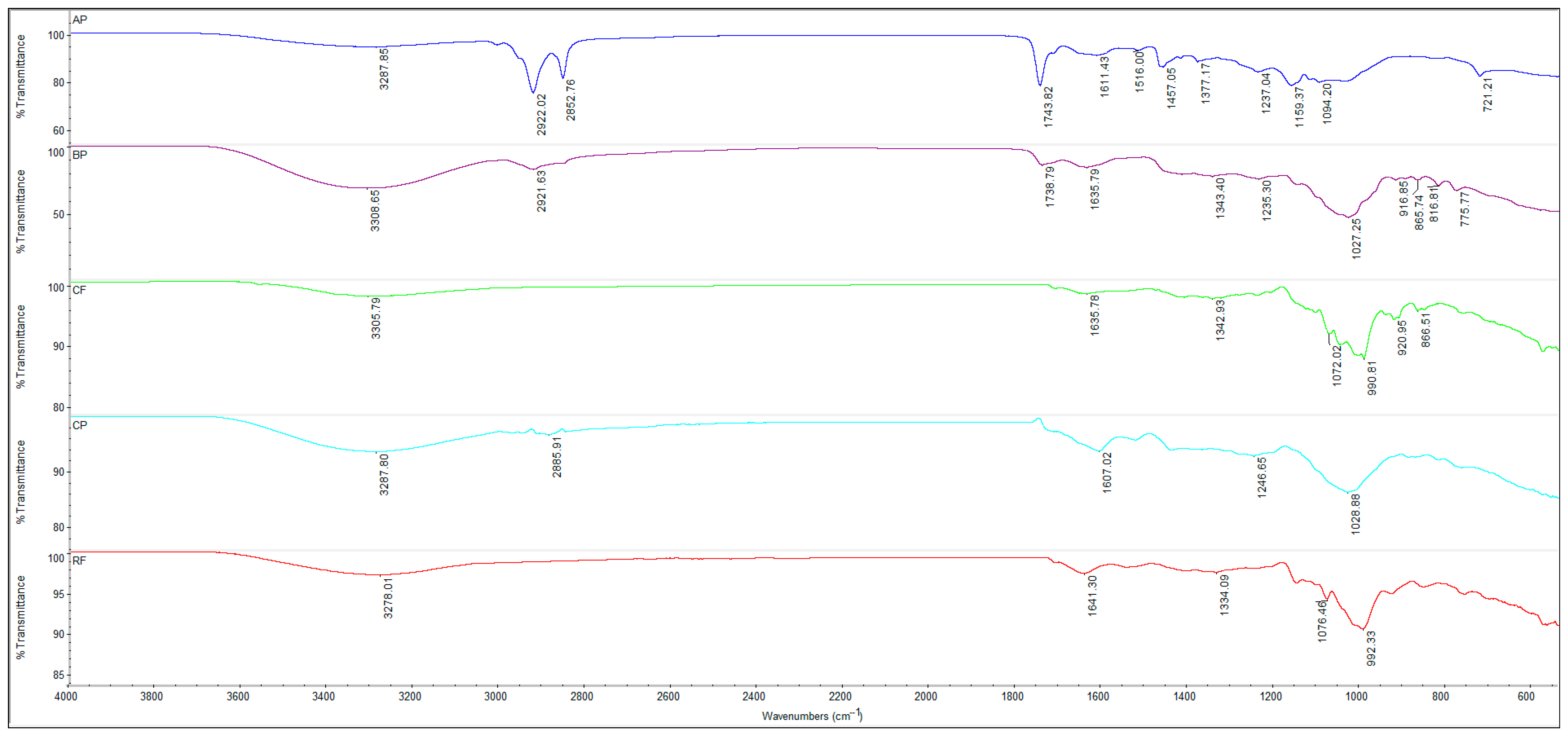
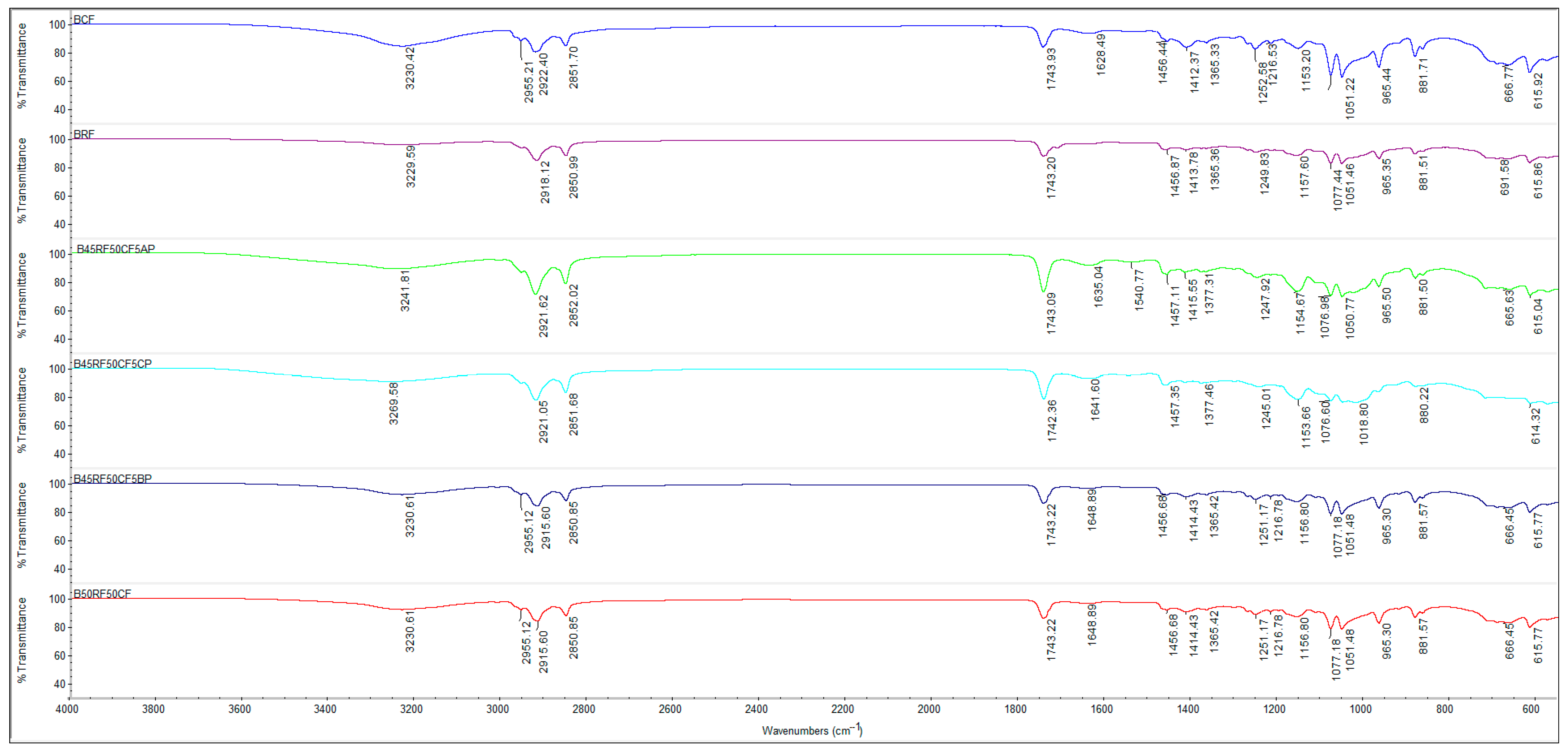
3.6. Characterization of Flours, Fruit Powders and Biscuit Formulations Using Fourier Transform Infrared Spectroscopy (FTIR)
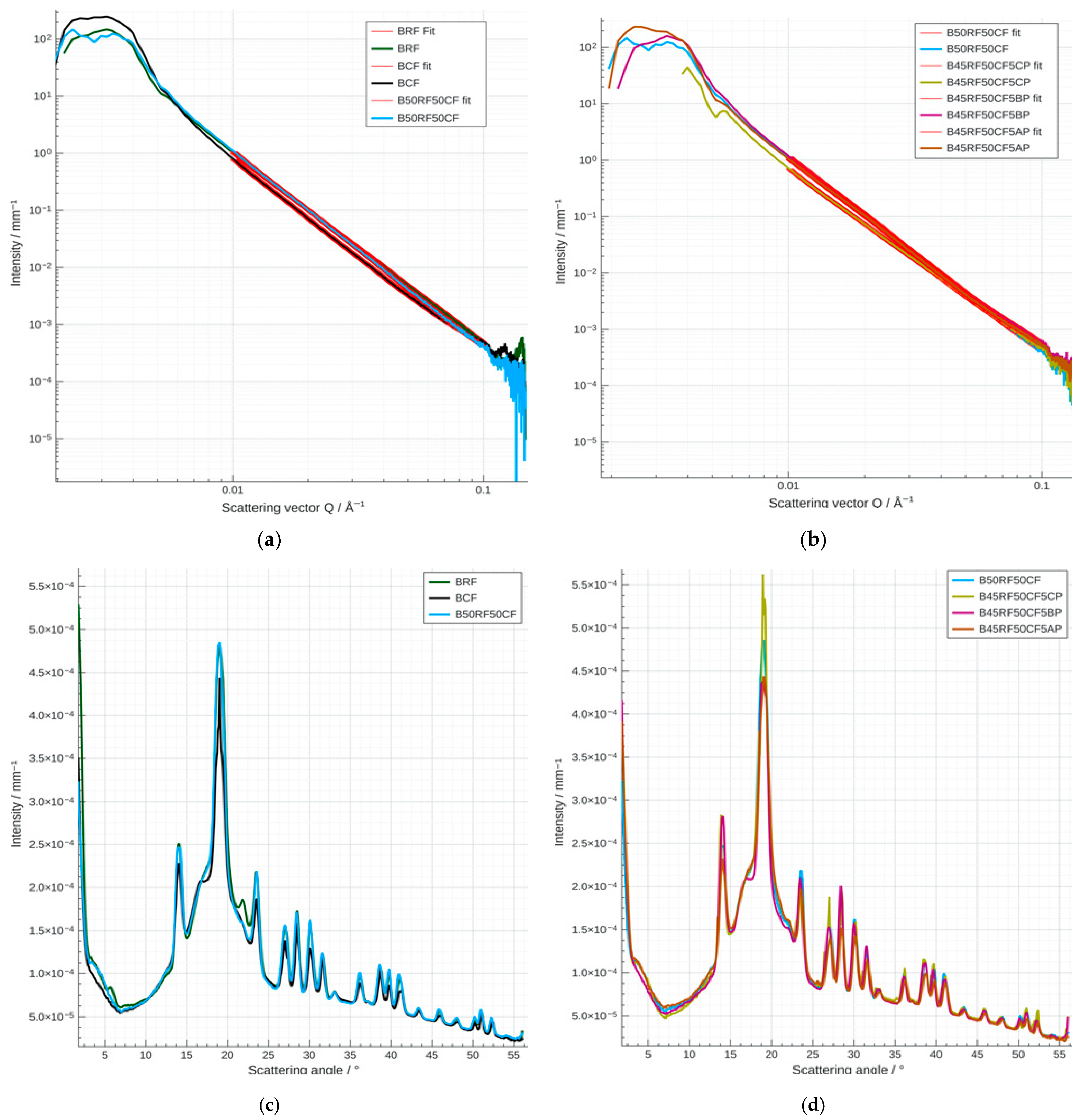
3.7. Small- and Wide-Angle X-Ray Scattering (SAXS/WAXS) Analysis of Flours, Fruit Powders and Biscuit Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boruah, B.; Ray, S. Current progress in the valorization of food industrial by-products for the development of functional food products. Food Sci. Appl. Biotechnol. 2024, 7, 289–317. [Google Scholar] [CrossRef]
- Siminiuc, R.; Țurcanu, D. Certain Aspects of Nutritional Security of People with Gluten-Related Disorders. Food Nutr. Res. 2020, 11, 1012–1031. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Oyesiji, O.O. Gluten free rice-soy pasta: Proximate composition, textural properties and sensory attributes. Heliyon 2021, 7, e06052. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Absi, Y.; Hernández-Jiménez, M.; Revilla, I. Nutritional value, mineral composition, fatty acid profile and bioactive compounds of commercial plant-based gluten-free flours. Appl. Sci. 2023, 13, 2309. [Google Scholar] [CrossRef]
- Demirkesen, I. Formulation of chestnut cookies and their rheological and quality characteristics. J. Food Qual. 2016, 39, 264–273. [Google Scholar] [CrossRef]
- Torra, M.; Belorio, M.; Ayuso, M.; Carocho, M.; Ferreira, I.C.; Barros, L.; Gómez, M. Chickpea and Chestnut Flours as Non-Gluten Alternatives in Cookies. Foods 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Khorshidian, N.; Yousefi, M.; Khaneghah, A.M. Physicochemical, rheological, and sensory properties of gluten-free cookie produced by flour of chestnut, date seed, and modified starch. J. Food Qual. 2022, 2022, 5159084. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Vasconcelos, M.D.C.D.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Michałowska, D. Effect of Coconut and Chestnut Flour Supplementations on Texture, Nutritional and Sensory Properties of Baked Wheat Based Bread. Molecules 2021, 26, 4641. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Attar, H. Effect of drying method on rheological, thermal, and structural properties of chestnut flour doughs. Food Hydrocoll. 2015, 51, 76–87. [Google Scholar] [CrossRef]
- Demirkesen, I.; Sumnu, G.; Sahin, S.; Uysal, N. Optimisation of formulations and infrared–microwave combination baking conditions of chestnut–rice breads. Int. J. Food Sci. Technol. 2011, 46, 1809–1815. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Rama, B. Influence of the chestnuts drying temperature on the rheological properties of their doughs. Food Bioprod. Process. 2013, 91, 7–13. [Google Scholar] [CrossRef]
- Silav-Tuzlu, G.; Tacer-Caba, Z. Influence of chia seed, buckwheat and chestnut flour addition on the overall quality and shelf life of the gluten-free biscuits. Food Technol. Biotechnol. 2021, 59, 463–474. [Google Scholar] [CrossRef]
- Sharma, C.; Devi, A. Effects of soy and water chestnut flour on the quality of cookies. Asian J. Dairy Food Res. 2021, 40, 332–336. [Google Scholar] [CrossRef]
- Kossyva, V.; Vrontaki, M.; Manouras, V.; Tzereme, A.; Meleti, E.; Dimitriou, L.; Maisoglou, I.; Alexandraki, M.; Koureas, M.; Malissiova, E.; et al. From Waste to Worth: Utilizing Downgraded Greek Chestnuts in Gluten-Free Functional Biscuits. Sci 2025, 7, 106. [Google Scholar] [CrossRef]
- Mert, S.; Sahin, S.; Sumnu, G. Development of gluten-free wafer sheet formulations. LWT-Food Sci. Technol. 2015, 63, 1121–1127. [Google Scholar] [CrossRef]
- Choi, J.H.; Ahn, H.D.; Hwang, J.M.; Kim, Y.J.; Kim, S.B.; Kim, I.B.; Choi, H.Y. Effects of chestnut powder content on the quality characteristics and antioxidant activity of rice muffins. Food Sci. Preserv. 2024, 31, 256–266. [Google Scholar] [CrossRef]
- Littardi, P.; Paciulli, M.; Carini, E.; Rinaldi, M.; Rodolfi, M.; Chiavaro, E. Quality evaluation of chestnut flour addition on fresh pasta. LWT 2020, 126, 109303. [Google Scholar] [CrossRef]
- Kruger, J.; Taylor, J.R.; Ferruzzi, M.G.; Debelo, H. What is food-to-food fortification? A working definition and framework for evaluation of efficiency and implementation of best practices. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3618–3658. [Google Scholar] [CrossRef]
- Borczak, B.; Kapusta-Duch, J.; Domagała, D.; Doskočil, I. Study on the Potential Antitumor Activity of Cookies Enriched with Sambucus nigra L., Aronia melanocarpa, Hippophae rhamnoides L., and Crataegus L., on WM793 Melanoma and MCF-7 Breast Cell Lines. Appl. Sci. 2023, 13, 12256. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, S.K.; Mo, E.K. Quality characteristics of cookies with acaiberry (Euterpe oleracea Mart.) powder added. Food Sci. Preserv. 2014, 21, 661–667. [Google Scholar] [CrossRef]
- Krajewska, A.; Dziki, D. Enrichment of cookies with fruits and their by-products: Chemical composition, antioxidant properties, and sensory changes. Molecules 2023, 28, 4005. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Zbikowska, A.; Lukasiak, P.; Kowalska, M.; Lukasiak, A.; Kozłowska, M.; Marciniak-Lukasiak, K. Incorporation of Chokeberry Pomace into Baked Products: Influence on the Quality of the Dough and the Muffins. Appl. Sci. 2024, 14, 9675. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Wu, J.; Wang, H.X.; Li, S.S.; Zheng, X.C.; Du, H.; Xu, Y.J.; Wang, L.S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Li, Z.; Ni, X.; Yang, J.; Awasthi, M.K. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Salihu, S.; Gashi, N.; Hasani, E. Effect of plant extracts addition on the physico-chemical and sensory properties of biscuits. Appl. Sci. 2023, 13, 9674. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Lal, A.B.; Charles, A.P.R.; Singh, A.P.; Khare, A.; Vashisht, P. Development of Gluten Free Cookie Using Chestnut and Foxnut Flour Blend: Composition Optimization through Response Surface Methodology. Asian J. Dairy Food Res. 2025, 44, 84–91. [Google Scholar] [CrossRef]
- Sady, S.; Sielicka-Różyńska, M. Quality assessment of experimental cookies enriched with freeze-dried black chokeberry. Acta Sci. Pol. Technol. Aliment. 2019, 18, 463–471. [Google Scholar] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- ISO 2171:2023; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2023.
- Borşa, A.R.; Păucean, A.; Fogarasi, M.; Ranga, F.; Borșa, A.; Tanislav, A.E.; Mureșan, V.; Semeniuc, C.A. Utilisation of rosehip waste powder as a functional ingredient to enrich waffle cones with fibres, polyphenols, and carotenoids. Foods 2025, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Drakos, A.; Andrioti-Petropoulou, L.; Evageliou, V.; Mandala, I. Physical and textural properties of biscuits containing jet milled rye and barley flour. J. Food Sci. Technol. 2019, 56, 367–375. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G.; et al. Lupin (Lupinus spp.)-Fortified Bread: A Sustainable, Nutritionally, Functionally, and Technologically Valuable Solution for Bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef]
- Litwinek, D.; Gumul, D.; Łukasiewicz, M.; Zieba, T.; Kowalski, S. The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread. Appl. Sci. 2023, 13, 7670. [Google Scholar] [CrossRef]
- Blanch, G.P.; Ruiz del Castillo, M.L. Effect of Baking Temperature on the Phenolic Content and Antioxidant Activity of Black Corn (Zea mays L.) Bread. Foods 2021, 10, 1202. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Radulov, I.; Raba, D.N.; Cocan, I.; Negrea, M.; Misca, C.D.; Dragomir, C.; Dossa, S.; Suster, G. Strategies to Formulate Value-Added Pastry Products from Composite Flours Based on Spelt Flour and Grape Pomace Powder. Foods 2023, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Pop, I.A.; Dossa, S.; Stoin, D.; Neagu, C.; Moigradean, D.; Alexa, E.; Poiana, M.A. Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour. Foods 2025, 14, 3343. [Google Scholar] [CrossRef]
- Raba, D.N.; Radulov, I.; Alexa, E.; Poiana, M.A.; Misca, C.D.; Cocan, I.; Negrea, M.; Suster, G.; Dragomir, C. Insights into the development of pastry products based on spelt flour fortified with lingonberry powder. Agronomy 2023, 13, 2609. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Sahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010, 101, 329–336. [Google Scholar] [CrossRef]
- Hegazi, N.A.; Kamil, M.M.; Hussein, A.M.S.; Bareh, G.F. Chemical and technological properties of improved biscuit by chestnut flour. Int. J. Food Sci. Nutr. 2014, 3, 7–15. Available online: https://ijfans.org/uploads/paper/88854a78274af9f88e4d9f6986aa5f8e.pdf (accessed on 15 October 2025).
- Loubet Filho, P.S.; de Arruda Melo, J.; Baptista, R.C.; de Lima, B.G.T.; Reguengo, L.M.; Falk, R.B.; Wagner, R.; Junior, S.B.; dos Santos, E.F.; Betim Cazarin, C.B.; et al. The effects of acai (Euterpe oleracea) intake on gut bacteria and their metabolites in obese mice. J. Funct. Foods 2025, 127, 106748. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, A.R.; Park, J.H.; Kim, Y.; Kwon, Y.; Hong, E.Y.; Han, N.S.; Eom, H.J. Nutritional compositions and physicochemical properties of two domestic aronia (A. melanocarpa) varieties. Korean J. Food Nutr. 2016, 29, 283–289. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Galvez, A.; Pasten, A.; Ah-Hen, K.S.; Mejias, N.; Sepúlveda, L.; Poblete, J.; Gomez-Perez, L.S. Drying: A practical technology for blueberries (Vaccinium corymbosum L.)—Processes and their effects on selected health-promoting properties. Antioxidants 2024, 13, 1554. [Google Scholar] [CrossRef]
- Alinovi, M.; Rinaldi, M.; Paciulli, M.; Littardi, P.; Chiavaro, E. Chestnut peels and wheat bran at different water level influence the physical properties of pan bread. Eur. Food Res. Technl. 2022, 248, 1227–1237. [Google Scholar] [CrossRef]
- Raczkowska, E.; Nowicka, P.; Wojdyło, A.; Styczyńska, M.; Lazar, Z. Chokeberry pomace as a component shaping the content of bioactive compounds and nutritional, health-promoting (anti-diabetic and antioxidant) and sensory properties of shortcrust pastries sweetened with sucrose and erythritol. Antioxidants 2022, 11, 190. [Google Scholar] [CrossRef]
- Lucas, B.F.; Guelpa, R.; Brunner, T.; Denkel, C.; Costa, J. Development of snacks enriched with Açai pulp powder. In Proceedings of the 7th Simposio de Segurança Alimentar, Bento Gonçalves, RS, Brazil, 29–31 October 2020; Available online: https://arbor.bfh.ch/handle/arbor/41735 (accessed on 15 October 2025).
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef]
- Singh, G.D.; Riar, C.S.; Saini, C.; Bawa, A.S.; Sogi, D.S.; Saxena, D.C. Indian water chestnut flour method optimization for preparation, its physicochemical, morphological, pasting properties and itspotential in cookies preparation. LWT-Food Sci. Technol. 2011, 44, 665–672. [Google Scholar] [CrossRef]
- Suriya, M.; Rajput, R.; Reddy, C.K.; Haripriya, S.; Bashir, M. Functional and physicochemical characteristics of cookies prepared from Amorphophallus paeoniifolius flour. J. Food Sci. Technol. 2017, 54, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Ganorkar, P.M.; Jain, R.K. Effect of flaxseed incorporation on physical, sensorial, textural and chemical attributes of cookies. Int. Food Res. J. 2014, 21, 1515–1521. [Google Scholar]
- Arun, K.B.; Persia, F.; Aswathy, P.S.; Chandran, J.; Sajeev, M.S.; Jayamurthy, P.; Nisha, P. Plantain peel-a potential source of antioxidant dietary fibre for developing functional cookies. J. Food Sci. Technol. 2015, 52, 6355–6364. [Google Scholar] [CrossRef]
- Choudhury, M.; Badwaik, L.S.; Borah, P.K.; Sit, N.; Deka, S.C. Influence of bamboo shoot powder fortification on physicochemical, textural and organoleptic characteristics of biscuits. J. Food Sci. Technol. 2015, 52, 6742–6748. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Ali, S.A.; Ali, R.; Sayeed, S.A.; Mobin, L. Exploring the potential of bottle gourd (Lagenaria siceraria) flour as a fat mimetic in biscuits with improved physicochemical and nutritional characteristics and anti-diabetic properties. Ital. J. Food Sci. 2022, 34, 50–66. [Google Scholar] [CrossRef]
- Mete, M.; Dülger Altiner, D. Chestnut flour and applications of utilization. IJFER 2017, 3, 9–16. [Google Scholar]
- Aglietti, C.; Cappelli, A.; Andreani, A. From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain. Sustainability 2022, 14, 12181. [Google Scholar] [CrossRef]
- Shalini, P.K.R. Formulation and sensory evaluation of water chestnut flour laddoo. JPHT 2024, 12, 99–105. [Google Scholar]
- D’Eusanio, V.; Marchetti, A.; Rivi, M.; Morelli, L.; Scarponi, P.; Forti, L.; Tassi, L. Mineral Composition Analysis of Red Horse-Chestnut (Aesculus × Carnea) Seeds and Hydroalcoholic Crude Extract Using ICP OES. Molecules 2025, 30, 819. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, M.; Chen, L.; Zheng, B. Impact of using whole chestnut flour as a substitute for cake flour on digestion, functional and storage properties of chiffon cake: A potential application study. Food Chem. 2024, 432, 137016. [Google Scholar] [CrossRef]
- Kömürcü, T.C.; Bilgiçli, N. Glutenli ve Glutensiz Bisküvilerin Kestane, Lüpen ve Balkabağı Unlarından Hazırlanan Kompozit Un ile Zenginleştirilmesi. JIST 2023, 13, 1724–1737. [Google Scholar] [CrossRef]
- Zlateva, D.; Stefanova, D.; Chochkov, R.; Ivanova, P. Mineral content of wheat bread enriched with chestnut and rosehip flour. Bulg. J. Agric. Sci. 2024, 30, 333–340. [Google Scholar]
- Catana, M.; Catana, L.; Iorga, E.; Asanica, A.C.; Belc, N. Bakery products fortified with dried fruits of Aronia melanocarpa. Sci. Pap.-Ser. B-Hortic. 2018, 62, 693–701. [Google Scholar]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.; Baba, W.N.; Masoodi, F.A.; Bazaz, R. Wheat-water chestnut flour blends: Effect of baking on antioxidant properties of cookies. J. Food Sci. Technol. 2016, 53, 4278–4288. [Google Scholar] [CrossRef]
- Conti, V.; Salusti, P.; Romi, M.; Cantini, C. Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours. Foods 2022, 11, 1364. [Google Scholar] [CrossRef]
- Donno, D.; Fabro, M.; Mellano, M.G.; Gamba, G.; Fioccardi, A.; Beccaro, G.L. Integrating Traditional Wheat-Based Foods with High Health Value Flours: Castanea spp. Agro-Biodiversity in Bakery Products. Agriculture 2022, 12, 946. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Widelska, G.; Wójtowicz, A.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Dib, A.; Matwijczuk, A. Content of Phenolic Compounds and Antioxidant Activity of New Gluten-Free Pasta with the Addition of Chestnut Flour. Molecules 2019, 24, 2623. [Google Scholar] [CrossRef]
- Tolić, M.T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.O.; Pintea, A.M.; Sconța, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Hort. Agrobot. 2011, 39, 70–176. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Ekiert, H. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant activity, total phenolic content and total flavonoid content in sweet chestnut (Castanea sativa Mill.) cultivars grown in Northwest Spain under different environmental conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.; Centeno, J.; Domínguez, R.; Pateiro, M.; Carballo, J.; Lorenzo, J. Total Phenol Content and Antioxidant Activity of Different Celta Pig Carcass Locations as Affected by the Finishing Diet (Chestnuts or Commercial Feed). Antioxidants 2021, 10, 5. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Cascone, G.; Oliviero, M.; Sorrentino, L.; Crescente, G.; Boscaino, F.; Sorrentino, A.; Volpe, M.G.; Moccia, S. Mild Approach for the Formulation of Chestnut Flour-Enriched Snacks: Influence of Processing Parameters on the Preservation of Bioactive Compounds of Raw Materials. Foods 2024, 13, 2651. [Google Scholar] [CrossRef] [PubMed]
- Varastegani, B.; Zzaman, W.; Yang, T.A. Investigation on physicochemical and sensory evaluation of cookies substituted with papaya pulp flour. J. Food Qual. 2015, 38, 175–183. [Google Scholar] [CrossRef]
- Bhaduri, S.; Navder, K. Freeze dried blueberry powder fortification improves the quality of gluten free snacks. J. Food Process Technol. 2014, 5, 396. [Google Scholar] [CrossRef]
- Manisha, I.; Punia, D.; Sindhu, S. Development of bakery products using composite flour of wheat and water chestnut and their sensory and nutritional evaluation. Pharma Innov. 2021, 10, 1038–1042. [Google Scholar]
- Najjar, Z.; Kizhakkayil, J.; Shakoor, H.; Platat, C.; Stathopoulos, C.; Ranasinghe, M. Antioxidant Potential of Cookies Formulated with Date Seed Powder. Foods 2022, 11, 448. [Google Scholar] [CrossRef]
- Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Brennan, C.S. The combined effect of blackcurrant powder and wholemeal flours to improve health promoting properties of cookies. Plant Food Hum. Nutr. 2017, 72, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Thakur, A.; Vaidya, D.; Kaushal, M.; Verma, A.; Gupta, A. Mineral composition physicochemical properties, FTIR spectra and scanning electron microscopy of rice flour. J. Vitam. Miner 2020, 9, 219–328. [Google Scholar]
- Ishikawa, D.; Yang, J.; Fujii, T. Quantification of starch order in physically modified rice flours using small-angle X-ray scattering (SAXS) and Fourier transform infrared (FT-IR) spectroscopy. Appl. Spectrosc. 2021, 75, 1033–1042. [Google Scholar] [CrossRef]
- Siregar, S.; Nurhikmat, A.; Amdani, R.Z.; Hatmi, R.U.; Kobarsih, M.; Kusumaningrum, A.; Karim, M.A.; Dameswari, A.H.; Siswanto, N.; Siswoprayogi, S.; et al. Estimation of proximate composition in rice using ATR-FTIR spectroscopy and Chemometrics. ACS Omega 2024, 9, 32760–32768. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Beigh, M.A.; Naseer, B.; Naik, H.R. Visco-thermal and structural characterization of water chestnut flour. J. Food Sci. Technol. 2020, 57, 2949–2959. [Google Scholar] [CrossRef]
- Wani, I.A.; Hamid, H.; Hamdani, A.M.; Gani, A.; Ashwar, B.A. Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting. J. Adv. Res. 2017, 8, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, R.; Derycke, V.; Delcour, J.A.; Goderis, B.; Reynaers, H.; Koch, M.H. Structural transformations during gelatinization of starches in limited water: Combined wide-and small-angle X-ray scattering study. Biomacromolecules 2006, 7, 1231–1238. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, P.; Zhao, Y.; Zhu, Y.; Xiao, X. The effect of protein–starch interaction on the structure and properties of starch, and its application in flour products. Foods 2025, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Fluerasu, D.; Neagu, C.; Dossa, S.; Negrea, M.; Jianu, C.; Berbecea, A.; Stoin, D.; Lalescu, D.; Brezovan, D.; Cseh, L.; et al. The Use of Whey Powder to Improve Bread Quality: A Sustainable Solution for Utilizing Dairy By-Products. Foods 2025, 14, 2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Wang, S.; Copeland, L. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Sci. Rep. 2016, 6, 28271. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Zhong, Y.; Zhou, Q.; Lv, Q.; Chen, L.; Liu, X. The effects of molecular fine structure on rice starch granule gelatinization dynamics as investigated by in situ small-angle X-ray scattering. Food Hydrocoll. 2021, 121, 107014. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Prieto, D.M. Influence of the particle size on the rheological behaviour of chestnut flour doughs. J. Food Eng. 2010, 100, 270–277. [Google Scholar] [CrossRef]
- Zheng, F.; Ren, F.; Zhu, X.; Han, Z.; Jia, Y.; Liu, X.; Liu, H. The interaction between starch and phenolic acids: Effects on starch physicochemical, digestibility and phenolic acids stability. Food Funct. 2025, 16, 4202–4225. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Shahid, M.; Muhammad, A.; Faiq, M. Compositional analysis, functional properties and microstructure of cookies fortified with olive leaves powder. J. Microbiol. Biotechnol. Food Sci. 2025, 15, e12215. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Chen, R.; Liu, F.; Wei, S. Recent advances in structural characterization of biomacromolecules in foods via small-angle X-ray scattering. Front. Nutr. 2022, 9, 1039762. [Google Scholar] [CrossRef]
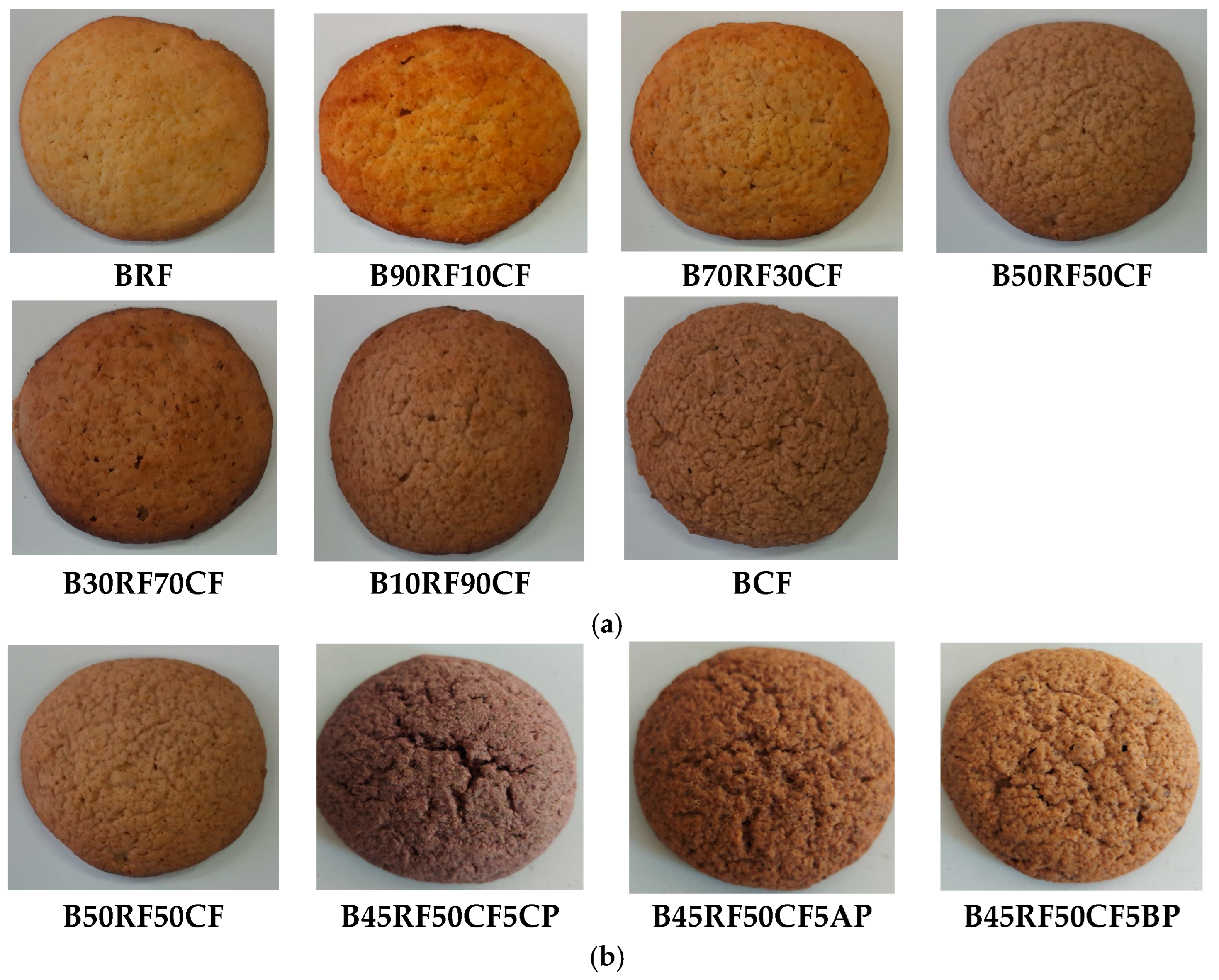

| Ingredients (g) | Gluten-Free Biscuit Formulations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Set I | Set II | ||||||||||
| BRF | B90RF10CF | B70RF30CF | B50RF50CF | B30RF70CF | B10RF90CF | BCF | B50RF50CF | B45RF50CF5CP | B45RF50CF5AP | B45RF50CF5BP | |
| RF | 160 | 144 | 112 | 80 | 48 | 16 | - | 80 | 72 | 72 | 72 |
| CF | - | 16 | 48 | 80 | 112 | 144 | 160 | 80 | 80 | 80 | 80 |
| Stevia | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Butter | 110 | 110 | 110 | 110 | 110 | 110 | 110 | 110 | 110 | 110 | 110 |
| Egg | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Salt | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baking powder | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| CP | - | - | - | - | - | - | - | - | 8 | - | - |
| AP | - | - | - | - | - | - | - | - | - | 8 | - |
| BP | - | - | - | - | - | - | - | - | - | - | 8 |
| Chemical Parameters | Energy Value (kcal/100 g) | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Moisture (%) | Protein (%) | Lipids (%) | Ash (%) | CRB (%) | Crude Fiber (%) | |
| RF | 10.33 ± 0.16 a | 5.75 ± 0.03 f | 1.65 ± 0.26 e | 2.57 ± 0.12 a | 79.70 ± 0.22 a | 2.65 ± 0.11 g | 356.61 ± 0.11 g |
| 90RF10CF | 10.04 ± 0.10 b | 5.85 ± 0.02 e | 1.86 ± 0.22 e | 2.59 ± 0.09 a | 79.66 ± 0.24 a | 3.31 ± 0.14 f | 358.76 ± 0.13 f |
| 70RF30CF | 9.46 ± 0.16 c | 6.04 ± 0.05 d | 2.29 ± 0.19 d | 2.64 ± 0.08 a | 79.57 ± 0.22 a | 4.62 ± 0.13 e | 363.06 ± 0.12 e |
| 50RF50CF | 8.88 ± 0.32 d | 6.23 ± 0.04 c | 2.72 ± 0.21c | 2.68 ± 0.10 a | 79.49 ± 0.23 a | 5.94 ± 0.15 d | 367.37 ± 0.10 d |
| 30RF70CF | 8.29 ± 0.14 e | 6.42 ± 0.03 b | 3.15 ± 0.14 b | 2.72 ± 0.10 a | 79.41 ± 0.25 a | 7.25 ± 0.08 c | 371.67 ± 0.11 c |
| 10RF90CF | 7.71 ± 0.16 f | 6.62 ± 0.15 a | 3.58 ± 0.27 a | 2.77 ± 0.07 a | 79.32 ± 0.23 a | 8.57 ± 0.12 b | 375.98 ± 0.14 b |
| CF | 7.42 ± 0.12 g | 6.71 ± 0.12 a | 3.79 ± 0.20 a | 2.79 ± 0.10 a | 79.28 ± 0.22 a | 9.23 ± 0.15 a | 378.13 ± 0.13 a |
| CP | 2.86 ± 0.17 c | 6.66 ± 0.17 c | 4.45 ± 0.11 b | 2.46 ± 0.08 c | 83.57 ± 0.12 a | 18.22 ± 0.14 b | 400.98 ± 0.04 b |
| AP | 5.56 ± 0.13 b | 9.02 ± 0.15 a | 45.11 ± 0.19 a | 4.87 ± 0.11 a | 35.44 ± 0.11 c | 20.30 ± 0.15 a | 583.83 ± 0.09 a |
| BP | 7.90 ± 0.14 a | 7.28 ± 0.16 b | 4.13 ± 0.18 c | 4.03 ± 0.14 b | 76.65 ± 0.10 b | 14.14 ± 0.12 c | 372.93 ± 0.05 c |
| 50RF50CF | 8.88 ± 0.32 a | 6.23 ± 0.04 a | 2.72 ± 0.21b | 2.68 ± 0.10 a | 79.49 ± 0.23 a | 5.94 ± 0.15 b | 367.37 ± 0.10 d |
| 45RF50CF5CP | 8.50 ± 0.30 a | 6.28 ± 0.14 a | 2.86 ± 0.29 b | 2.72 ± 0.09 a | 79.68 ± 0.34 a | 6.72 ± 0.13 a | 369.59 ± 0.12 b |
| 45RF50CF5AP | 8.64 ± 0.29 a | 6.40 ± 0.15 a | 4.89 ± 0.27 a | 2.80 ± 0.08 a | 77.28 ± 0.32 b | 6.82 ± 0.12 a | 378.73 ± 0.12 a |
| 45RF50CF5BP | 8.75 ± 0.31 a | 6.31 ± 0.12 a | 2.84 ± 0.30 b | 2.75 ± 0.11 a | 79.34 ± 0.33 a | 6.51 ± 0.13 a | 368.19 ± 0.13 c |
| Sample | Chemical Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Protein (%) | Lipids (%) | Ash (%) | Total Carbohydrates (%) | Crude Fiber (%) | Energy Value (kcal/100 g) | |
| BRF | 8.39 ± 0.21 a | 6.88 ± 0.16c | 11.49 ± 0.32 f | 2.89 ± 0.12 e | 70.39 ± 0.02 a | 2.73 ± 0.11 b | 412.36 ± 0.11 g |
| B90RF10CF | 8.16 ± 0.14 a | 6.96 ± 0.06 c | 11.87 ± 0.06 e | 2.97 ± 0.10 e | 70.04 ± 0.06 b | 3.30 ± 0.14 c | 414.82 ± 0.17 f |
| B70RF30CF | 7.72 ± 0.19 b | 7.17 ± 0.14 b | 12.64 ± 0.19 d | 3.15 ± 0.07 d | 69.33 ± 0.10 c | 4.44 ± 0.16 d | 419.73 ± 0.12 e |
| B50RF50CF | 7.28 ± 0.18 c | 7.39 ± 0.17 b | 13.40 ± 0.14 c | 3.32 ± 0.05 c | 68.62 ± 0.08 d | 5.58 ± 0.12 e | 424.64 ± 0.09 d |
| B30RF70CF | 6.83 ± 0.14 d | 7.60 ± 0.11b | 14.17 ± 0.12 b | 3.49 ± 0.02 c | 67.90 ± 0.04 e | 6.72 ± 0.13 f | 429.55 ± 0.12 c |
| B10RF90CF | 6.39 ± 0.15 e | 7.82 ± 0.10 a | 14.93 ± 0.21 a | 3.66 ± 0.09 a | 67.20 ± 0.02 f | 7.87 ± 0.12 g | 434.46 ± 0.11 b |
| BCF | 6.16 ± 0.12 e | 7.93 ± 0.15 a | 15.32 ± 0.23 a | 3.75 ± 0.13 a | 66.84 ± 0.09 g | 8.44 ± 0.13 a | 436.92 ± 0.16 a |
| B50RF50CF | 7.28 ± 0.18 a | 7.39 ± 0.17 a | 13.40 ± 0.14 b | 3.32 ± 0.05 a | 68.62 ± 0.08 c | 5.58 ± 0.12 b | 424.64 ± 0.21 b |
| B45RF50CF5CP | 7.01 ± 0.15 a | 7.43 ± 0.26 a | 13.45 ± 0.07 b | 3.34 ± 0.05 a | 69.27 ± 0.16 a | 6.36 ± 0.17 a | 424.07 ± 0.20 c |
| B45RF50CF5AP | 7.13 ± 0.17 a | 7.50 ± 0.16 a | 15.08 ± 0.05 a | 3.42 ± 0.01 a | 66.87 ± 0.18 d | 6.46 ± 0.14 a | 433.21 ± 0.24 a |
| B45RF50CF5BP | 7.25 ± 0.12 a | 7.46 ± 0.23 a | 13.50 ± 0.06 b | 3.38 ± 0.07 a | 68.93 ± 0.16 b | 6.15 ± 0.09 a | 422.67 ± 0.19 d |
| Sample | Physical Properties | |||
|---|---|---|---|---|
| Diameter (mm) | Thickness (mm) | Weight (g) | Spread Ratio | |
| BRF | 60.10 ± 0.19 a | 8.60 ± 0.17 a | 25.92 ± 0.15 a | 6.98 ± 0.03 a |
| B90RF10CF | 59.41 ± 0.18 b | 8.73 ± 0.12 a | 25.83 ± 0.18 a | 6.80 ± 0.02 b |
| B70RF30CF | 58.13 ± 0.09 c | 8.99 ± 0.15 a | 25.66 ± 0.17 a | 6.46 ± 0.05 c |
| B50RF50CF | 56.74 ± 0.13 d | 9.25 ± 0.16a | 25.49 ± 0.11 a | 6.13 ± 0.06 d |
| B30RF70CF | 55.45 ± 0.10 e | 9.51 ± 0.16 a | 25.31 ± 0.15 a | 5.83 ± 0.09 e |
| B10RF90CF | 54.04 ± 0.18 f | 9.77 ± 0.19 a | 25.14 ± 0.12 a | 5.53 ± 0.04 f |
| BCF | 53.42 ± 0.19 g | 9.90 ± 0.18 a | 25.06 ± 0.17 a | 5.39 ± 0.08 g |
| B50RF50CF | 56.74 ± 0.13 a | 9.25 ± 0.16 a | 25.49 ± 0.11 a | 6.13 ± 0.02 a |
| B45RF50CF5CP | 56.44 ± 0.25 a | 9.29 ± 0.22 a | 25.47 ± 0.15 a | 6.08 ± 0.09 a |
| B45RF50CF5AP | 56.49 ± 0.26 a | 9.27 ± 0.20 a | 25.46 ± 0.13 a | 6.09 ± 0.03 a |
| B45RF50CF5BP | 56.40± 0.18 a | 9.30 ± 0.22 a | 25.45 ± 0.19 a | 6.06 ± 0.04 a |
| Sample | Macro and Microelement Content (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| K | P | Mg | Ca | Fe | Zn | Mn | Cu | |
| RF | 1050.47 ± 0.10 g | 630.63 ± 0.37 g | 636.08 ± 0.11 g | 110.86 ± 0.12 g | 29.47 ± 0.09 g | 14.34 ± 0.09 g | 13.74 ± 0.08 g | 3.07 ± 0.08 g |
| 90RF10CF | 1191.06 ± 0.16 f | 689.59 ± 0.28 f | 656.54 ± 0.09 f | 149.23 ± 0.09 f | 29.70 ± 0.04 f | 14.90 ± 0.03 f | 14.18 ± 0.28 f | 3.32 ± 0.02 f |
| 70RF30CF | 1472.25 ± 0.12 e | 807.51 ± 0.32 e | 697.46 ± 0.04 e | 225.97 ± 0.18 e | 30.25 ± 0.02 e | 16.06± 0.07 e | 15.08 ± 0.16 e | 3.81 ± 0.09 e |
| 50RF50CF | 1753.43 ± 0.18 d | 925.44 ± 0.26 d | 738.38 ± 0.18 d | 302.72 ± 0.19 d | 30.73 ± 0.12 d | 17.20 ± 0.13 d | 15.99 ± 0.09 d | 4.40 ± 0.13 d |
| 30RF70CF | 2034.62 ± 0.16 c | 1043.36 ± 0.27 c | 779.29 ± 0.05 c | 379.46 ± 0.12 c | 31.22 ± 0.04 c | 18.30 ± 0.04 c | 16.88 ± 0.14 c | 4.93 ± 0.04 c |
| 10RF90CF | 2315.80 ± 0.19 b | 1161.20 ± 0.28 b | 820.21 ± 0.19 b | 456.20 ± 0.14 b | 31.80 ± 0.10 b | 19.50 ± 0.16 b | 17.78 ± 0.08 b | 5.46 ± 0.02 b |
| CF | 2456.39 ± 0.15 a | 1220.25 ± 0.25 a | 840.67 ± 0.02 a | 494.58± 0.09 a | 32.07 ± 0.03 a | 20.06 ± 0.04 a | 18.18 ± 0.03 a | 5.72 ± 0.06 a |
| CP | 2514.21 ± 0.14 a | 2326.87 ± 0.26 a | 823.05 ± 0.25 b | 1256.66 ± 0.12 c | 84.57 ± 0.18 a | 135.05 ± 0.19 a | 7.48 ± 0.21 c | 9.46 ± 0.20 b |
| AP | 650.86 ± 0.19 c | 550.85 ± 0.22 b | 1560.82 ± 0.26 a | 2210.73 ± 0.25 a | 40.81 ± 0.09 c | 65.77 ± 0.26 b | 24.07 ± 0.09 b | 10.84 ± 0.26 a |
| BP | 994.78 ± 0.15 b | 82.55 ± 0.27 c | 675.07 ± 0.24 c | 1852.13 ± 0.22 b | 75.07 ± 0.19 b | 24.95 ± 0.23 c | 26.46 ± 0.25 a | 5.31 ± 0.13 c |
| 50RF50CF | 1753.43 ± 0.18 b | 925.44 ± 0.16 b | 738.38 ± 0.08 d | 302.72 ± 0.19 d | 30.73 ± 0.12 d | 17.20 ± 0.13 d | 15.99 ± 0.09 b | 4.40 ± 0.13 b |
| 45RF50CF5CP | 1826.62 ± 0.13 a | 1010.25 ± 0.25 a | 746.61 ± 0.03 b | 360.01 ± 0.14 c | 33.52 ± 0.09 a | 23.19 ± 0.04 a | 15.66 ± 0.20 c | 4.72 ± 0.05 a |
| 45RF50CF5AP | 1732.56 ± 0.06 d | 921.45 ± 0.04 c | 784.61 ± 0.02 a | 407.71 ± 0.22 a | 31.30 ± 0.02 c | 19.74 ± 0.15 b | 16.48 ± 0.02 a | 4.78 ± 0.11 a |
| 45RF50CF5BP | 1750.65 ± 0.08 c | 898.03 ± 0.28 d | 740.32 ± 0.09 c | 388.78 ± 0.14 b | 33.04 ± 0.06 b | 17.72 ± 0.07 c | 16.58 ± 0.09 a | 4.48 ± 0.17 b |
| Sample | Macro and Microelement Content (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| K | P | Mg | Ca | Fe | Zn | Mn | Cu | |
| BRF | 889.26 ± 0.28 g | 389.26 ± 0.26 g | 489.22 ± 0.16 g | 79.14 ± 0.36 g | 18.45 ± 0.22 g | 10.32 ± 0.30 g | 8.42 ± 0.17 g | 1.23 ± 0.06 g |
| B90RF10CF | 1019.62 ± 0.21f | 429.60 ± 0.03 f | 501.74 ± 0.08 f | 103.28 ± 0.12 f | 18.80 ± 0.05 f | 10.93 ± 0.05 f | 8.83 ± 0.03 f | 1.43 ± 0.02 f |
| B70RF30CF | 1280.21 ± 0.35 e | 510.32 ± 0.14 e | 526.76 ± 0.15 e | 151.24 ± 0.05 e | 19.51 ± 0.26 e | 12.17 ± 0.19 e | 9.66 ± 0.28 e | 1.83 ± 0.29 e |
| B50RF50CF | 1541.04 ± 0.29 d | 591.03 ± 0.06 d | 551.79 ± 0.07 d | 201.01 ± 0.14 d | 20.33 ± 0.09 d | 13.42 ± 0.14 d | 10.51 ± 0.14 d | 2.22 ± 0.07 d |
| B30RF70CF | 1801.66 ± 0.30 c | 671.73 ± 0.16 c | 576.80 ± 0.12 c | 249.69 ± 0.09 c | 21.09 ± 0.12 c | 14.66 ± 0.20 c | 11.33 ± 0.08 c | 2.62 ± 0.17 c |
| B10RF90CF | 2062.38 ± 0.27 b | 752.41 ± 0.11 b | 600.88 ± 0.06 b | 297.54 ± 0.15 b | 21.75 ± 0.09 b | 15.97 ± 0.12 b | 12.20 ± 0.11 b | 3.01 ± 0.04 b |
| BCF | 2192.83 ± 0.29 a | 792.84 ± 0.07 a | 614.24 ± 0.21 a | 322.92 ± 0.26 a | 22.22 ± 0.12 a | 16.59 ± 0.04 a | 12.64 ± 0.09 a | 3.21 ± 0.05 a |
| B50RF50CF | 1541.04 ± 0.09 c | 591.03 ± 0.06 c | 551.79 ± 0.07 d | 201.01 ± 0.14 d | 20.33 ± 0.09 c | 13.42 ± 0.14 d | 10.51 ± 0.11 c | 2.22 ± 0.04 d |
| B45RF50CF5CP | 1621.71 ± 0.16 a | 686.93 ± 0.14 a | 567.50 ± 0.19 b | 258.42 ± 0.05 c | 23.24 ± 0.16 a | 19.56 ± 0.07 a | 10.42 ± 0.06 c | 2.58 ± 0.03 b |
| B45RF50CF5AP | 1528.13 ± 0.07 d | 598.12 ± 0.09 b | 604.60 ± 0.28 a | 306.45 ± 0.02 a | 20.96 ± 0.24 b | 16.23 ± 0.23 b | 11.24 ± 0.02 b | 2.69 ± 0.06 a |
| B45RF50CF5BP | 1546.27 ± 0.15 b | 574.10 ± 0.02 d | 560.73 ± 0.14 c | 288.47 ± 0.17 b | 23.08 ± 0.01 a | 13.95 ± 0.05 c | 11.41 ± 0.12 a | 2.39 ± 0.12 c |
| Sample | TPC (mg GAE/100 g d.s.) | TFC (mg QE/100 g d.s.) | FRAP (μM Fe2+/g d.s.) |
|---|---|---|---|
| RF | 59.10 ± 0.14 g | 44.46 ± 0.19 g | 8.09 ± 0.22 g |
| 90RF10CF | 67.54 ± 0.29 f | 51.69 ± 0.27 f | 8.73 ± 0.17 f |
| 70RF30CF | 86.23 ± 0.08 e | 68.42 ± 0.24 e | 10.42 ± 0.30 e |
| 50RF50CF | 104.72 ± 0.12 d | 83.34 ± 0.22 d | 12.08 ± 0.12 d |
| 30RF70CF | 124.52 ± 0.25 c | 100.24 ± 0.18 c | 13.50 ± 0.27 c |
| 10RF90CF | 143.16 ± 0.19 b | 115.76 ± 0.08 b | 14.92 ± 0.09 b |
| CF | 152.44 ± 0.08 a | 123.57 ± 0.27 a | 16.18 ± 0.14 a |
| CP | 5512.44 ± 0.24 a | 3966.48 ± 0.36 a | 449.63 ± 0.27 a |
| AP | 5060.04 ± 0.25 c | 2442.03 ± 0.31 c | 377.90 ± 0.15 c |
| BP | 5482.71 ± 0.33 b | 3711.44 ± 0.14 b | 412.08 ± 0.19 b |
| 50RF50CF | 104.72 ± 0.12 d | 83.34 ± 0.22 d | 12.08 ± 0.12 d |
| 45RF50CF5CP | 377.41 ± 0.15 a | 280.40 ± 0.14 a | 32.51 ± 0.25 a |
| 45RF50CF5AP | 354.62 ± 0.35 c | 203.86 ± 0.28 c | 28.64 ± 0.06 c |
| 45RF50CF5BP | 376.04 ± 0.28 b | 266.48 ± 0.30 b | 30.46 ± 0.04 b |
| Sample | Dilution of Extract | DPPH Inhibition (%) |
|---|---|---|
| RF | 1:100 | 28.14 ± 0.17 g |
| 90RF10CF | 1:100 | 31.64 ± 0.23 f |
| 70RF30CF | 1:100 | 37.87 ± 0.25 e |
| 50RF50CF | 1:100 | 44.86 ± 0.13 d |
| 30RF70CF | 1:100 | 52.25 ± 0.24 c |
| 10RF90CF | 1:100 | 59.94 ± 0.08 b |
| CF | 1:100 | 62.62 ± 0.12 a |
| CP | 1:2000 | 143.65 ± 0.26 a |
| AP | 1:2000 | 126.63 ± 0.07 c |
| BP | 1:2000 | 131.72 ± 0.18 b |
| 50RF50CF | 1:100 | 44.86 ± 0.13 d |
| 45RF50CF5CP | 1:100 | 51.36 ± 0.26 a |
| 45RF50CF5AP | 1:100 | 50.27 ± 0.09 c |
| 45RF50CF5BP | 1:100 | 51.02 ± 0.03 b |
| Sample | Dilution of Extract | DPPH Inhibition (%) |
|---|---|---|
| BRF | 1:100 | 27.54 ± 0.09 g |
| B90RF10CF | 1:100 | 30.29 ± 0.13 f |
| B70RF30CF | 1:100 | 37.49 ± 0.21 e |
| B50RF50CF | 1:100 | 44.89 ± 0.28 d |
| B30RF70CF | 1:100 | 51.25 ± 0.06 c |
| B10RF90CF | 1:100 | 59.29 ± 0.31 b |
| BCF | 1:100 | 62.45 ± 0.23 a |
| B50RF50CF | 1:100 | 44.89 ± 0.28 d |
| B45RF50CF5CP | 1:100 | 50.36 ± 0.32 a |
| B45RF50CF5AP | 1:100 | 48.57 ± 0.05 c |
| B45RF50CF5BP | 1:100 | 49.14 ± 0.16 b |
| Sample | Median of the Distribution, nm | Surface Equivalent Mean Size, nm | Goodness of Fit |
|---|---|---|---|
| RF | 850.25 | 40.31 | 1.06 |
| CF | 912.98 | 134.13 | 1.10 |
| CP | 821.78 | 35.68 | 1.00 |
| AP | 784.48 | 25.69 | 1.11 |
| BP | 757.67 | 24.31 | 1.18 |
| BRF | 866.67 | 71.49 | 1.04 |
| B50RF50CF | 884.21 | 87.44 | 1.03 |
| BCF | 864.74 | 55.36 | 1.10 |
| B45RF50CF5CP | 847.38 | 52.85 | 1.00 |
| B45RF50CF5AP | 867.71 | 65.59 | 1.00 |
| B45RF50CF5BP | 872.78 | 68.91 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoin, D.; Poiana, M.-A.; Alexa, E.; Cocan, I.; Negrea, M.; Jianu, C.; Radulov, I.; Suba, M.; Ianasi, C. Current Trends in Gluten-Free Biscuit Formulation Using Rice Flour Enriched with Chestnut Flour and Fruit Powders. Foods 2025, 14, 4074. https://doi.org/10.3390/foods14234074
Stoin D, Poiana M-A, Alexa E, Cocan I, Negrea M, Jianu C, Radulov I, Suba M, Ianasi C. Current Trends in Gluten-Free Biscuit Formulation Using Rice Flour Enriched with Chestnut Flour and Fruit Powders. Foods. 2025; 14(23):4074. https://doi.org/10.3390/foods14234074
Chicago/Turabian StyleStoin, Daniela, Mariana-Atena Poiana, Ersilia Alexa, Ileana Cocan, Monica Negrea, Calin Jianu, Isidora Radulov, Mariana Suba, and Catalin Ianasi. 2025. "Current Trends in Gluten-Free Biscuit Formulation Using Rice Flour Enriched with Chestnut Flour and Fruit Powders" Foods 14, no. 23: 4074. https://doi.org/10.3390/foods14234074
APA StyleStoin, D., Poiana, M.-A., Alexa, E., Cocan, I., Negrea, M., Jianu, C., Radulov, I., Suba, M., & Ianasi, C. (2025). Current Trends in Gluten-Free Biscuit Formulation Using Rice Flour Enriched with Chestnut Flour and Fruit Powders. Foods, 14(23), 4074. https://doi.org/10.3390/foods14234074












