Advances in Optical Sensing Technologies for On-Site Detection of Harmful Residues in Food: Principles and Recent Applications
Abstract
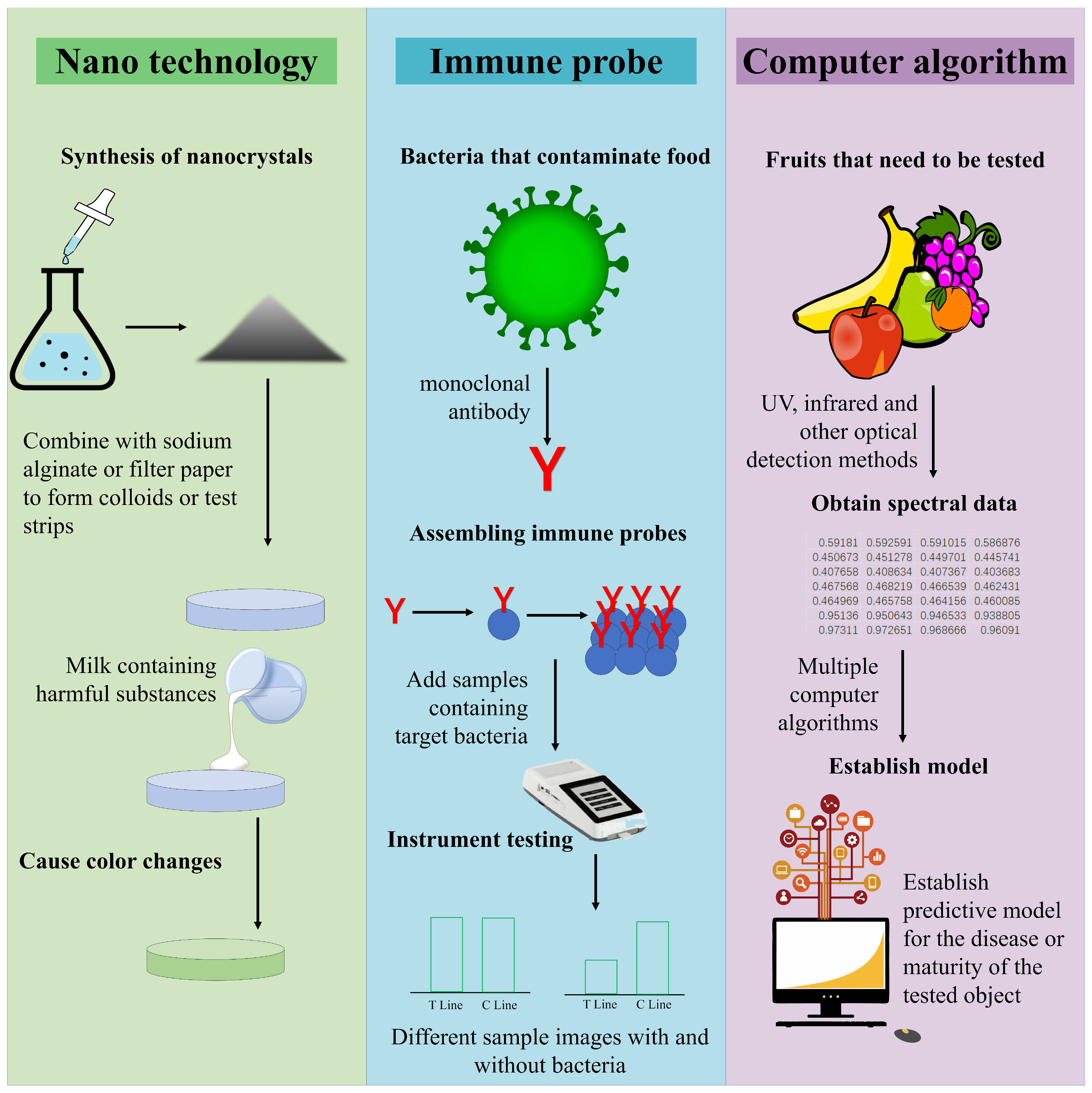
1. Introduction
2. Principles of Optical Sensing Technology
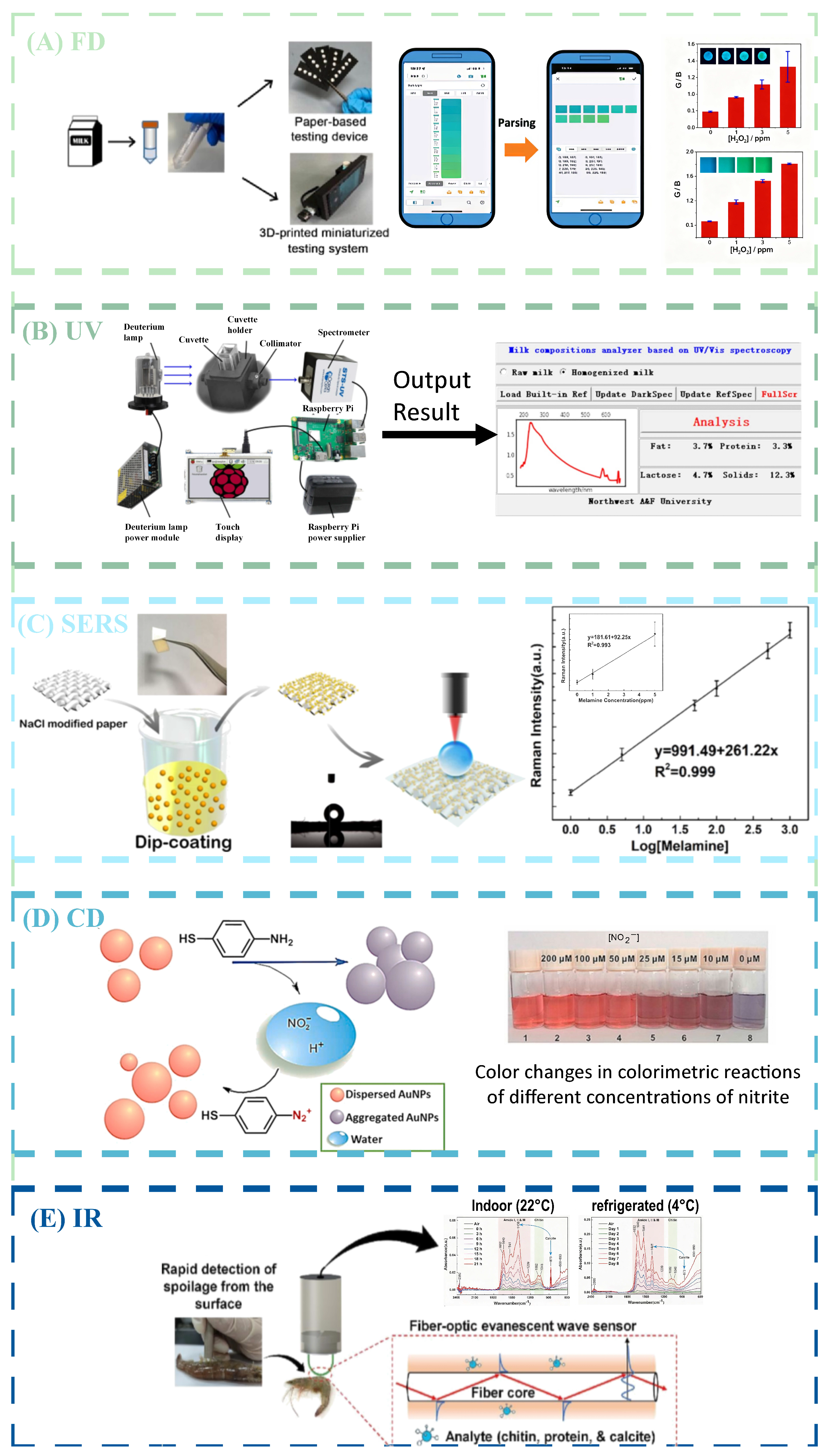
2.1. Fluorescence Imaging
2.2. Ultraviolet–Visible Absorption Spectroscopy
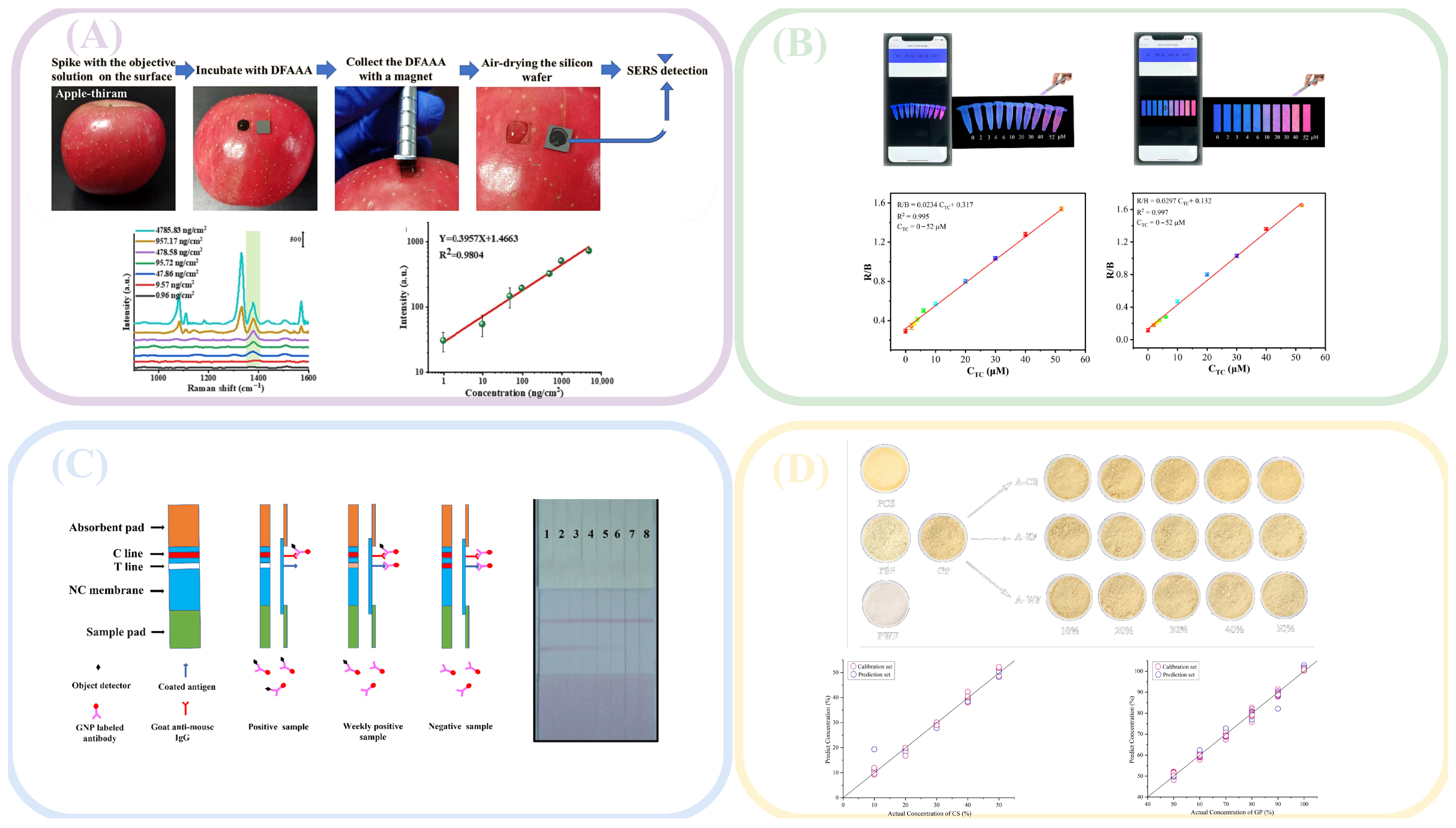
2.3. Surface-Enhanced Raman Spectroscopy
2.4. Colorimetric Method
2.5. Mid-Infrared Spectroscopy
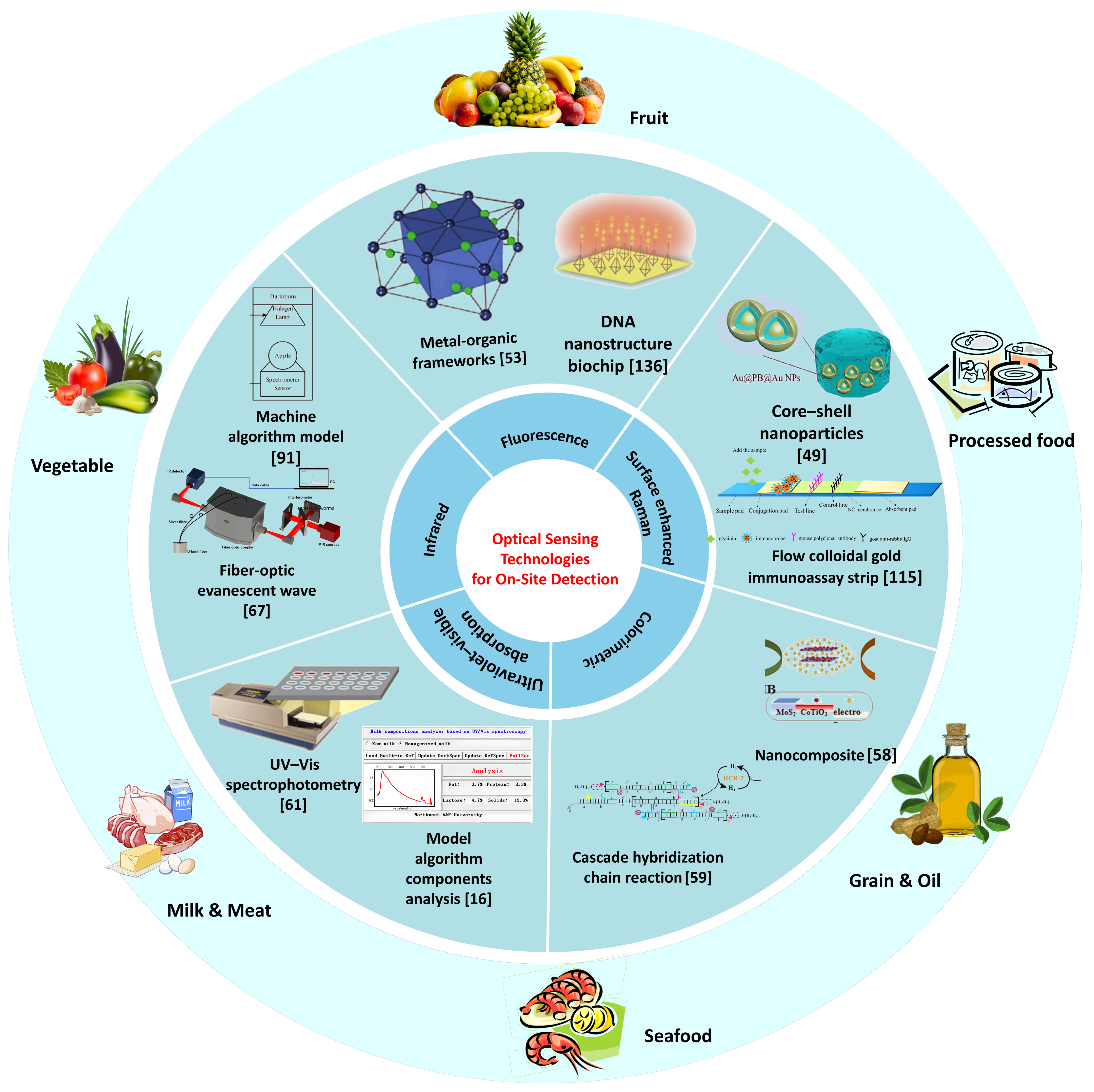
3. Applications for On-Site Food Detection
3.1. Harmful Residues in Fruit and Vegetables
3.2. Harmful Residues in Animal Farming
3.3. Harmful Residues in Grain and Oil
3.4. Harmful Residues in Processed Food
3.5. Optical Detection of Food Raw Materials
4. Discussion
| Detection | Methods | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fluorescence | Nanozymes | Simple, highly selective, and sensitive | Potential cytotoxicity and food safety to be verified | [110] |
| DNA probe | High stability and good universality in small molecule detection | The preparation process is complex, and the cost is higher compared to general food testing | [111] | |
| UV-VIS Spectroscopy | Molecular absorption spectrophotometry | Easy to operate, high quantitative selectivity | The preprocessing method is complex | [112] |
| Data processing algorithm | Comprehensive and accurate analysis, fast detection speed | Requires a large number of samples for calibration | [113] | |
| Surface-Enhanced Raman | Genetic tools | Strong specificity and strong signal and high sensitivity, specificity, and accuracy | The substrate is expensive, subject to interference in complex environments | [114] |
| Immune | Highly sensitive and specific | The synthesis process is complex; antigens are easily destroyed during food processing | [115] | |
| Colorimetric | Nucleic acid amplification techniques | Easy to operate, low equipment requirements, and simple sensor design | Low accuracy and stability need improvement | [116] |
| Nanozymes | Capable of detecting target molecules quickly, sensitively, and conveniently | There is relatively little research on the detailed mechanism; nonspecific adsorption affects the accuracy of the results | [116] | |
| Infrared | Machine algorithm model | Comprehensive assessment without injury; economical and portable | Model interpretability needs to be developed | [117] |
| Fourier transform infrared spectroscopy | Quick, no damage to the tested object, efficient and simple | Wide band with severe overlap between bands | [118] |
| Analyte | Analyte | Optical Sensing Technology | Support Materials | Detected Data | Reference |
|---|---|---|---|---|---|
| fruit and vegetables | Maturity of kiwifruit | Infrared | Fourier transform infrared spectroscopy | Rp2 0.92 | [121] |
| Capsicum fruit defect | Infrared | Machine algorithm model | accuracy 98% | [122] | |
| animal farming | Vibrio parahaemolyticus in cod | Fluorescence | DNA probe | LOD 39.00 CFU/mL | [123] |
| Clenbuterol | SERS | Immune | LOD 0.48 pg/mL | [124] | |
| Milk powder adulteration | Infrared | Fourier transform infrared spectroscopy | LOD 0.25% | [125] | |
| grain and oil | Adulteration of perilla oil | UV-VIS spectroscopy | Data processing algorithm | Rp 0.99 | [126] |
| Genetically modified soybean products | Fluorescence | DNA probe | LOD 0.20% | [127] | |
| processed food | Wine-producing area | UV-VIS spectroscopy | Data processing algorithm | accuracy 100% | [128] |
| Benzoates in beverages | Colorimetric | Nanozymes | LOD 0.33 μM | [129] | |
| Coffee acid produced by wine | Fluorescence | Nanozymes | LOD 18.90 nM | [130] | |
| Casein phosphopeptide | Colorimetric | Nanozymes | LOD 0.27 μg/mL | [131] |
| Analyte | Optical Sensing Technology | LOD | National Food Safety Standards | Corresponding Standards | Reference |
|---|---|---|---|---|---|
| hydrogen peroxide | Colorimetric | 0.01 µM | 25 to 50 ppm | US Environmental Protection Agency | [58] |
| rice yeast acid | UV-VIS spectroscopy | 3.43 nM | 0.25 mg/kg | China’s national food safety standard | [40] |
| cyfluoxate | Colorimetric | 100 μg/kg | 0.1 mg/kg | China’s maximum residue limits | [72] |
| amine vapors | Fluorescence | 1.2 ppb | 50 to 100 ppm | US Food and Drug Administration | [78] |
| tetracycline | Fluorescence | 14 nM | 100 μg/kg | EU food safety standards | [83] |
| ampicillin | Fluorescence | 3.9 ng/mL | 50 μg/L | EU food safety standards | [85] |
| AFB1 | Raman | 0.094 pg/mL | 10 μg/kg | Chinese Pharmacopoeia Commission | [87] |
5. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Kshetri, N. Blockchain’s Role in Enhancing Quality and Safety and Promoting Sustainability in the Food and Beverage Industry. Sustainability 2023, 15, 16223. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and human safety: The impact of microplastics. Crit. Rev. Food Sci. Nutr. 2024, 64, 3502–3521. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pu, H.; Sun, D.W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2800–2813. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414. [Google Scholar] [CrossRef]
- Wahed, P.; Razzaq, M.A.; Dharmapuri, S.; Corrales, M. Determination of formaldehyde in food and feed by an in-house validated HPLC method. Food Chem. 2016, 202, 476–483. [Google Scholar] [CrossRef]
- Pavlicek, V.; Tuma, P. The use of capillary electrophoresis with contactless conductivity detection for sensitive determination of stevioside and rebaudioside A in foods and beverages. Food Chem. 2017, 219, 193–198. [Google Scholar] [CrossRef]
- Bougadi, E.T.; Kalogianni, D.P. Paper-based DNA biosensor for food authenticity testing. Food Chem. 2020, 322, 126758. [Google Scholar] [CrossRef]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Wei, S.; Su, Z.; Bu, X.; Shi, X.; Pang, B.; Zhang, L.; Li, J.; Zhao, C. On-site colorimetric detection of Salmonella typhimurium. npj Sci. Food 2022, 6, 48. [Google Scholar] [CrossRef]
- Willett, D.R.; Rodriguez, J.D. Quantitative Raman assays for on-site analysis of stockpiled drugs. Anal. Chim. Acta 2018, 1044, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, J.Y.; Wang, Y.T.; Zhang, H.C.; Chen, M.L.; Yang, T.; Wang, J.H. M13 phage-based nanoprobe for SERS detection and inactivation of Staphylococcus aureus. Talanta 2021, 221, 121668. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Guercetti, J.; Geballa-Koukoula, A.; Tsagkaris, A.S.; Nelis, J.L.D.; Marco, M.P.; Salvador, J.P.; Gerssen, A.; Hajslova, J.; Elliott, C.; et al. ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers. Foods 2021, 10, 1399. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines 2020, 11, 356. [Google Scholar] [CrossRef]
- Yang, T.; Luo, Z.; Bewal, T.; Li, L.; Xu, Y.; Mahdi Jafari, S.; Lin, X. When smartphone enters food safety: A review in on-site analysis for foodborne pathogens using smartphone-assisted biosensors. Food Chem. 2022, 394, 133534. [Google Scholar] [CrossRef]
- Shan, Y.; Lu, Y.-N.; Yi, W.; Wang, B.; Li, J.; Guo, J.; Li, W.; Yin, Y.; Wang, S.; Liu, F. On-site food safety detection: Opportunities, advancements, and prospects. Biosens. Bioelectron. X 2023, 14, 100350. [Google Scholar] [CrossRef]
- Fu, W.; Fu, X.; Li, Z.; Liu, Z.; Li, X. Advances in smartphone assisted sensors for on-site detection of food safety based on fluorescence on-off-on mode: A review. Chem. Eng. J. 2024, 489, 151225. [Google Scholar] [CrossRef]
- Xie, C.; Meng, C.; Liu, H.; Sun, B. Progress in research on smartphone-assisted MIP optosensors for the on-site detection of food hazard factors. TrAC Trends Anal. Chem. 2024, 170, 117459. [Google Scholar] [CrossRef]
- Wu, G.; Qiu, H.; Liu, X.; Luo, P.; Wu, Y.; Shen, Y. Nanomaterials-based fluorescent assays for pathogenic bacteria in food-related matrices. Trends Food Sci. Technol. 2023, 142, 104214. [Google Scholar] [CrossRef]
- Hassan, D.; Bakhsh, H.; Khurram, A.M.; Bhutto, S.A.; Jalbani, N.S.; Ghumro, T.; Solangi, A.R. Fluorescent Nanotechnology: An Evolution in Optical Sensors. Curr. Anal. Chem. 2022, 18, 176–185. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X. Emerging strategies in fluorescent aptasensor toward food hazard aflatoxins detection. Trends Food Sci. Technol. 2022, 129, 621–633. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Meng, Q.; Zhu, J.; Jia, X.; Zhao, Q.; Tang, C.; Yu, Y.; Zhang, J. A naphthimide fluorescent probe for the detection of selenols in selenium-enriched Tan sheep. Food Chem. 2022, 373, 131647. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, Y.; Yu, H.; Cheng, Y.; Xie, Y.; Yao, W. Sulfhydryl-functionalized carbon dots as effective probes for fluorescence enhancement detection of patulin. Food Chem. 2023, 420, 136037. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N,S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Wu, Z.; Pu, H.; Wei, Q. On-off-on fluorescent nanosensing: Materials, detection strategies and recent food applications. Trends Food Sci. Technol. 2022, 119, 243–256. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Zhai, X.; Shen, T.; Shi, J.; He, Y.; et al. A novel sustainable biomass-based fluorescent probe for sensitive detection of salicylic acid in rice. Food Chem. 2024, 434, 137260. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Yuan, X.; Xiong, J.; Zong, M.H.; Wu, X.; Lou, W.Y. A dual-mode sensing platform based on metal-organic framework for colorimetric and ratiometric fluorescent detection of organophosphorus pesticide. Food Chem. 2024, 432, 137272. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Tang, H.; Cao, D. A novel chromophore reaction-based pyrrolopyrrole aza-BODIPY fluorescent probe for H2S detection and its application in food spoilage. Food Chem. 2023, 427, 136591. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Zhao, Y.; Zhu, J.; Wang, H. Rapid detection of zearalenone in cereals using La3+-doped upconversion nanoparticles-based immunochromatographic assay. Food Control 2023, 153, 109904. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Yang, M.; Liu, A.; Xu, W.; He, P. Silver nanocluster-based aptasensor for the label-free and enzyme-free detection of ochratoxin A. Food Chem. 2024, 431, 137126. [Google Scholar] [CrossRef]
- Campmajo, G.; Navarro, G.J.; Nunez, N.; Puignou, L.; Saurina, J.; Nunez, O. Non-Targeted HPLC-UV Fingerprinting as Chemical Descriptors for the Classification and Authentication of Nuts by Multivariate Chemometric Methods. Sensors 2019, 19, 1388. [Google Scholar] [CrossRef]
- Rahman, A.; Kondo, N.; Ogawa, Y.; Suzuki, T.; Shirataki, Y.; Wakita, Y. Prediction of K value for fish flesh based on ultraviolet–visible spectroscopy of fish eye fluid using partial least squares regression. Comput. Electron. Agric. 2015, 117, 149–153. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. A novel, green and safe ultrasound-assisted emulsification liquid phase microextraction based on alcohol-based deep eutectic solvent for determination of patulin in fruit juices by spectrophotometry. J. Food Compos. Anal. 2019, 82, 103256. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Alawsi, T.; Mattia, G.P.; Al-Bawi, Z.; Beraldi, R. Smartphone-based colorimetric sensor application for measuring biochemical material concentration. Sens. Bio-Sens. Res. 2021, 32, 100404. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, W.; He, G.; Li, G.; Lin, L. “M+N” theory and UV–Vis-NIR transmission spectroscopy used in quantitative analysis of total bilirubin. Infrared Phys. Technol. 2018, 94, 65–68. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Hu, Y.; Zhou, C.; Lan, W.; Fu, H.; She, Y. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality and authenticity. Sens. Actuators B Chem. 2021, 329, 129135. [Google Scholar] [CrossRef]
- Yang, B.; Guo, W.; Liang, W.; Zhou, Y.; Zhu, X. Design and evaluation of a miniature milk quality detection system based on UV/Vis spectroscopy. J. Food Compos. Anal. 2022, 106, 104341. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, S.; Song, H.; Luo, X.; Wu, D.; Zheng, F.; Liu, W.; Ji, S. The dual-mode platform based on cysteamine-stabilized gold nanoparticles for the high throughput and on-site detection of bongkrekic acid. Food Control 2022, 136, 108887. [Google Scholar] [CrossRef]
- Karthick Kannan, P.; Shankar, P.; Blackman, C.; Chung, C.H. Recent Advances in 2D Inorganic Nanomaterials for SERS Sensing. Adv. Mater. 2019, 31, e1803432. [Google Scholar] [CrossRef] [PubMed]
- Akinoglu, G.E.; Mir, S.H.; Gatensby, R.; Rydzek, G.; Mokarian-Tabari, P. Block Copolymer Derived Vertically Coupled Plasmonic Arrays for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 23410–23416. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D.-W. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.R.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2023, 403, 134384. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, H.R.; Guo, Z.; Zou, X.; Cai, J. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, T.; Liu, J.; Zhou, G. In-Situ Grown Silver Nanoparticles on Nonwoven Fabrics Based on Mussel-Inspired Polydopamine for Highly Sensitive SERS Carbaryl Pesticides Detection. Nanomaterials 2019, 9, 384. [Google Scholar] [CrossRef]
- Zhang, C.; You, T.; Yang, N.; Gao, Y.; Jiang, L.; Yin, P. Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem. 2019, 287, 363–368. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, X.; Jayan, H.; Yin, L.; Xue, S.; El-Seedi, H.R.; Zou, X. Recent developments and applications of surface enhanced Raman scattering spectroscopy in safety detection of fruits and vegetables. Food Chem. 2024, 434, 137469. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L.; Li, H.; Yuan, R.; Yang, X. Construction of a SERS platform for sensitive detection of aflatoxin B1 based on CRISPR strategy. Food Chem. 2023, 415, 135768. [Google Scholar] [CrossRef]
- Najafi, R.; Mukherjee, S.; Hudson, J., Jr.; Sharma, A.; Banerjee, P. Development of a rapid capture-cum-detection method for Escherichia coli O157 from apple juice comprising nano-immunomagnetic separation in tandem with surface enhanced Raman scattering. Int. J. Food Microbiol. 2014, 189, 89–97. [Google Scholar] [CrossRef]
- Kim, S.Y.; Seo, H.Y.; Ha, J.H. A colorimetric sensor array for the discrimination of glucosinolates. Food Chem. 2020, 328, 127149. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, L.; Liu, Y.; Liu, P.; Wang, W.; Luo, X. A morphology-based ultrasensitive multicolor colorimetric assay for detection of blood glucose by enzymatic etching of plasmonic gold nanobipyramids. Anal. Chim. Acta 2019, 1071, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Wang, S.; Yu, J.; Chen, J.; Liao, D.; Liu, M.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Liu, J. Detection mechanism and the outlook of metal-organic frameworks for the detection of hazardous substances in milk. Food Chem. 2024, 430, 136934. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, M.; Ghasemi, F.; Seyed Hosseini, H.M. Plasmonic nanoparticles for colorimetric detection of nitrite and nitrate. Food Chem. Toxicol. 2021, 149, 112025. [Google Scholar] [CrossRef]
- Qi, Y.; Xie, H.; Liu, J.; Fu, X.; Lu, M.; Liu, M.; Riaz, S.; Wei, P.; Xie, Y. Detection of NO2− with a colorimetric sensor based on etching Au NBPs. J. Food Compos. Anal. 2023, 124, 105681. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Z.; Fauconnier, M.-L.; Li, C.; Chen, L.; Zheng, X.; Zhang, D. Bimodal single-atom iron nanozyme biosensor for volatile amine and food freshness detection. Nano Today 2023, 53, 102025. [Google Scholar] [CrossRef]
- He, Z.; Zhu, J.; Weng, G.J.; Li, J.J.; Zhao, J.W. Detection of ferrous ion by etching-based multi-colorimetric sensing of gold nanobipyramids. Nanotechnology 2020, 31, 335505. [Google Scholar] [CrossRef]
- Ullah, I.; Yaqub, A.; Haq, M.Z.U.; Ajab, H.; Jafry, A.T.; Khan, M.K. Sensitive and cost-effective colorimetric sensor based on enzyme mimic MoS2@CoTiO3 nanocomposite for detection of hydrogen peroxide in milk and tap water. J. Food Compos. Anal. 2023, 124, 105689. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.; Zou, H.; Li, Y. Plasma colorimetric aptasensor for the detection of chloramphenicol in honey based on cage Au@AuNPs and cascade hybridization chain reaction. Food Chem. 2022, 377, 132031. [Google Scholar] [CrossRef]
- Sendin, K.; Williams, P.J.; Manley, M. Near infrared hyperspectral imaging in quality and safety evaluation of cereals. Crit. Rev. Food Sci. Nutr. 2018, 58, 575–590. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, Z.; Liu, J.; Xu, Z.; Du, Z. Determination of the food dye indigotine in cream by near-infrared spectroscopy technology combined with random forest model. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117551. [Google Scholar] [CrossRef]
- Corro-Herrera, V.A.; Gomez-Rodriguez, J.; Hayward-Jones, P.M.; Barradas-Dermitz, D.M.; Aguilar-Uscanga, M.G.; Gschaedler-Mathis, A.C. In-situ monitoring of Saccharomyces cerevisiae ITV01 bioethanol process using near-infrared spectroscopy NIRS and chemometrics. Biotechnol. Prog. 2016, 32, 510–517. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Ali, S.; Li, H.; Ouyang, Q.; Wu, X.; Hashim, M.M.; Chen, Q. Recent advances in assessing qualitative and quantitative aspects of cereals using nondestructive techniques: A review. Trends Food Sci. Technol. 2021, 116, 815–828. [Google Scholar] [CrossRef]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Genangeli, A.; Allasia, G.; Bindi, M.; Cantini, C.; Cavaliere, A.; Genesio, L.; Giannotta, G.; Miglietta, F.; Gioli, B. A Novel Hyperspectral Method to Detect Moldy Core in Apple Fruits. Sensors 2022, 22, 4479. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiao, L.; Wu, J.; Zhang, Y.; Zhu, Q.; Dong, D. Non-destructive and in-situ detection of shrimp freshness using mid-infrared fiber-optic evanescent wave spectroscopy. Food Chem. 2023, 422, 136189. [Google Scholar] [CrossRef]
- Liang, M.; Chen, R.; Xian, Y.; Hu, J.; Hou, X.; Wang, B.; Wu, Y.; Wang, L. Determination of bongkrekic acid and isobongkrekic acid in rice noodles by HPLC-Orbitrap HRMS technology using magnetic halloysite nanotubes. Food Chem. 2021, 344, 128682. [Google Scholar] [CrossRef]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Q.; Wu, J.; Dai, Z.; Wang, Y. Naked-eyes detection of Shigella flexneri in food samples based on a novel gold nanoparticle-based colorimetric aptasensor. Food Control 2019, 98, 333–341. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control 2022, 131, 108399. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Wu, A.; Xu, C.; Liu, L.; Qu, A.; Kuang, H. Rapid and sensitive detection of cyhalofop-butyl in foods using a gold nanoparticle-based lateral-flow strip. Food Biosci. 2023, 55, 102986. [Google Scholar] [CrossRef]
- Lv, M.; Pu, H.; Sun, D.W. A durian-shaped multilayer core-shell SERS substrate for flow magnetic detection of pesticide residues on foods. Food Chem. 2024, 433, 137389. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, Q.; Yin, L.; Wu, W.; Han, E.; Wang, C.; Zhou, R.; Bai, J.; Cai, J. Simultaneous detection of acetamiprid and carbendazim based on Raman-silent spectral window tags-mediated surface-enhanced Raman scattering aptasensor coupled with magnetic separation. Sens. Actuators B Chem. 2024, 400, 134792. [Google Scholar] [CrossRef]
- Waseem, H.; Williams, M.R.; Jameel, S.; Hashsham, S.A. Antimicrobial Resistance in the Environment. Water Env. Res. 2018, 90, 865–884. [Google Scholar] [CrossRef]
- Effah, C.Y.; Ding, L.; Tan, L.; He, S.; Li, X.; Yuan, H.; Li, Y.; Liu, S.; Sun, T.; Wu, Y. A SERS bioassay based on vancomycin-modified PEI-interlayered nanocomposite and aptamer-functionalized SERS tags for synchronous detection of Acinetobacter baumannii and Klebsiella pneumoniae. Food Chem. 2023, 423, 136242. [Google Scholar] [CrossRef]
- Janci, T.; Valinger, D.; Gajdos Kljusuric, J.; Mikac, L.; Vidacek, S.; Ivanda, M. Determination of histamine in fish by Surface Enhanced Raman Spectroscopy using silver colloid SERS substrates. Food Chem. 2017, 224, 48–54. [Google Scholar] [CrossRef]
- Mi, J.; Guo, Y.; Gong, Y.; Liu, S.; Zhao, M.; Hu, Q.; Yu, L. Highly sensitively detection of amine vapors released during shrimp spoilage by fluorescent molecules locked in covalent organic frameworks. Food Chem. 2023, 424, 136370. [Google Scholar] [CrossRef]
- Li, Y.-F.; Sun, Y.-M.; Beier, R.C.; Lei, H.-T.; Gee, S.; Hammock, B.D.; Wang, H.; Wang, Z.; Sun, X.; Shen, Y.-D.; et al. Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. TrAC Trends Anal. Chem. 2017, 88, 25–40. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Duan, C.; Dou, L.; Wen, K.; Wang, Z.; Yu, X.; Shen, J. Comparison of two fluorescence quantitative immunochromatographic assays for the detection of amantadine in chicken muscle. Food Chem. 2022, 377, 131931. [Google Scholar] [CrossRef]
- Warner, A.J.; Hathaway-Schrader, J.D.; Lubker, R.; Davies, C.; Novince, C.M. Tetracyclines and bone: Unclear actions with potentially lasting effects. Bone 2022, 159, 116377. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wang, Y.; Yang, J.; Li, H.; Han, X.; Wang, S. Carbon dots-based fluorescent molecularly imprinted photonic crystal hydrogel strip: Portable and efficient strategy for selective detection of tetracycline in foods of animal origin. Food Chem. 2024, 433, 137407. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, X.; Zhang, X.; Fan, J.; Zhang, R.; Feng, X. A smartphone-assisted paper-based ratio fluorescent probe for the rapid and on-site detection of tetracycline in food samples. Talanta 2023, 265, 124874. [Google Scholar] [CrossRef]
- de Faria, L.V.; Lisboa, T.P.; Campos, N.D.S.; Alves, G.F.; Matos, M.A.C.; Matos, R.C.; Munoz, R.A.A. Electrochemical methods for the determination of antibiotic residues in milk: A critical review. Anal. Chim. Acta 2021, 1173, 338569. [Google Scholar] [CrossRef]
- Chen, C.; Lei, H.; Liu, N.; Yan, H. An aptasensor for ampicillin detection in milk by fluorescence resonance energy transfer between upconversion nanoparticles and Au nanoparticles. Food Chem. X 2022, 15, 100439. [Google Scholar] [CrossRef]
- He, H.; Sun, D.W.; Pu, H.; Huang, L. Bridging Fe3O4@Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1. Food Chem. 2020, 324, 126832. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.; Song, Z.; Khan, I.M.; Wang, Z.; Jiang, C.; Ma, X. Satellite nanostructures composed of CdTe quantum dots and DTNB-labeled AuNPs used for SERS-fluorescence dual-signal detection of AFB1. Food Control 2024, 156, 110112. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Song, J.; Liu, Q.; Song, L.; Fei, X.; Li, X. A novel multimode biosensor for sensitive detection of AFB1 in food based on Mxenes nano enzymes. Food Chem. 2023, 426, 136645. [Google Scholar] [CrossRef]
- Niu, X.; He, H.; Ran, H.; Wu, Z.; Tang, Y.; Wu, Y. Rapid colorimetric sensor for ultrasensitive and highly selective detection of Fumonisin B1 in cereal based on laccase-mimicking activity of silver phosphate nanoparticles. Food Chem. 2023, 429, 136903. [Google Scholar] [CrossRef]
- Luo, S.; Song, X.; Wang, J.; Huang, X. Field specific capture of Pb(II) in aqueous samples with three channels in-tip microextraction apparatus based on ion-imprinted polymer. Talanta 2023, 262, 124676. [Google Scholar] [CrossRef]
- Liu, M.; Zareef, M.; Zhu, A.; Wei, W.; Li, H.; Chen, Q. SERS-based Au@Ag core-shell nanoprobe aggregates for rapid and facile detection of lead ions. Food Control 2024, 155, 110078. [Google Scholar] [CrossRef]
- Jiang, S.T.; Gao, D.J.; Yu, Y.; Yu, Y. A portable front face fluorescent system for in situ detection of aluminum in flour foods. Food Chem. 2023, 418, 135986. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.S.; Tanaka, T. Comparison of quantitative methods based on SYBR Green real-time qPCR to estimate pork meat adulteration in processed beef products. Food Chem. 2018, 269, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Sahar, A.; Pasha, I.; Shahid, M. Determination of Adulteration of Chicken Meat into Minced Beef Mixtures using Front Face Fluorescence Spectroscopy Coupled with Chemometric. Food Sci. Anim. Resour. 2022, 42, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.X.; Guo, S.; Zhang, X.; Yan, H.; Zhang, Z.Y.; Chen, X.; Chen, J.Y.; Jin, S.J.; Yang, J.; Duan, J.A. Rapid detection of adulteration in powder of ginger (Zingiber officinale Roscoe) by FT-NIR spectroscopy combined with chemometrics. Food Chem. X 2022, 15, 100450. [Google Scholar] [CrossRef]
- Daszykowski, M.; Kula, M.; Stanimirova, I. Quantification and Detection of Ground Garlic Adulteration Using Fourier-Transform Near-Infrared Reflectance Spectra. Foods 2023, 12, 3377. [Google Scholar] [CrossRef]
- Mburu, M.; Komu, C.; Paquet-Durand, O.; Hitzmann, B.; Zettel, V. Chia Oil Adulteration Detection Based on Spectroscopic Measurements. Foods 2021, 10, 1798. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Raypah, M.E.; Omar, A.F.; Muncan, J.; Zulkurnain, M.; Abdul Najib, A.R. Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics. Molecules 2022, 27, 2324. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, J.; Dou, X.; Deng, Z.; Wang, X.; Ma, F.; Yu, L.; Yun, Y.H.; Li, P.; Zhang, L. Comprehensive adulteration detection of sesame oil based on characteristic markers. Food Chem. X 2023, 18, 100745. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Ma, F.; Yu, L.; Mao, J.; Zhang, W.; Jiang, J.; Zhang, L.; Li, P. Rapid detection of sesame oil multiple adulteration using a portable Raman spectrometer. Food Chem. 2023, 405, 134884. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Sharifuzzaman, M.; Moro, G.; Sinibaldi, A.; Altintas, Z.; Kumar, S.; Chiavaioli, F.; Marques, C. Advances in optical devices for the detection of contaminants in food and water. TrAC Trends Anal. Chem. 2025, 184, 118139. [Google Scholar] [CrossRef]
- Martines-Arano, H.; Vera-Ku, M.; Álvarez-Espino, R.; Vivanco-Benavides, L.E.; Martínez-González, C.L.; Torres-Torres, C. Thrinax radiata Seed Germplasm Dynamics Analysis Assisted by Chaos Theory. Math. Comput. Appl. 2025, 30, 113. [Google Scholar] [CrossRef]
- Qiu, H.; Zhou, F.; Guo, K.; Chu, R.; Shui, L.; Liu, Y. Photothermal convection-driven highly sensitive in situ detection of nanoplastics in water using an optical fiber SERS probe. J. Hazard. Mater. 2025, 498, 139895. [Google Scholar] [CrossRef]
- Cabrera, D.H.; Gallardo, V.C.; Basurto-Pensado, M.A.; Agarwal, V.; Antunez, E.E. Edible gelatin-based hydrosol for optical detection of metal ions in water. Optik 2025, 336, 172453. [Google Scholar] [CrossRef]
- Nuzzi, C.; Pasinetti, S.; Bassi, I.; Bello, V. On the applicability of speckle pattern imaging combined with AI for raw milk classification. Measurement 2026, 258, 119246. [Google Scholar] [CrossRef]
- Miyazaki, K.; Fujita, H.; Yasuda, K.; Saiki, K.; Watanabe, T.M. Comparative analysis of autofluorescence spectra in a filet of three fish species during chilled storage for raw consumption. Food Chem. 2025, 493, 145577. [Google Scholar] [CrossRef]
- Sahu, D.; Ghosh, S.; Jayaraman, S.; Neelapu, B.C.; Pal, K. Low-cost machine learning-integrated optical spectrophotometer for non-destructive color and shelf-life analysis: A study on sliced bread. Food Chem. 2025, 492, 145379. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Sun, C.; Majeed, U.; Wang, C.; Jiang, S.; Zou, X. Optical properties of multilayered tissues of different varieties of apples and inspection models of internal quality. J. Food Compos. Anal. 2025, 146, 107942. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Han, M.; Zhang, R.; Li, H.; Wu, F.; Wu, A.; Wang, X. Selenium-based nanozyme as a fluorescence-enhanced probe and imaging for chlortetracycline in living cells and foods. Food Chem. 2024, 432, 137147. [Google Scholar] [CrossRef]
- Yang, M.; Gao, Y.; Xu, L.; Zong, C.; Wang, R. Universal and ultrasensitive assay of multiple microRNAs based on HCR probe and tetrahedral DNA nanostructure engineered plasmon-enhanced fluorescence biochip. Microchem. J. 2024, 205, 111242. [Google Scholar] [CrossRef]
- Morschbacher, A.P.; Dullius, A.; Dullius, C.H.; Bandt, C.R.; Kuhn, D.; Brietzke, D.T.; Malmann Kuffel, F.J.; Etgeton, H.P.; Altmayer, T.; Goncalves, T.E.; et al. Validation of an analytical method for the quantitative determination of selenium in bacterial biomass by ultraviolet-visible spectrophotometry. Food Chem. 2018, 255, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Johnson, J.B.; Zhang, K.; Guo, Y.; Liu, D.; Wang, Z.; Bian, X. Variational mode decomposition unfolded partial least squares regression for ultraviolet–visible spectral analysis of edible oil blend, fuel oil and aqueous samples. Microchem. J. 2024, 196, 109587. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Lu, M.; Li, X.; Liang, Y.; Wang, R.; Zhang, W.; Man, S.; Ma, L. Advances in surface-enhanced Raman scattering detection of foodborne pathogens: From recognition-based fingerprint to molecular diagnosis. Coord. Chem. Rev. 2024, 518, 216083. [Google Scholar] [CrossRef]
- Huang, P.; Shang, A.; Liu, D.; Xi, J. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for rapid detection of glycinin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122407. [Google Scholar] [CrossRef]
- Yang, T.; Luo, Z.; Tian, Y.; Qian, C.; Duan, Y. Design strategies of AuNPs-based nucleic acid colorimetric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115795. [Google Scholar] [CrossRef]
- Khan, M.A.; Asadi, H.; Zhang, L.; Qazani, M.R.C.; Oladazimi, S.; Loo, C.K.; Lim, C.P.; Nahavandi, S. Application of artificial intelligence in cognitive load analysis using functional near-infrared spectroscopy: A systematic review. Expert Syst. Appl. 2024, 249, 123717. [Google Scholar] [CrossRef]
- Chu, C.; Wang, H.; Luo, X.; Fan, Y.; Nan, L.; Du, C.; Gao, D.; Wen, P.; Wang, D.; Yang, Z.; et al. Rapid detection and quantification of melamine, urea, sucrose, water, and milk powder adulteration in pasteurized milk using Fourier transform infrared (FTIR) spectroscopy coupled with modern statistical machine learning algorithms. Heliyon 2024, 10, e32720. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Xiao, Z.; Xu, H.; Wang, L.; Pan, T.; Liao, J.; Tian, Y. Dual-mode colorimetric/fluorometric test paper for rapid on-site sulfur dioxide quantification in food and environmental samples. J. Environ. Chem. Eng. 2025, 13, 115115. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Dong, W.; Gong, X.; Dong, C.; Shuang, S.; Song, S. N, P and S co-doped red fluorescent carbon dots sensing for nitrite by fluorescent/colorimetric/smart phone/test strip methods through diazo coupling reaction in food samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 343, 126550. [Google Scholar] [CrossRef]
- Ding, G.; Jin, K.; Chen, X.; Li, A.; Guo, Z.; Zeng, Y. Non-destructive prediction of ready-to-eat kiwifruit firmness based on Fourier transform near-infrared spectroscopy. Postharvest Biol. Technol. 2024, 212, 112908. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Kim, Y.-N.; Kim, H.; Akter, T.; Kim, H.; Park, M.-S.; Kim, M.S.; Baek, I.; Cho, B.-K. Hyperspectral imaging system for pre- and post-harvest defect detection in paprika fruit. Postharvest Biol. Technol. 2024, 218, 113151. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhang, C.; Zhao, L.; Huang, Y. Triplex DNA-based aggregation-induced emission probe: A new platform for hybridization chain reaction-based fluorescence sensing assay. Anal. Chim. Acta 2024, 1299, 342406. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Bai, W.; Zheng, S.; Wang, Q.; Zhang, L.; Hu, Q.; Liu, Y.; Wang, C.; Wang, S. Fabrication of Raman reporter molecule–embedded magnetic SERS tag for ultrasensitive immunochromatographic monitoring of Cd ions and clenbuterol in complex samples. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135159. [Google Scholar] [CrossRef]
- Lafi, A.G.A.; Naser, I.; Abboud, H. Application of two dimensional correlation Fourier Transform Infrared spectroscopy (2D-COS-FTIR) to the quantification of carbohydrates in milk powder. J. Food Compos. Anal. 2024, 134, 106541. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, W.; Liu, P.; Tan, X.; Bian, X. Rapid quantification of single component oil in perilla oil blends by ultraviolet-visible spectroscopy combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 321, 124710. [Google Scholar] [CrossRef] [PubMed]
- Bo-Hong, W.; Chang, P.-C.; Yen-Peng, H. Analysis of genetically modified soya and soya products using a fluorescence probe based on PCR-initiated isothermal amplification of G-quadruplex DNA. J. Food Compos. Anal. 2024, 125, 105828. [Google Scholar] [CrossRef]
- Gu, H.-W.; Zhou, H.-H.; Lv, Y.; Wu, Q.; Pan, Y.; Peng, Z.-X.; Zhang, X.-H.; Yin, X.-L. Geographical origin identification of Chinese red wines using ultraviolet-visible spectroscopy coupled with machine learning techniques. J. Food Compos. Anal. 2023, 119, 105265. [Google Scholar] [CrossRef]
- Qin, S.; Li, Z.; Zhou, B.; Liu, B.; Xue, Y.; Zhao, R.; Zheng, L.; Chen, Z.; Zuo, X. Colorimetric detection of benzoate in the beverage using the peroxidase-like properties of single-atom Fe-N-C nanozymes. Microchem. J. 2024, 206, 111614. [Google Scholar] [CrossRef]
- Liao, X.; Li, B.; Wang, L.; Chen, Y. Boric acid functionalized Fe3O4@CeO2/Tb-MOF as a luminescent nanozyme for fluorescence detection and degradation of caffeic acid. Biosens. Bioelectron. 2024, 264, 116637. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Peng, X.; Qi, W.; Wang, M. A facile nanozyme-based colorimetric method to realize the quantitative and specific detection of casein phosphopeptides in food samples. Talanta 2024, 276, 126212. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, Z.; Lu, M.; Gao, P.; Li, J.; Zhao, J.; Hu, J. A Vis/NIR device for detecting moldy apple cores using spectral shape features. Comput. Electron. Agric. 2024, 220, 108898. [Google Scholar] [CrossRef]
- Shin, H.J.; Lim, J.-H.; Park, K.-J.; Ok, G. State-of-the-art nondestructive high-speed raster scanning inspection for food safety and quality using terahertz refractive index mapping. Food Chem. Adv. 2024, 4, 100685. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Hsu, S.-H.; Ko, C.-Y.; Hsu, K.-H. Real-time defect and freshness inspection on chicken eggs using hyperspectral imaging. Food Control 2023, 150, 109716. [Google Scholar] [CrossRef]
- Shi, S.; Wei, C.; Ma, T.; Zhao, L.; Guo, W.; Sun, Y.; Li, Z. Penny-per-Test Colorimetric Kit for Rapid On-Site Detection of Edible Oil Spoilage to Prevent Waste and Health Risks. Sens. Actuators B Chem. 2025, 449, 139122. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, C.; Zhao, Y.; Zhou, M.; Lv, T.; Song, C.; Qin, T.; Wang, L.; Liu, B.; Peng, X. Smartphone-based on-site detection of hydrogen peroxide in milk by using a portable ratiometric fluorescent probe. Food Chem. 2023, 410, 135381. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, Y.; Yang, H.; Su, Q.; Li, L.; Meng, X.; Li, M.; Jia, X.; Ma, P.; Fan, B.; et al. Advances in Optical Sensing Technologies for On-Site Detection of Harmful Residues in Food: Principles and Recent Applications. Foods 2025, 14, 4073. https://doi.org/10.3390/foods14234073
Liu Q, Liu Y, Yang H, Su Q, Li L, Meng X, Li M, Jia X, Ma P, Fan B, et al. Advances in Optical Sensing Technologies for On-Site Detection of Harmful Residues in Food: Principles and Recent Applications. Foods. 2025; 14(23):4073. https://doi.org/10.3390/foods14234073
Chicago/Turabian StyleLiu, Qinghua, Yuanyuan Liu, Huihui Yang, Qian Su, Linglei Li, Xiangqi Meng, Minmin Li, Xiaoxue Jia, Peihua Ma, Bei Fan, and et al. 2025. "Advances in Optical Sensing Technologies for On-Site Detection of Harmful Residues in Food: Principles and Recent Applications" Foods 14, no. 23: 4073. https://doi.org/10.3390/foods14234073
APA StyleLiu, Q., Liu, Y., Yang, H., Su, Q., Li, L., Meng, X., Li, M., Jia, X., Ma, P., Fan, B., Wang, F., & Li, L. (2025). Advances in Optical Sensing Technologies for On-Site Detection of Harmful Residues in Food: Principles and Recent Applications. Foods, 14(23), 4073. https://doi.org/10.3390/foods14234073





