Electrochemical Behaviour of the Antioxidant Gallic Acid Using a Low-Cost Screen-Printed Carbon Sensor and Its Exploitation for Banana Wine Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Apparatus
2.2. Chemicals and Reagents
2.3. Banana Wine Preparation
2.4. Voltammetric Characterisation Procedure of Gallic Acid Using Different pH Values
2.5. Calibration Procedure
2.6. Determination of Original Gallic Acid Concentration in Banana Wine and Recovery Study Using the Standard Addition Method
2.7. Statistical Analysis
3. Results and Discussion
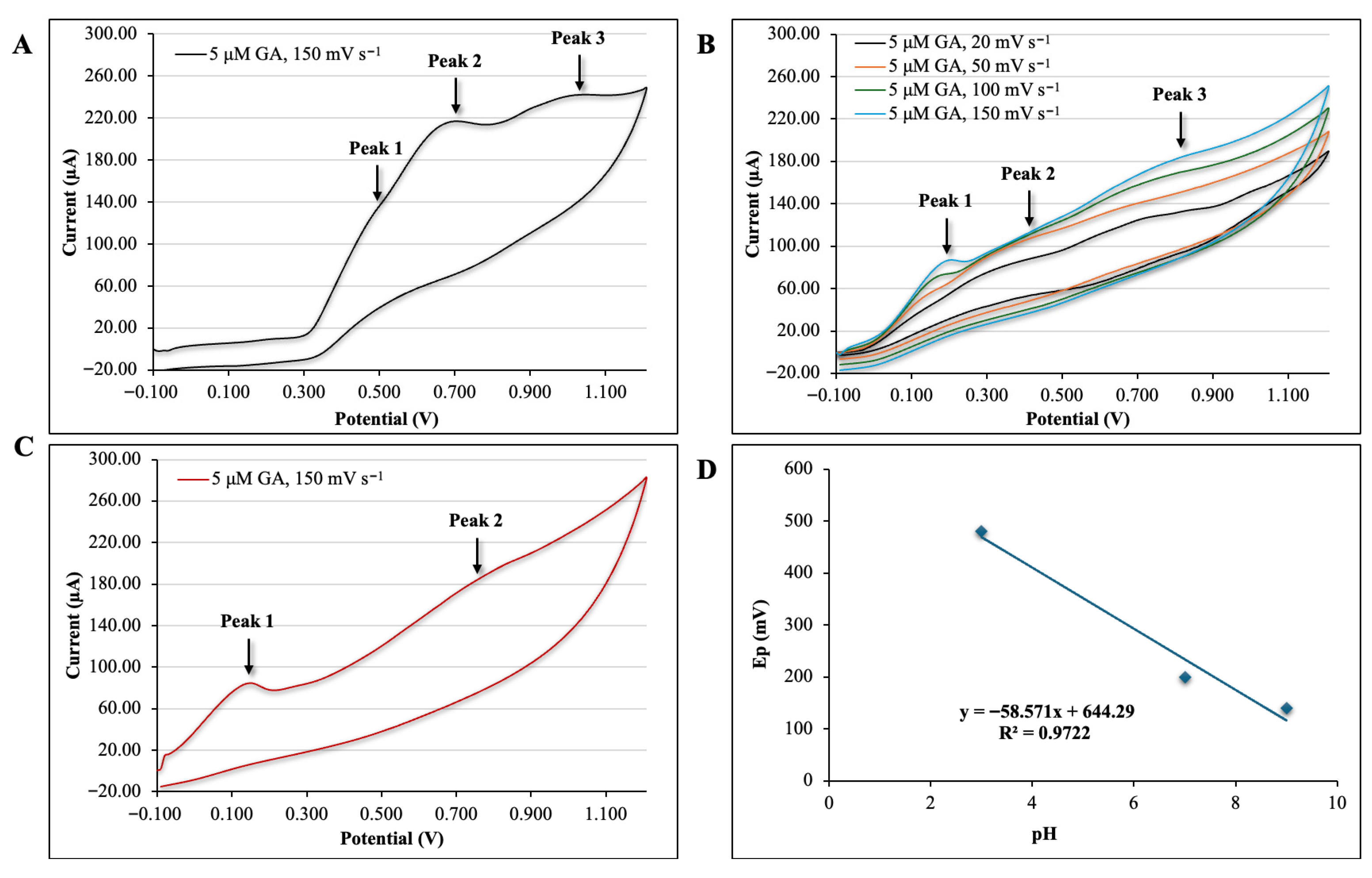
3.1. Cyclic Voltammetric Characterisation of Gallic Acid Using Plain SPCE Sensors Under Acidic, Neutral and Alkaline Conditions
3.1.1. Effect of pH on the Oxidation of Gallic Acid
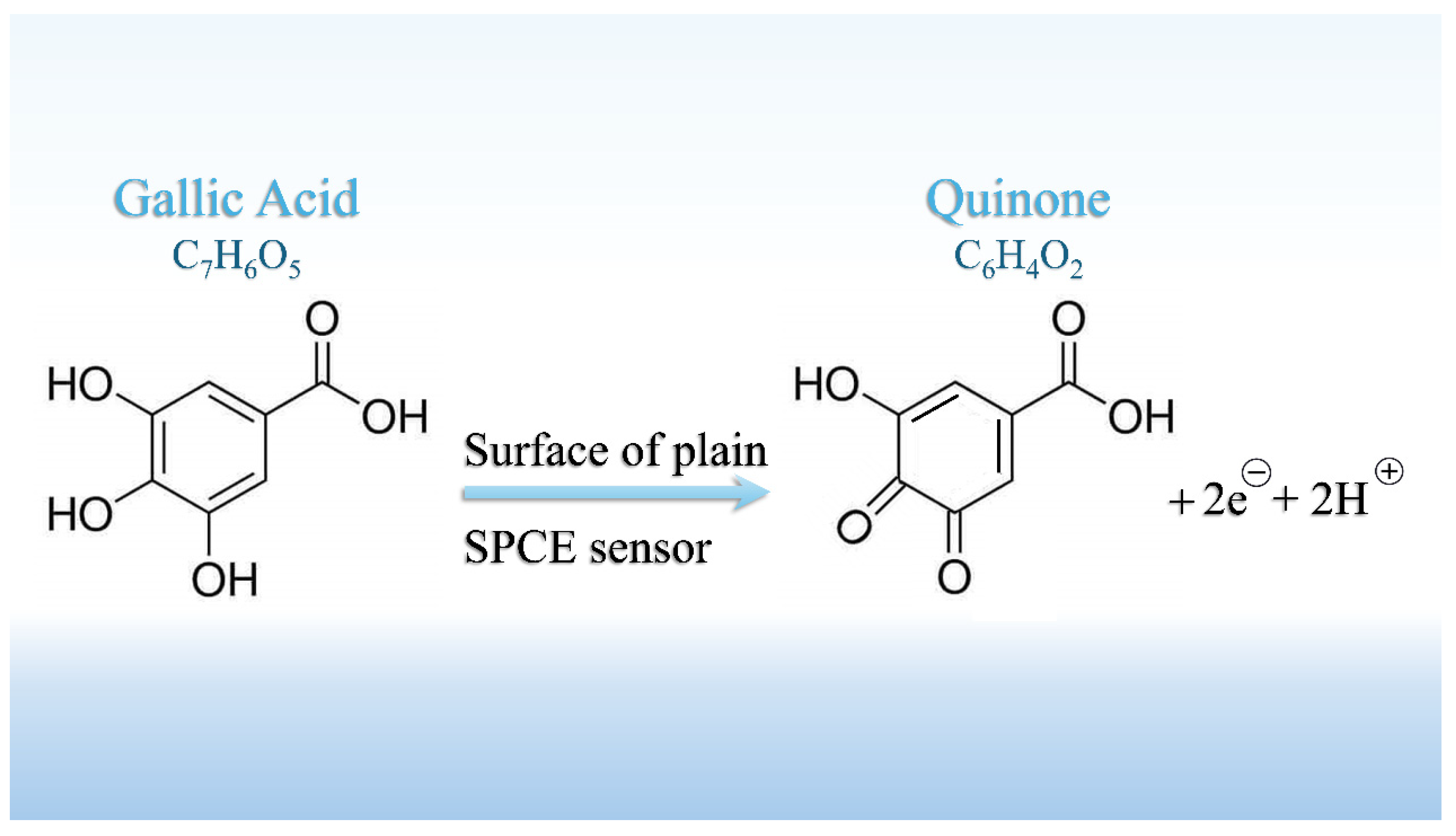
3.1.2. Mechanism of Gallic Acid Oxidation
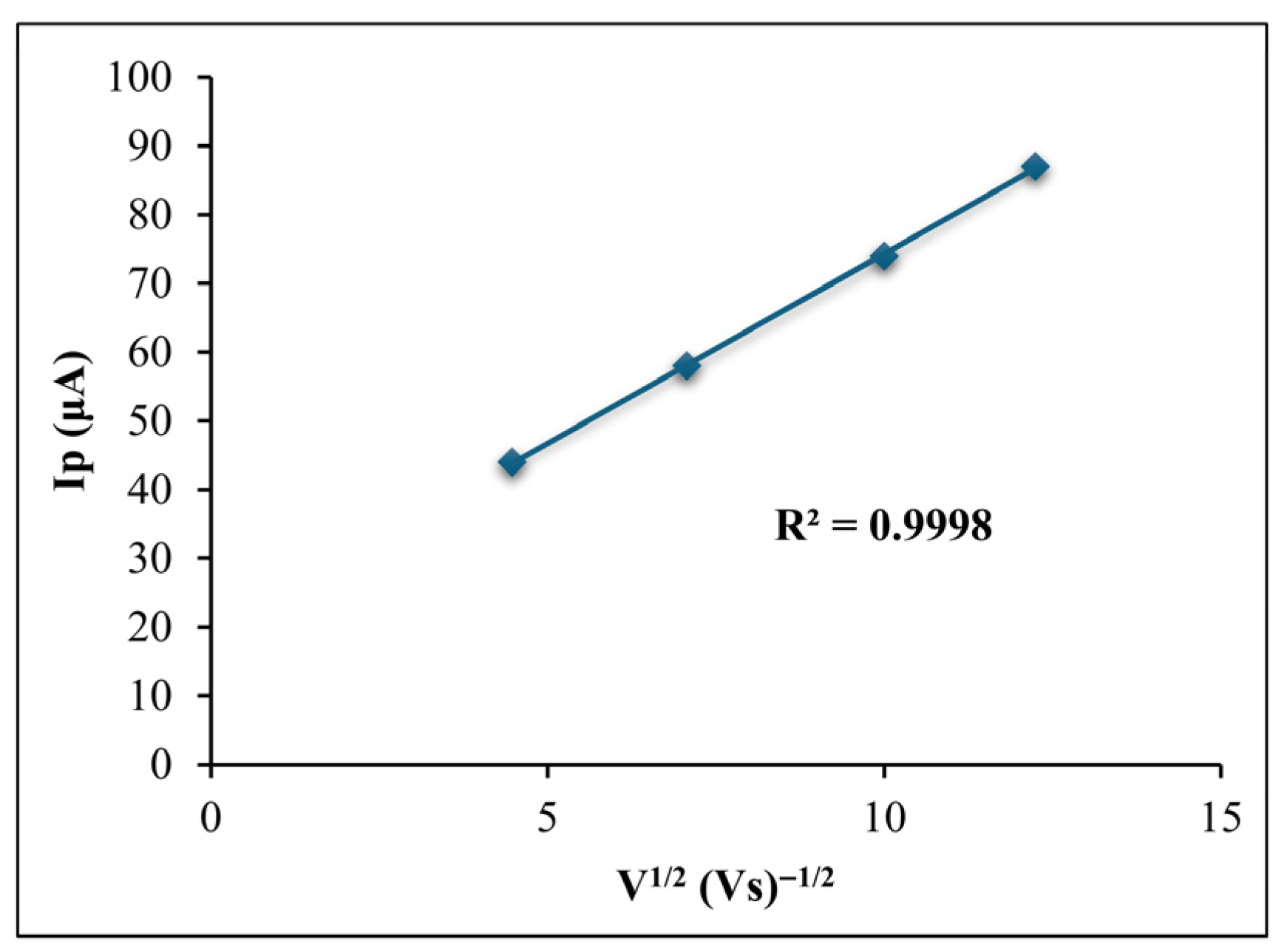
3.1.3. Effect of Scan Rates on Anodic Peak Current
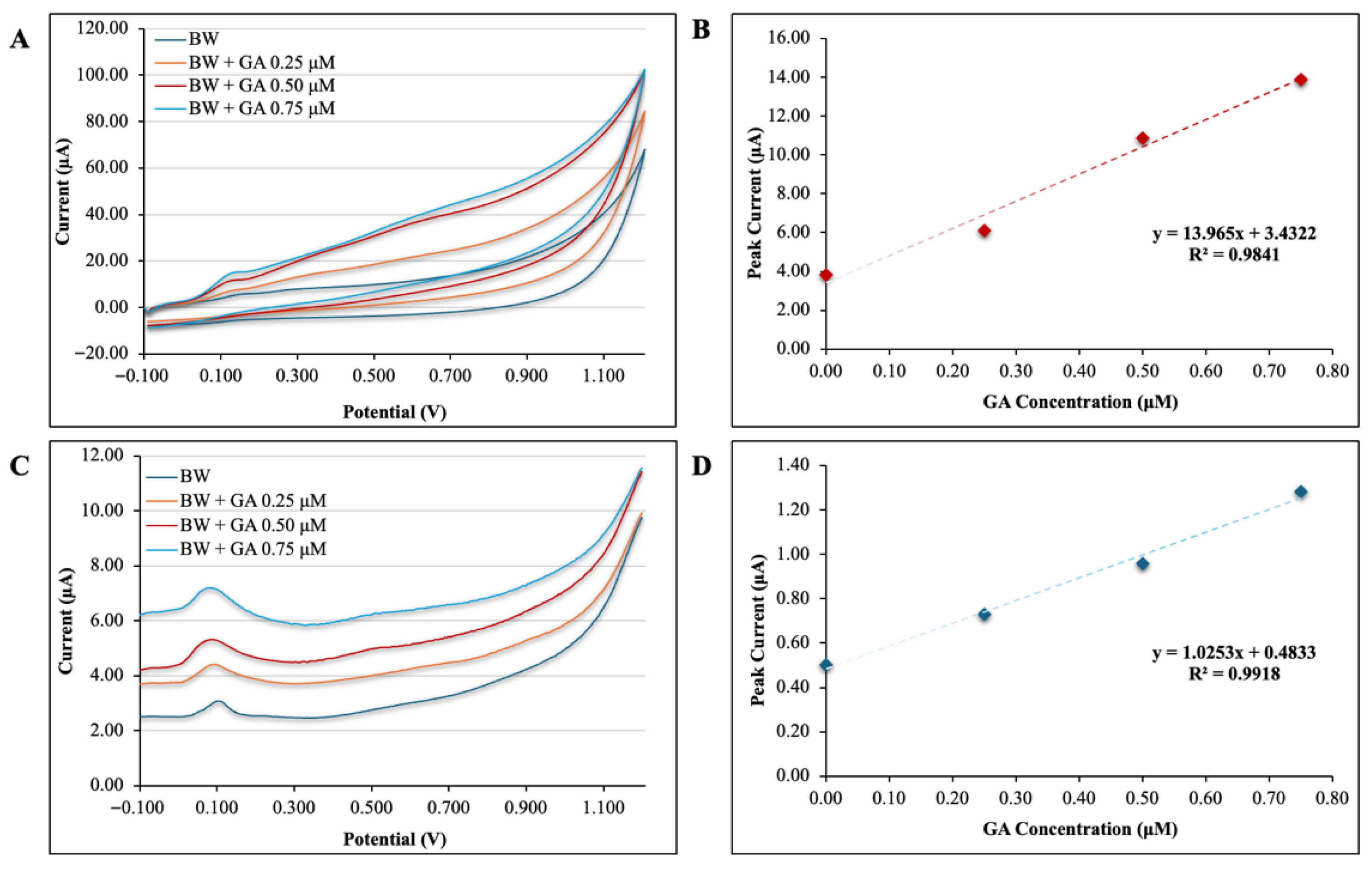
3.2. Calibration Study of Gallic Acid
3.3. Analytical Application of the Plain SPCE Sensor to the Determination of Gallic Acid in Banana Wine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPCE | Screen-printed Carbon Electrode |
| GA | Gallic Acid |
| BW | Banana Wine |
| CV | Cyclic Voltammetry |
| DPV | Differential-Pulsed Voltammetry |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HPLC | High-performance Liquid Chromatography |
| TLC FT-IR | Thin-layer Chromatography |
| DSC | Differential Scanning Calorimetry |
| TG | Thermogravimetry |
| LOD | Limit of Detection |
| STEYX | Standard Error of the Regression Line |
References
- FAO. Banana Market Review: Preliminary Results 2019; FAO: Rome, Italy, 2022; pp. 1–21. [Google Scholar]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of Ultrasound-Assisted Extraction Conditions for Recovery of Phenolic Compounds and Antioxidant Capacity from Banana (Musa cavendish) Peel. J. Food Process. Preserv. 2017, 41, e13148. [Google Scholar] [CrossRef]
- Li, Z.; Qin, C.; He, X.; Chen, B.; Tang, J.; Liu, G.; Li, L.; Yang, Y.; Ye, D.; Li, J.; et al. Development of Green Banana Fruit Wines: Chemical Compositions and In Vitro Antioxidative Activities. Antioxidants 2023, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Dave, S.; Parihar, N. Removal of Copper (Ii) from Aqueous Solution Using Chemically Activated Banana Peels as an Adsorbent. Poll Res. 2020, 39, 287–291. [Google Scholar]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana By-Products: An under-Utilized Renewable Food Biomass with Great Potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Seccia, A.; Nardone, G. Wine market structure and consumer demand. In Proceedings of the 19th Annual World Symposium of IAMA (International Food and Agribusiness Management Association), Budapest, Hungary, 20–23 June 2009; pp. 1–2. [Google Scholar]
- Oliveira, M.J.; Botelho, G. Degradation of polyamide 11 in rotational moulding. Polym. Degrad. Stab. 2008, 93, 139–146. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and Functional Foods: Whole versus Processed Foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Kamal, A.M.; Taha, M.S.; Mousa, A.M. The Radioprotective and Anticancer Effects of Banana Peels Extract on Male Mice. J. Food Nutr. Res. 2019, 7, 827–835. [Google Scholar] [CrossRef]
- Rita, W.S.; Swantara, I.M.D.; Astiti Asih, I.A.R.; Puspawati, N.M. Antibacterial Activity and Antioxidant Capacity of Selected Local Banana Peel (Musa sp.) Methanol Extracts Cultivated in Bali. Int. J. Agric. Environ. Bioresearch 2020, 5, 242–251. [Google Scholar] [CrossRef]
- Oliveira, M.d.S.; Furlong, E.B. Screening of Antifungal and Antimycotoxigenic Activity of Plant Phenolic Extracts. World Mycotoxin J. 2008, 1, 139–146. [Google Scholar] [CrossRef]
- Pramote, B.; Waranuch, N.; Kritsunankul, O. Simultaneous Determination of Gallic Acid and Catechins in Banana Peel Extract by Reversed-Phase High Performance Liquid Chromatography. Asian Health Sci. Technol. Reports 2018, 26, 189–200. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Prejanò, M.; Marino, T.; Russo, N.; Tao, Y.; Li, Y. Gallic Acid Improves Color Quality and Stability of Red Wine via Physico-Chemical Interaction and Chemical Transformation as Revealed by Thermodynamics, Real Wine Dynamics and Benchmark Quantum Mechanical Calculations. Food Res. Int. 2024, 188, 114510. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, H.; Wang, D.; Qian, L.; Zhu, Y.; Tao, S. Determination of Gallic Acid by Flow Injection Analysis Based on Luminol-AgNO 3-Ag NPs Chemiluminescence System. Chin. J. Chem. 2012, 30, 837–841. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal Characterization of Dried Extract of Medicinal Plant by DSC and Analytical Techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Wei, G.L.; Zeng, E.Y. Gas Chromatography-Mass Spectrometry and High-Performance Liquid Chromatography-Tandem Mass Spectrometry in Quantifying Fatty Acids. TrAC Trends Anal. Chem. 2011, 30, 1429–1436. [Google Scholar] [CrossRef]
- Bester, K. Quantification with HPLC-MS/MS for Environmental Issues: Quality Assurance and Quality Assessment. Anal. Bioanal. Chem. 2008, 391, 15–20. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Doran, O.; Hart, J.P. Studies Towards the Development of a Novel, Screen-Printed Carbon-Based, Biosensor for the Measurement of Polyunsaturated Fatty Acids. Appl. Sci. 2020, 10, 7779. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Crew, A.; Doran, O.; Hart, J.P. Development of a Disposable, Amperometric Glycerol Biosensor Based on a Screen-Printed Carbon Electrode, Modified with the Electrocatalyst Meldolas Blue, Coated with Glycerol Dehydrogenase and NAD+: Application to the Analysis of Wine Quality. Appl. Sci. 2024, 14, 6118. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Li, Y.; Zhao, P.; Fei, J.; Xie, Y. Detection of Gallic Acid in Food Using an Ultra-Sensitive Electrochemical Sensor Based on Glass Carbon Electrode Modified by Bimetal Doped Carbon Nanopolyhedras. Food Chem. 2023, 429, 136900. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An Electrochemical Sensor for Gallic Acid Based on Fe2O3/Electro-Reduced Graphene Oxide Composite: Estimation for the Antioxidant Capacity Index of Wines. Mater. Sci. Eng. C 2015, 57, 279–287. [Google Scholar] [CrossRef]
- Hawkridge, F.M. Fundamentals of Electrochemical Analysis (Galus, Zbigniew). J. Chem. Educ. 1978, 55, A248. [Google Scholar] [CrossRef]
- Gilmartin, M.A.T.; Ewen, R.J.; Hart, J.P.; Honeybourne, C.L. Voltammetric and Photoelectron Spectral Elucidation of the Electrocatalytic Oxidation of Hydrogen Peroxide at Screen-printed Carbon Electrodes Chemically Modified with Cobalt Phthalocyanine. Electroanalysis 1995, 7, 547–555. [Google Scholar] [CrossRef]
- Hadzhiev, D.T.; Dodevska, T.M.; Shterev, I.G. Electrochemical Response of Gallic Acid on Activated Glassy Carbon Electrode. Bulg. Chem. Commun. 2024, 56, 118–120. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.M.; Robinson, J. Instrumental Methods in Electrochemistry; Horwood Series in Chemical Science; Horwood Publishing: Chichester, UK, 2001; ISBN 1898563802. [Google Scholar]
- da Silva, W.; Ghica, M.E.; Ajayi, R.F.; Iwuoha, E.I.; Brett, C.M.A. Tyrosinase Based Amperometric Biosensor for Determination of Tyramine in Fermented Food and Beverages with Gold Nanoparticle Doped Poly(8-Anilino-1-Naphthalene Sulphonic Acid) Modified Electrode. Food Chem. 2019, 282, 18–26. [Google Scholar] [CrossRef]
- Chikere, C.O.; Hobben, E.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Electroanalytical Determination of Gallic Acid in Red and White Wine Samples Using Cobalt Oxide Nanoparticles-Modified Carbon-Paste Electrodes. Microchem. J. 2021, 160, 105668. [Google Scholar] [CrossRef]
- Hart, J.P. Electroanalysis of Biologically Important Compounds; Ellis Horwood limited: Chichester, UK, 1990; ISBN 0132521075. [Google Scholar]
- Prasad, B.B.; Madhuri, R.; Tiwari, M.P.; Sharma, P.S. Imprinted Polymer–Carbon Consolidated Composite Fiber Sensor for Substrate-Selective Electrochemical Sensing of Folic Acid. Biosens. Bioelectron. 2010, 25, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Sarıbaş, P.; Yıldız, C.; Eskiköy Bayraktepe, D.; Pekin Turan, M.; Yazan, Z. Gold Nanoparticles Decorated Kaolinite Mineral Modified Screen-Printed Electrode: Use for Simple, Sensitive Determination of Gallic Acid in Food Samples. Food Chem. 2024, 453, 139701. [Google Scholar] [CrossRef] [PubMed]
- Alipour, E.; Mirzae Bolali, F.; Norouzi, S.; Saadatirad, A. Electrochemically Activated Pencil Lead Electrode as a Sensitive Voltammetric Sensor to Determine Gallic Acid. Food Chem. 2022, 375, 131871. [Google Scholar] [CrossRef]
| Method | Equation | R2 | STEYX | LOD (μM) | Slope |
|---|---|---|---|---|---|
| CV | y = 21.437x + 5.3253 | 0.9939 | 3.194 | 0.149 | 21.437 |
| DPV | y = 0.2031x + 0.0493 | 0.9787 | 0.023 | 0.118 | 0.2031 |
| BW | Original Concentration in BW (μM) | |
|---|---|---|
| CV | DPV | |
| 1 | 7.113 | 7.647 |
| 2 | 7.165 | 6.891 |
| 3 | 7.434 | 6.671 |
| 4 | 7.571 | 8.602 |
| 5 | 7.561 | 8.037 |
| Average | 7.369 a | 7.570 a |
| SD | 0.194 | 0.715 |
| RSD (%) | 2.60 | 9.40 |
| BW | Concentration Found in BW (μM) | GA Added (μM) | Recovery (Found—Added Concentration) in BW (μM) | ||
|---|---|---|---|---|---|
| CV | DPV | CV | DPV | ||
| 1 | 14.529 | 14.669 | 7.500 | 7.029 | 7.169 |
| 2 | 15.065 | 15.157 | 7.500 | 7.565 | 7.657 |
| 3 | 14.241 | 14.942 | 7.500 | 6.741 | 7.442 |
| 4 | 14.357 | 14.786 | 7.500 | 6.857 | 7.286 |
| 5 | 14.713 | 15.196 | 7.500 | 7.213 | 7.696 |
| Recovery (%) | - | - | - | 94.41 | 99.33 |
| Average | 14.581 a | 14.950 a | 7.500 | 7.081 | 7.450 |
| SD | 0.290 | 0.204 | n/a | 0.290 | 0.204 |
| RSD (%) | 2.00 | 1.40 | n/a | 4.10 | 2.70 |
| Sensor Type | Technique | LOD | Linear Range | Sample Type | References |
|---|---|---|---|---|---|
| Plain SPCE sensor | CV Electrolyte: 10% ethanol—0.1 M PBS pH 7.0 | 0.149 μM | 0.25 to 5.00 µM | BW | Our approach Foods, 2025 |
| Plain SPCE sensor | DPV Electrolyte: 10% ethanol—0.1 M PBS pH 7.0 | 0.118 μM | 0.25 to 5.00 µM | BW | Our approach Foods, 2025 |
| Gold NPs decorated Kaolinite mineral modified SPCE | DPV Electrolyte: Britton-Robinson buffer pH 2.0 | 0.50 nM | 0.002 µM to 40 µM | Black tea and pomegranate juice | [32] |
| Glassy carbon electrode modified with Co/FeOx@NC-800 | DPV Electrolyte: 0.1 M PBS pH 3.0 | 1.30 nM | 5.00 nM to 4.50 µM | Strawberries, grapes, tomatoes, and red wine | [22] |
| Cobalt oxide NPs-modified carbon-paste electrode | DPV Electrolyte: 0.1 M phosphate buffer pH 2.0 | 0.15 µM | 0.10 µM to 1.00 µM | Red and white wine | [29] |
| Activated pencil lead electrode | DPV Electrolyte: 0.25 M phosphate-buffer solution pH 2.0 | 0.25 µM | 0.49 to 24.30 µM | Black tea, green tea, and mango juice | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekonomou, S.I.; Doran, O.; Crew, A.; Hart, J.P. Electrochemical Behaviour of the Antioxidant Gallic Acid Using a Low-Cost Screen-Printed Carbon Sensor and Its Exploitation for Banana Wine Analysis. Foods 2025, 14, 4070. https://doi.org/10.3390/foods14234070
Ekonomou SI, Doran O, Crew A, Hart JP. Electrochemical Behaviour of the Antioxidant Gallic Acid Using a Low-Cost Screen-Printed Carbon Sensor and Its Exploitation for Banana Wine Analysis. Foods. 2025; 14(23):4070. https://doi.org/10.3390/foods14234070
Chicago/Turabian StyleEkonomou, Sotirios I., Olena Doran, Adrian Crew, and John P. Hart. 2025. "Electrochemical Behaviour of the Antioxidant Gallic Acid Using a Low-Cost Screen-Printed Carbon Sensor and Its Exploitation for Banana Wine Analysis" Foods 14, no. 23: 4070. https://doi.org/10.3390/foods14234070
APA StyleEkonomou, S. I., Doran, O., Crew, A., & Hart, J. P. (2025). Electrochemical Behaviour of the Antioxidant Gallic Acid Using a Low-Cost Screen-Printed Carbon Sensor and Its Exploitation for Banana Wine Analysis. Foods, 14(23), 4070. https://doi.org/10.3390/foods14234070









