Metabolic Pathway Analysis in Chicken Induced by Selenium-Enriched Yeast: Insights from Flavoromics and Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Treatments
2.2. Sample Collection and Preparation
2.3. Chemicals and Reagents
2.4. Extraction of VOCs Using Headspace Solid-Phase Microextraction (HS-SPME)
2.5. GC-MS Analysis
2.6. Analysis of VOCs
2.7. Calculation of Odor Active Value (OAV)
2.8. Amino Acid Analysis
2.9. Fatty Acids Analysis
2.10. Cholesterol Analysis
2.11. Untargeted Metabolomics Analysis
2.12. Data Analysis
3. Results and Discussion
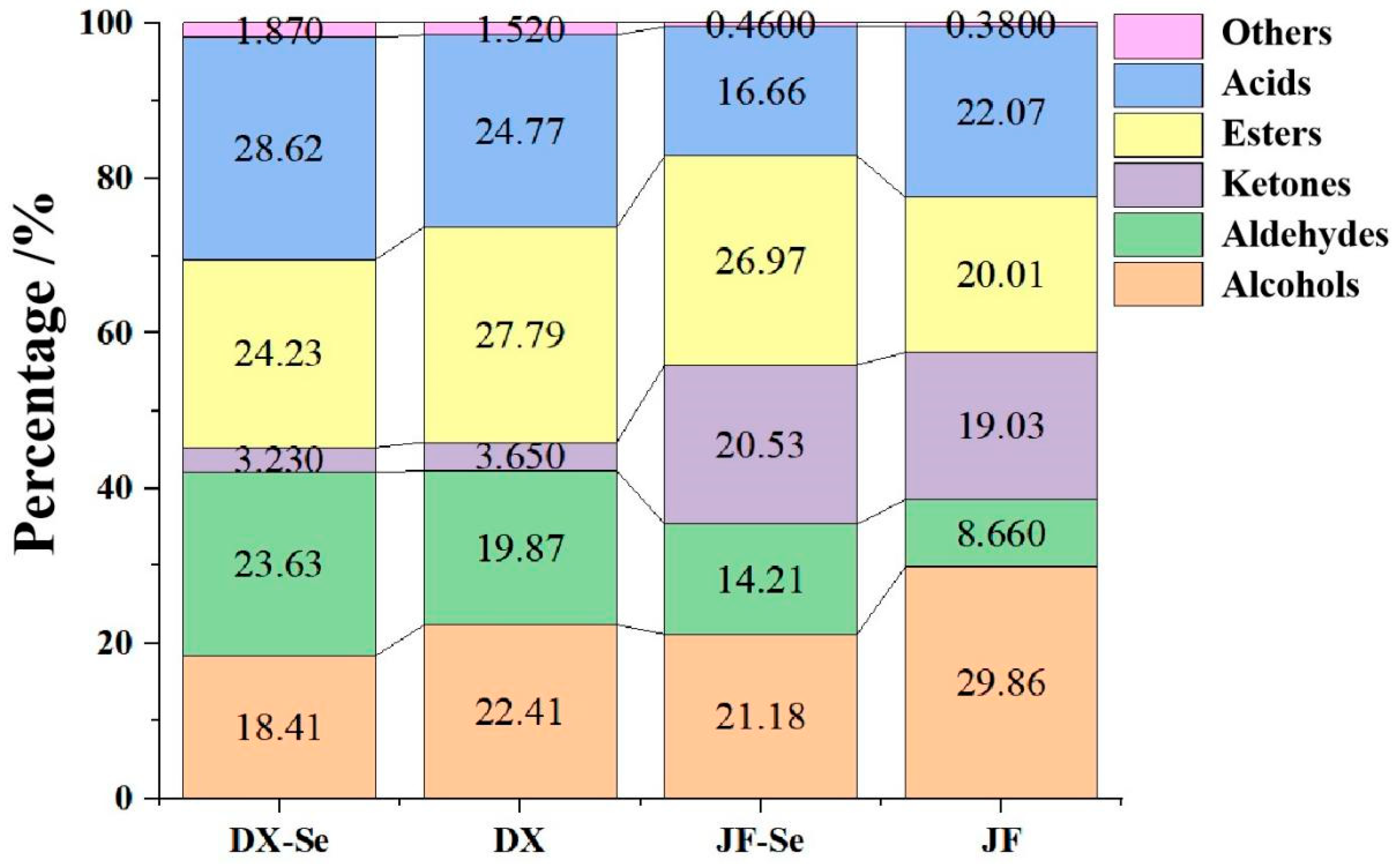
3.1. VOCs Analysis
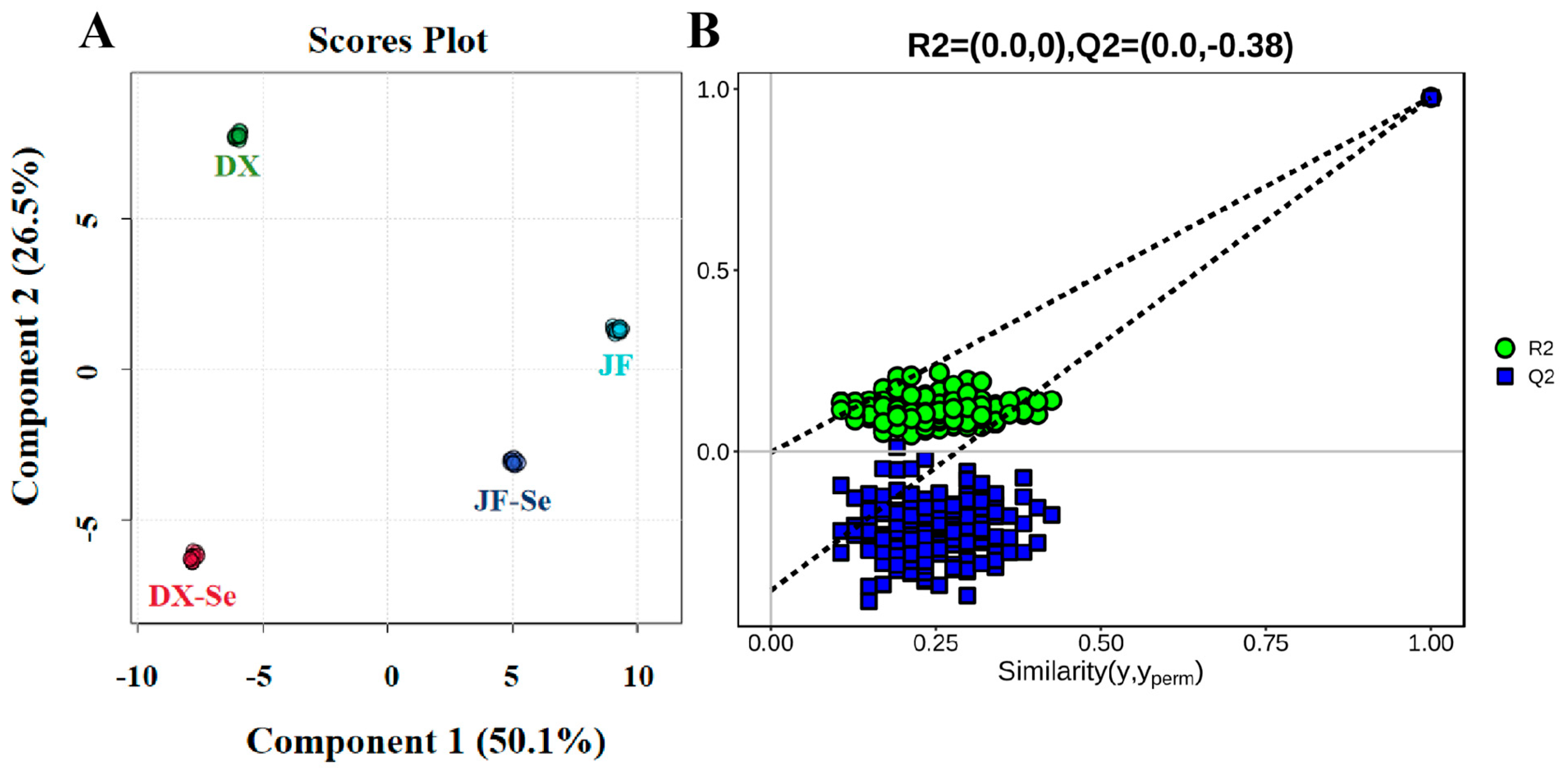
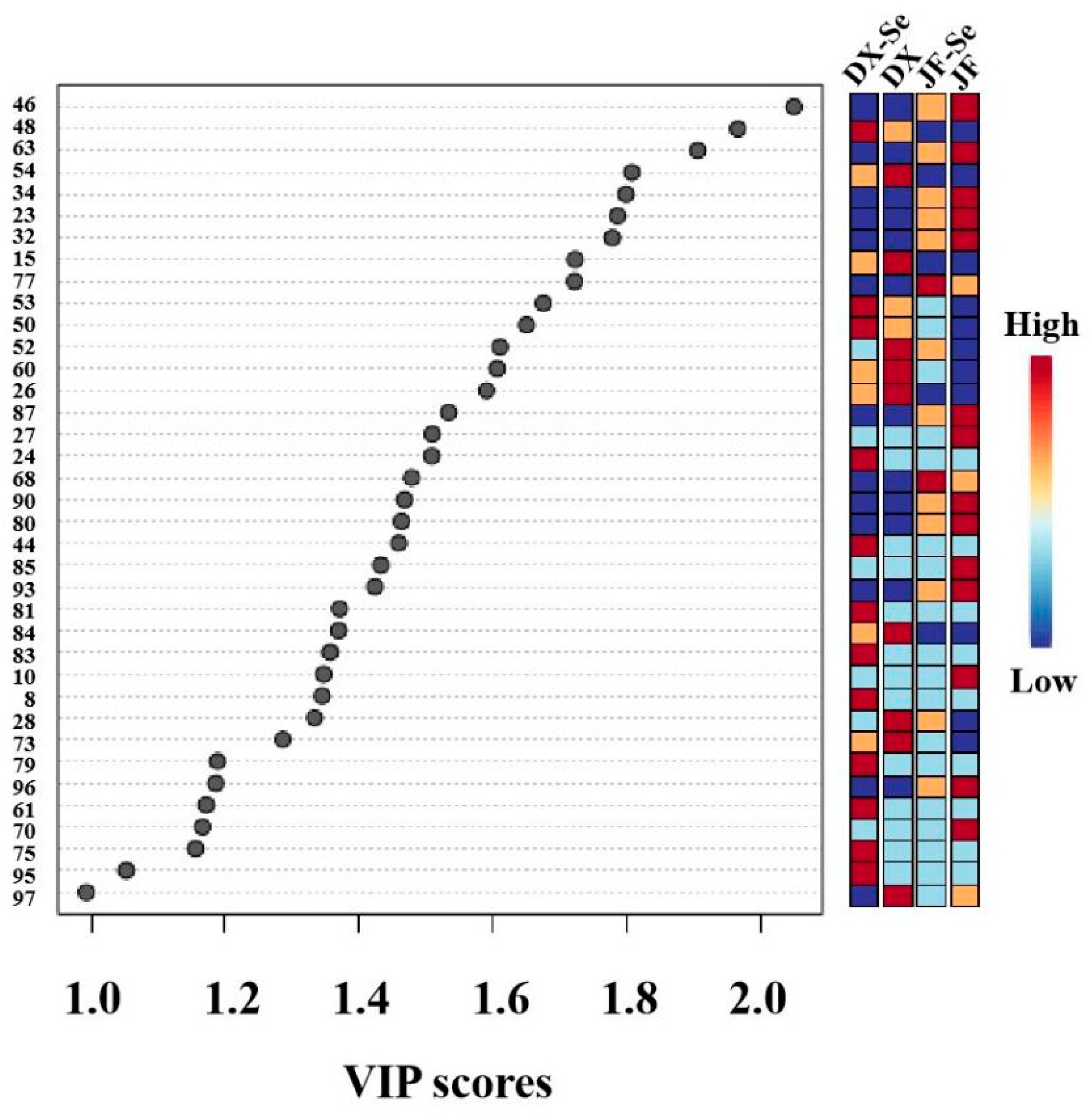
3.2. Chemometric Analysis of VOCs
3.3. Characteristic VOCs Analysis
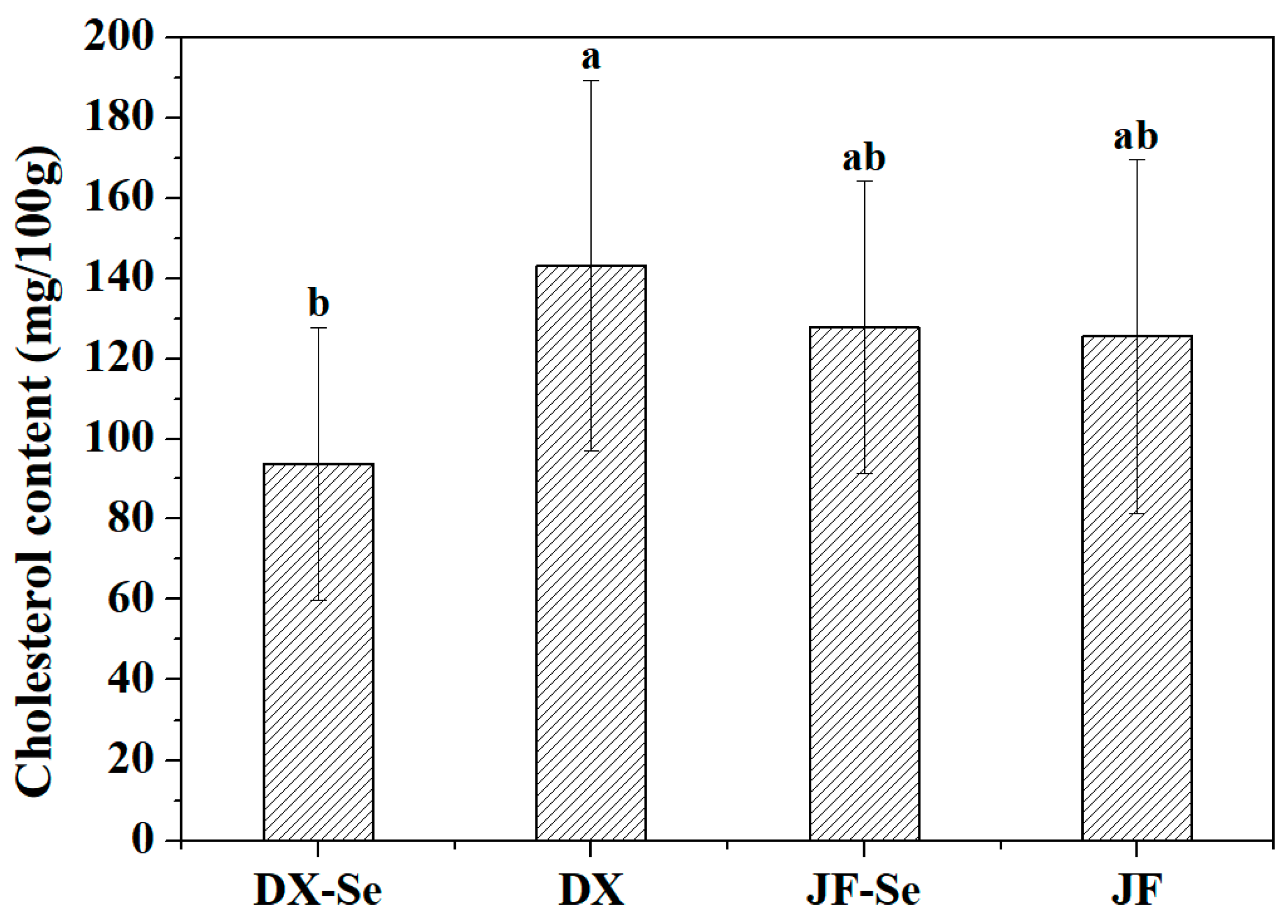
3.4. Fatty Acid Profiles and Cholesterol Analysis
3.5. Amino Acids Analysis
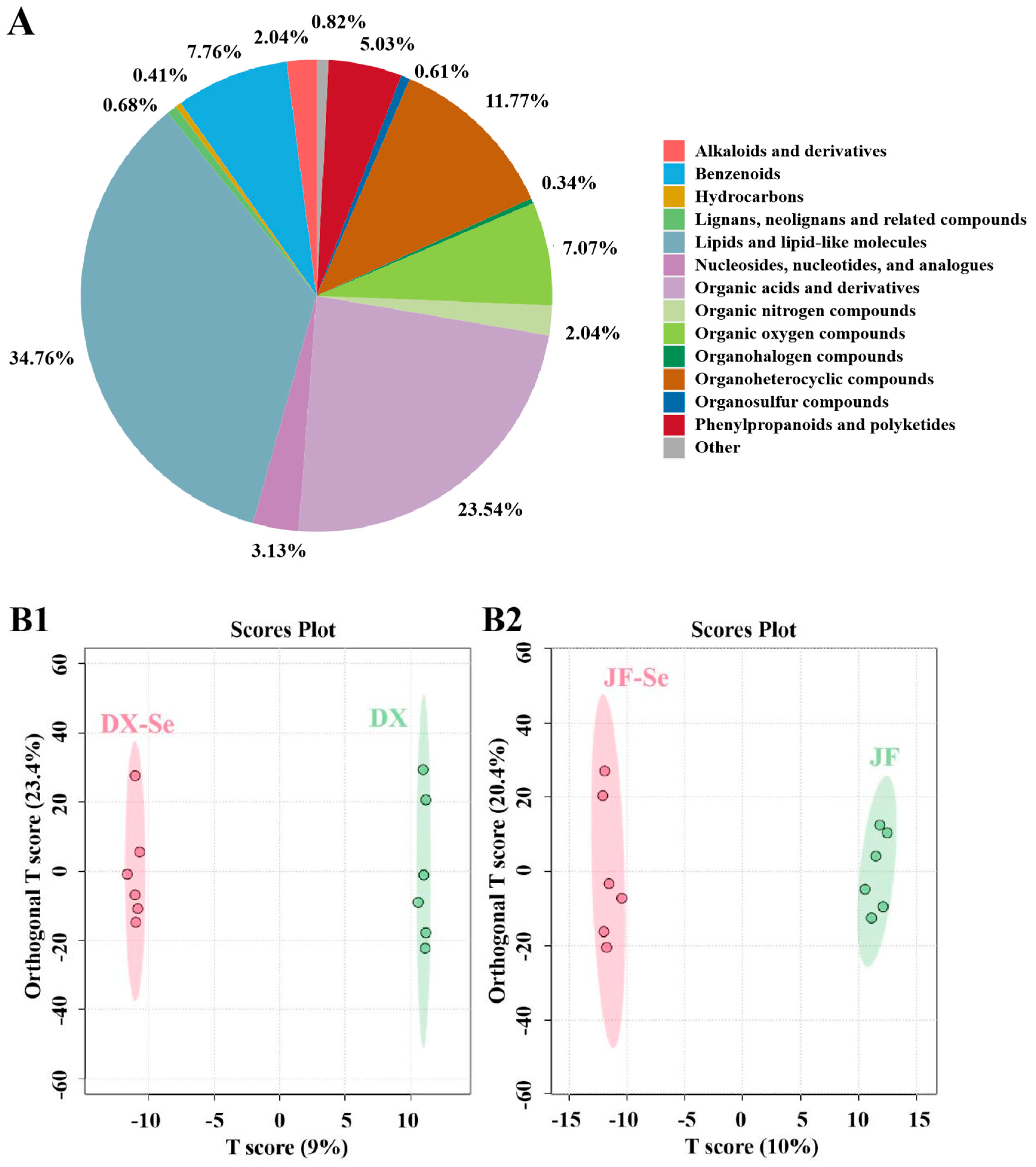
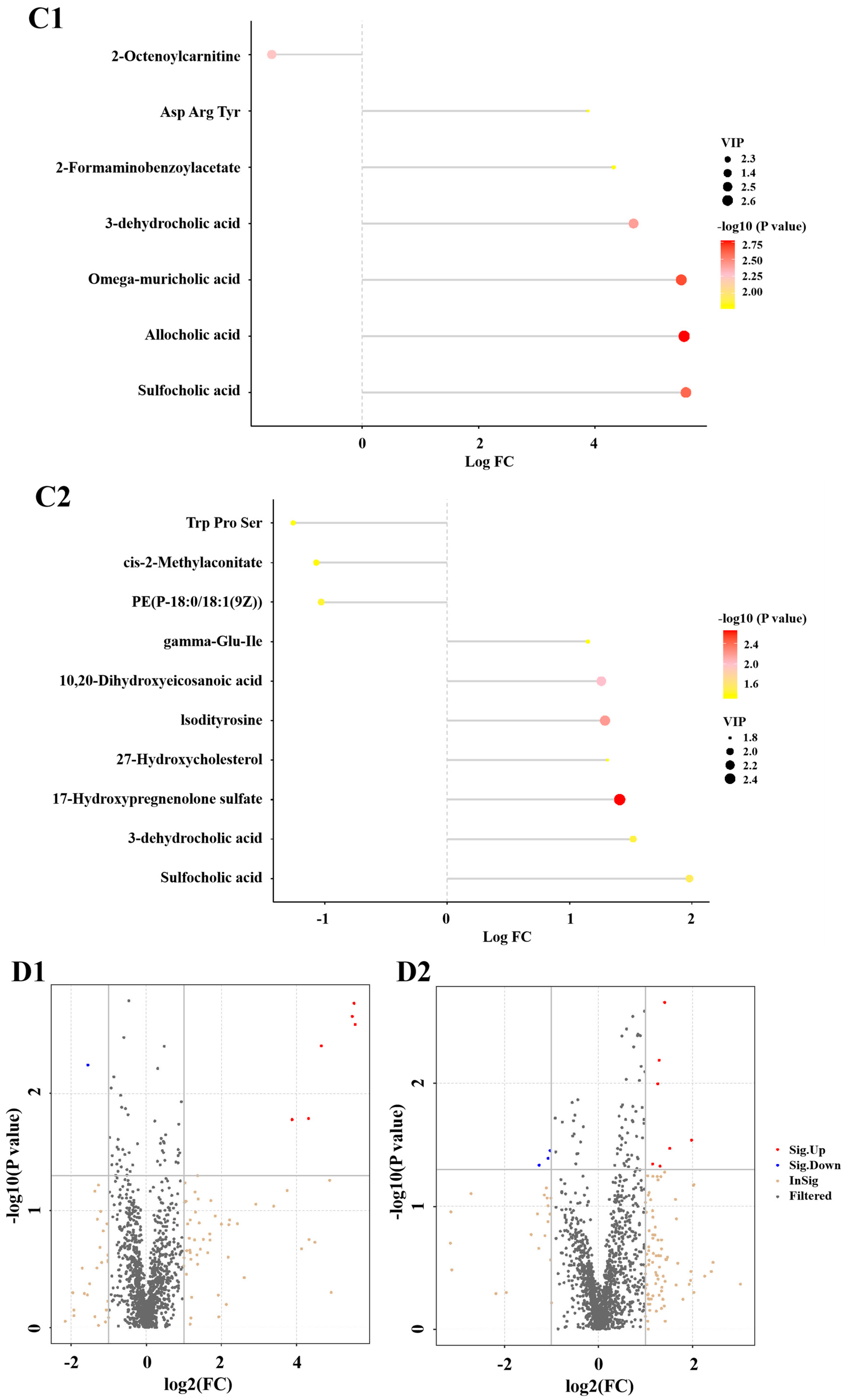
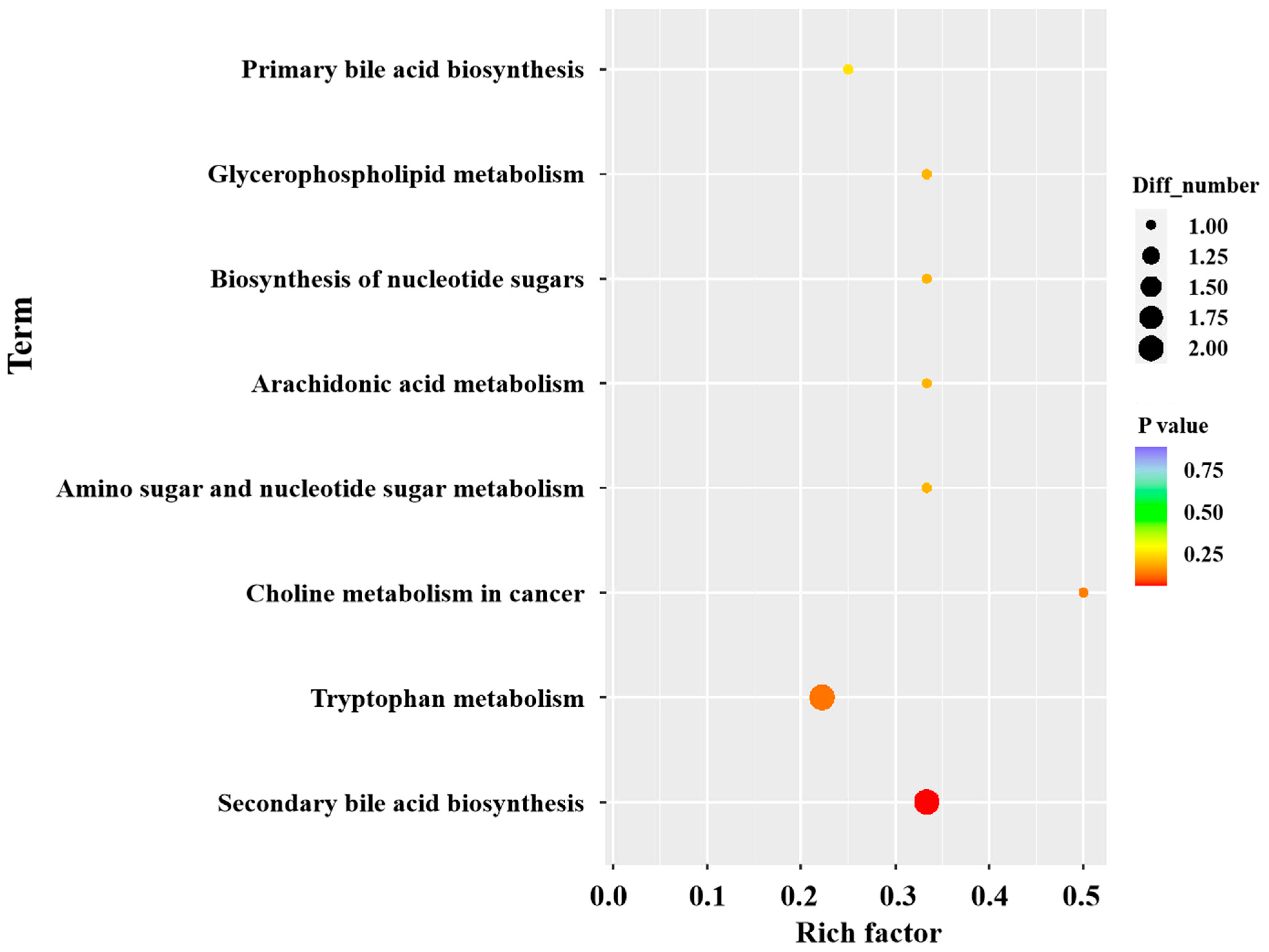
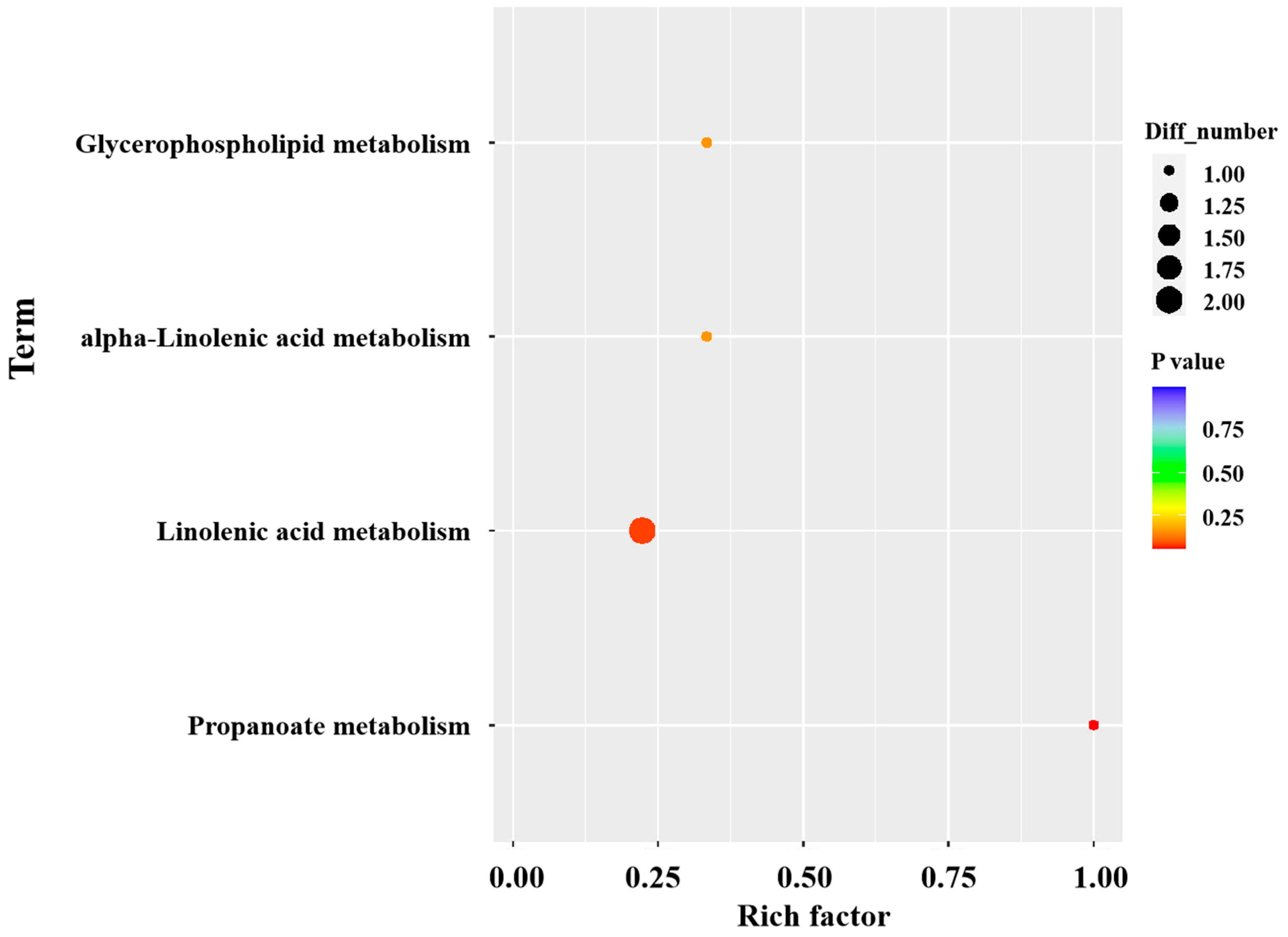
3.6. Metabolomics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Sun, B.; Hu, P.; Zhou, M.; Sun, S.; Du, P.; Ru, Y.; Suvorov, A.; Li, Y.; Liu, Y. Comparison of differential flavor metabolites in meat of lubei white goat, Jining Gray Goat and Boer Goat. Metabolites 2019, 9, 176. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A.; Teranishi, R.; Buttery, R.G. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. 1986, 24, 141–243. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lu, Y.; Lü, Y. Recent advances in the application of headspace gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2018, 36, 962–971. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreiraa, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef]
- Chen, G.; Su, Y.; He, L.; Shui, S. Analysis of volatile compounds in pork from four different pig breeds using headspace solid-phase micro-extraction/gas chromatography-mass spectrometry. Food Sci. Nutr. 2019, 7, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, M.; Scalzo, R.L.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography-Olfactometry (GC-O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Anal. Method. 2008, 1, 270–282. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Zhang, J.; Le, T.; Wang, W.; Xue, J.; Jiang, H. Research Progress of Metabomics applied to tea processing and quality analysis. Food Sci. Technol. 2022, 47, 74–81. [Google Scholar]
- Zhang, T.; Zhang, S.; Chen, L.; Ding, H.; Wang, J. UHPLC-MS/MS-Based nontargeted metabolomics analysis reveals biomarkers related to the freshness of chilled chicken. Foods 2020, 9, 1326. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.G.; Liao, G.Z. lH NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Beauclercq, S.; Desbarats, L.; Antier, C.; Collin, A.; Tesseraud, S. Serum and muscle metabolomics for the prediction of ultimate pH, a key factor for chicken-meat quality. J. Proteome Res. 2016, 15, 1168–1178. [Google Scholar] [CrossRef]
- Kim, C.; Ko, J.; Jo, C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chem. 2021, 342, 128316. [Google Scholar] [CrossRef]
- Peng, L.M.; Li, S.N.; He, Q.Q.; Zhao, J.L.; Li, L.L.; Ma, H.T. Based serum metabolomics analysis reveals simultaneous interconnecting changes during chicken embryonic development. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1210–1219. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aromacompounds in chicken broths of Beijing Youji and commercial broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Q.; Yin, L.; Tang, Y.; Lin, Z.; Zhang, D.; Liu, Y. A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens. Foods 2023, 12, 3680. [Google Scholar] [CrossRef]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of dietary incorporation of n-3 polyunsaturated fatty acids rich oil sources on fatty acid profile, keeping quality and sensory attributes of broiler chicken meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Farmer, L.J.; Derry, G.C.; Lewis, P.D.; Nute, G.R.; Piggott, J.R.; Patterson, R.L.S. Responses of two genotypes of chicken to the diets and stocking densities of conventional UK and Label Rouge production systems-II. Sensory attributes. Meat Sci. 1997, 47, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.E.; Alagawany, M.; Taha, A.E.; Puvaa, N.; Tufarelli, V. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood haematology, immunity and antioxidant status of broiler chickens. Anim. Biosci. 2020, 34, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik-Kalinowska, I.; Guzek, D.; G’orska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT—Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Shini, S.; Sultan, A.; Bryden, W. Selenium Biochemistry and Bioavailability: Implications for Animal Agriculture. Agriculture 2015, 5, 1277–1288. [Google Scholar] [CrossRef]
- Wang, Y.B. Differential effects of sodium selenite and nanoSe on growth performance, tissue Se distribution, and glutathione peroxidase activity of avian broiler. Biol. Trace Elem. Res. 2009, 128, 184–190. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, B.H. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim. Feed Sci. Technol. 2008, 144, 306–314. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Bi, X.; Zhang, Y.; Yin, H. Effects of various selenium-enriched yeasts, selenomethionine, and nanoselenium on production performance, quality, and antioxidant capacity in laying hens. Poultry Sci. 2024, 103, 103387. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Qiu, H.; Sun, P.; Zhu, L.Q.; Chen, F.; Qin, S.Y. Selenium-enriched Saccharomyces cerevisiae improves the meat quality of broiler chickens via activation of the glutathione and thioredoxin systems—Science Direct. Poultry Sci. 2020, 99, 6045–6054. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Wen, K.; Xue, Y.; Liu, L.; Geng, T.Y.; Gong, D.Q.; Yu, L. Probing the effects of dietary selenised glucose on the selenium concentration quality and antioxidant activity of eggs and production performances of laying hens. Animal 2021, 15, 100374. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.; Xie, M.; Guang, Y.L.; Xu, J.; Wu, N.; Cai, J.; Lai, Y.; Zhou, Y.M. HS-SPME-GC-MS Combined with Multivariate Analysis Assay for Volatile Organic Compounds of Commercial Eggs and Native Eggs. Food Anal. Method. 2025, 18, 347–358. [Google Scholar] [CrossRef]
- Huang, X.H.; Qi, L.B.; Fu, B.S.; Chen, Z.H.; Zhang, Y.Y.; Du, M.; Dong, X.P.; Zhu, B.W.; Lei, Q. Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem. 2019, 286, 241–249. [Google Scholar] [CrossRef]
- Ye, Y.H.; Zheng, S.Y.; Wang, Y.X. Analysis of aroma components changes in Gannan navel orange at different growth stages by HS-SPME-GC-MS, OAV, and multivariate analysis. Food Res. Int. 2024, 175, 113622. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour thresholds. In Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Shakoor, A.; Al-Dalali, S.; Xie, J.; Zhang, C.; Hossen, I. Insight into the effect of GSH curing treatment on the flavor formation of chicken meat. Food Chem. 2025, 468, 142488. [Google Scholar] [CrossRef]
- Koakoski, D.L.; Bordin, T.; Cavallini, D.; Buonaiuto, G. A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage. Fermentation 2024, 10, 398. [Google Scholar] [CrossRef]
- Bordin, C.; Raspa, F.; Greppi, M.; Harris, P.; Ellis, A.D.; Roggero, A.; Palestrini, C.; Cavallini, D.; Bergero, D.; Valle, E. Pony feeding management: The role of morphology and hay feeding methods on intake rate, ingestive behaviors and mouth shaping. Front. Vet. Sci. 2024, 11, 14. [Google Scholar] [CrossRef]
- Jin, Y.X.; Cui, H.A.; Yuan, X.Y.; Liu, L.; Liu, X.J.; Wang, Y.L.; Ding, J.Q.; Xiang, H.; Zhang, X.X.; Liu, J.F.; et al. Identification of the main aroma compounds in Chinese local chickenhigh-quality meat. Food Chem. 2021, 359, 129930. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.X.; Yuan, X.Y.; Liu, J.F. Inhibition of cholesterol biosynthesis promotes the production of 1-octen-3-ol through mevalonic acid. Food Res. Int. 2022, 158, 111392. [Google Scholar] [CrossRef] [PubMed]
- Merlo, T.C.; Lorenzo, J.M.; Saldana, E.; Patinho, I.; Oliveira, A.C.; Menegali, B.S.; Selani, M.M.; Dominguez, R.; Contreras-Castillo, C.J. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Yeung, C.; Kim, J.S.; Chen, F. Static headspace analysis-olfactometry (SHA-O) of odor impact components in salted-dried white herring (Ilisha elongata). Food Chem. 2007, 104, 842–851. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavor formation in meat and meat products:a review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Purrinos, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lac6n”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Li, M.; Huang, J.; Zu, X.; Liao, T.; Xiong, G.Q. Effects of temporary rearing with organic selenium on the muscle flavor and texture properties of large mouth bass (Micropterus salmonides). Food Chem. 2022, 397, 133747. [Google Scholar] [CrossRef]
- Keizo, S.; Hiromichi, O.; Shigeru, A. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta 1982, 713, 68–72. [Google Scholar] [CrossRef]
- Lehner, T.; Siegmund, B. The impact of ventilation during postharvest ripening on the development of flavour compounds and sensory quality of mangoes (Mangifera indica L.) cv. Kent. Food Chem. 2020, 320, 126608. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, H.; Pang, X. Comparative investigation on aroma profiles of five different mint (Mentha) species using a combined sensory, spectroscopic and chemometric study. Food Chem. 2022, 371, 131104. [Google Scholar] [CrossRef]
- Dong, J.H.; Qiu, H.L.; Gao, S.S.; Hou, L.L.; Liu, H.W.; Zhu, L.Q.; Chen, F. A combination of selenium and Bacillus subtilis improves the quality and flavor of meat and slaughter performance of broilers. Front. Vet. Sci. 2023, 10, 1259760. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Hamid Nbejhit, A.E.D.; Robertson, J.; Law, T.F. Evaluation of pre-rigor injection of beef with proteases on cooked meat volatile profile after 1 day and 21 day post-mortem storage. Meat Sci. 2012, 92, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.K.; Ziegler, P. A comparison of the volatile fractions from cured and uncured meat. J. Food Sci. 1965, 30, 610–614. [Google Scholar] [CrossRef]
- Lian, F.; Cheng, J.-H.; Sun, D.W. Effects of combined roasting with steam cooking on fat content, physicochemical properties and in vitro protein digestion of chicken wings as compared with other conventional cooking methods. LWT 2023, 183, 114941. [Google Scholar] [CrossRef]
- Chen, J.N.; Zhang, Y.Y.; Huang, X.H.; Dong, M.; Dong, X.P.; Zhou, D.Y.; Zhu, B.W.; Qin, L. Integrated volatolomics and metabolomics analysis reveals thecharacteristic flavor formation in Chouguiyu, a traditional fermented mandarin fish of China. Food Chem. 2023, 418, 135874. [Google Scholar] [CrossRef]
- Souza, H.A.L.; Mariutti, L.R.B.; Bragagnolo, N. Microwave assisted direct saponification for the simultaneous determination of cholesterol and cholesterol oxides in shrimp. J. Steroid Biochem. 2016, 169, 88–95. [Google Scholar] [CrossRef]
- Wei, N.B.; Liu, H.Y.; Wang, H.F.; Ren, D.X.; Liu, X.J.; Wang, C. Research Progress of Mechanism of Action of Proteins in Cholesterol Metabolism of Sterol Regulatory Element Binding. Chin. J. Anim. Sci. 2013, 49, 80–84. [Google Scholar]
- Bjarnadottir, O.; Romero, Q.; Bendahl, P.O.; Jirstrom, K.; Ryden, L. Targeting HM G-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 2013, 138, 449–508. [Google Scholar] [CrossRef]
- Yeganeh, B.; Wiechec, E.; Ande, S.R.; Sharma, P.; Ghavami, S. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol. Therapeut. 2014, 143, 87–110. [Google Scholar] [CrossRef]
- Wang, Z.M. Effects of Selenium Source on the Performance, Egg Quality and Blood Biochemical Parameters of Laying Hens; Chinese Academy of Agricultural Sciences: Beijing, China, 2013. [Google Scholar]
- World Health Organization. Energy and Protein Requirements: Report of a Joint FAO/WHO Ad Hoc Expert Committee; WHO: Geneva, Switzerland, 1973. [Google Scholar]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.Y.L. Farnesoid X receptor induces Takeda G-protein receptor 5 Crosstalk to regulate Bile Acid Synthesis and Hepatic Metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fuchs, C.D.; Halilbasic, E.; Trauner, M. Bile acids in regulation of inflammation and immunity: Friend or foe? Clin. Exp. Rheumatol. 2016, 34, 25–31. [Google Scholar] [PubMed]
- Wallace, B.D.; Redinbo, M.R. Xenobiotic-sensing nuclear receptors involved indrug metabolism: A structural perspective. Drug Metab. Rev. 2013, 45, 79–100. [Google Scholar] [CrossRef]
- Chen, L.; Ma, S.; Cao, A.; Zhao, R. Bile acids promote lipopolysaccharide clearance via the hepato-biliary pathway in broiler chickens. Ecotox. Environ. Safe 2024, 282, 116767. [Google Scholar] [CrossRef]
- Callejón-Leblic, B.; Arias-Borrego, A.; de Fátima Vélez-Pérez, R.; García-Barrera, T. Selenium Bioavailability in Food and the Impact in Mammals Gut Microbiota Environmental Science and Engineering; Springer: Cham, Switzerland, 2025; pp. 693–746. [Google Scholar] [CrossRef]
- Yu, T.; Guo, J.; Zhu, S.; Li, M.; Zhu, Z.Z.; Cheng, S.Y.; Sun, Y.M.; Cong, X. Protective effects of selenium-enriched peptides from cardamine violifolia against high fat diet induced obesity and its associated metabolic disorders in mice. RSC Adv. 2020, 10, 31411. [Google Scholar] [CrossRef]
- Gema, R.M.; Raúl, C.R.; Marta, S.R.; José, A.G.M.; Maria, C.C.; Nieves, A.; Tamara, G.B. Modulation of the gut microbiota and the microbial-produced gut metabolites by diclofenac exposure and selenium supplementation. Environ. Sci. Pollut. Res. 2025, 32, 16945–16957. [Google Scholar] [CrossRef]
- Yu, X.L.; Cao, T.; Ipemba, E.; Bakala, G.B.; Leveut, L.G.D.; Peng, W.Q.; Ji, F.J.; Li, H.F.; Xu, L.M.; Wu, H.A. Effects of dietary supplementation with bile acids on growth performance, antioxidant capacity, lipid metabolism, and cecal microbiota of Danzhou chickens. Poultry Sci. 2025, 104, 105276. [Google Scholar] [CrossRef]
- Fan, Q.S.; Ge, Y.L.; Fu, Y.M.; Gao, Y.F.; Zhou, G.H.; Liu, Z.H.; Yuan, X.J.; Yang, W.R.; Jiao, N.; Ding, Y.; et al. Research note: Effects of dietary bile acids supplementation on hepatic lipid accumulation and fatty acid profile of hens at late laying cycle. Poultry Sci. 2025, 104, 106026. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilledred and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. 12-Methyltridecanal, a Species-Specific Odorant of Stewed Beef. LWT—Food Sci. Technol. 1993, 26, 171–177. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Dodson, A.T.; Mottram, D.S. Volatile flavour profile of goat meat extracted by three widely used techniques. Food Chem. 2009, 115, 1081–1087. [Google Scholar] [CrossRef]
- Yan, J.; Pan, Y.; Shao, W.; Wang, C.; Wang, R.; He, Y.; Zhang, M.; Wang, Y.; Li, T.; Wang, Z. Beneficial effect of the short-chain fatty acid propionate onvascular calcification through intestinal microbiota remodelling. Microbiome 2022, 10, 195. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Odor Description | Content/ng/g | p-Value | |||
|---|---|---|---|---|---|---|---|
| DX-Se | DX | JF-Se | JF | ||||
| 1 | Butyl acetate | pear | 1810.03 ± 531.99 | 2136.44 ± 937.68 | 1487.32 ± 331.50 | 1595.55 ± 265.59 | 0.051 |
| 2 | Hexanal | grass, tallow, fat | 1221.01 ± 268.77 b | 1092.00 ± 193.35 b | nd * | 3446.35 ± 476.03 a | <0.001 |
| 3 | 2-Methylbutyl acetate | fruit | nd | 291.15 ± 67.96 | nd | nd | - |
| 4 | p-Xylene | sweet | 121.60 ± 31.77 ab | 140.59 ± 38.15 a | 96.84 ± 16.15 b | 104.98 ± 17.76 b | 0.002 |
| 5 | m-Xylene | plastic | 164.51 ± 52.09 a | 158.83 ± 25.20 a | 95.27 ± 14.90 b | 107.50 ± 21.23 b | <0.001 |
| 6 | 1-Butanol | medicine, fruit | 726.40 ± 236.39 a | 500.22 ± 180.03 b | 374.10 ± 98.98 bc | 298.62 ± 59.62 c | <0.001 |
| 7 | 1-Penten-3-ol | butter, pungent | 417.58 ± 181.11 b | 742.04 ± 247.98 a | 358.02 ± 90.54 b | 377.63 ± 104.20 b | <0.001 |
| 8 | alpha-Terpinene | lemon | 331.53 ± 65.18 | nd | nd | nd | - |
| 9 | o-Xylene | geranium | 147.75 ± 48.31 a | 138.90 ± 17.90 a | 68.36 ± 17.12 b | 99.46 ± 19.11 b | <0.001 |
| 10 | 2-Heptanone | soap | nd | nd | nd | 434.17 ± 36.65 | - |
| 11 | 3-Methyl-3-butenyl acetate | - | 1658.68 ± 287.87 a | 1430.58 ± 429.59 a | 1621.29 ± 404.37 a | 783.77 ± 66.62 b | <0.001 |
| 12 | Limonene | lemon, orange | 1353.80 ± 250.82 a | 1028.29 ± 256.75 b | 970.88 ± 253.78 b | 582.81 ± 86.24 c | <0.001 |
| 13 | 2-Methyl-1-butanol | wine, onion, malt | 1486.69 ± 368.86 a | 1533.29 ± 348.90 a | 744.96 ± 147.15 b | 575.96 ± 85.71 b | <0.001 |
| 14 | Isoamyl alcohol | whiskey, malt, burnt | 892.65 ± 459.75 b | 1696.21 ± 582.47 a | 499.71 ± 107.56 c | 491.42 ± 56.16 c | <0.001 |
| 15 | 2-Methylpyridine | sweat | 560.12 ± 83.82 | 628.45 ± 213.46 | nd | nd | 0.313 |
| 16 | Styrene | balsamic, gasoline | 232.05 ± 56.24 | 214.01 ± 47.03 | 249.33 ± 49.77 | 273.77 ± 67.50 | 0.073 |
| 17 | 1-Pentanol | balsamic | 1366.88 ± 329.89 b | 2040.63 ± 695.08 a | 722.07 ± 107.14 c | 2192.49 ± 544.42 a | <0.001 |
| 18 | 3-Octanone | herb, butter, resin | 671.70 ± 141.06 b | 438.94 ± 57.51 c | 495.04 ± 98.41 c | 1608.21 ± 225.89 a | <0.001 |
| 19 | Hexyl acetate | fruit, herb | nd | 605.76 ± 133.16 | nd | nd | - |
| 20 | 4-Methylthiazole | roasted meat | nd | 467.66 ± 84.66 | nd | nd | - |
| 21 | 2-Octanone | soap, gasoline | 294.03 ± 58.32 b | nd | 239.70 ± 23.61 b | 844.42 ± 129.40 a | <0.001 |
| 22 | Octanal | fat, soap, lemon, green | 17,067.57 ± 3507.217 a | nd | nd | 6529.79 ± 787.96 b | <0.001 |
| 23 | trans-2-Penten-1-ol | mushroom | nd | nd | 464.48 ± 57.39 b | 1857.27 ± 655.44 a | <0.001 |
| 24 | trans-2-Heptenal | soap, fat, almond | 2566.85 ± 171.88 | nd | nd | nd | - |
| 25 | 1-Hexanol | resin, flower, green | 2195.81 ± 593.00 ab | 2862.71 ± 970.55 a | 3429.51 ± 1055.22 a | 3628.86 ± 1606.35 a | 0.014 |
| 26 | 4,5-Dimethylthiazole | roast, smoke | 158.65 ± 9.07 b | 327.93 ± 26.61 a | nd | nd | <0.001 |
| 27 | Heptyl acetate | pear, fruity, aromatic, sweet | nd | nd | nd | 3194.82 ± 428.94 | - |
| 28 | cis-3-Hexen-1-ol | grass | 305.62 ± 64.93 c | 502.56 ± 122.01 b | 817.54 ± 334.69 a | nd | <0.001 |
| 29 | 2-Nonanone | hot milk, soap, green | nd | nd | 10,467.27 ± 699.69 | nd | - |
| 30 | 2-Ethylhexyl acetate | fruit | 728.53 ± 181.76 c | 416.17 ± 55.88 d | 1930.10 ± 282.89 b | 2388.98 ± 137.31 a | <0.001 |
| 31 | Nonanal | fat, citrus, green | 764.73 ± 181.80 c | 738.00 ± 172.49 c | 1474.86 ± 580.12 b | 4568.94 ± 746.25 a | <0.001 |
| 32 | trans-3-Octen-2-one | nut, crushed bug | nd | nd | 328.35 ± 62.61 b | 1669.13 ± 116.17 a | <0.001 |
| 33 | 1,2,4,5-Tetramethylbenzene | rancid, sweet | 22.02 ± 4.96 ab | 25.94 ± 6.71 a | 22.66 ± 2.71 ab | 19.02 ± 4.51 b | 0.015 |
| 34 | trans-2-Octenal | green, nut, fat | nd | nd | 252.97 ± 49.18 b | 2206.44 ± 443.62 a | <0.001 |
| 35 | (Z)-Linalool oxide | flower | nd | nd | 1957.02 ± 857.52 | nd | - |
| 36 | Acetic acid | sour | 54,966.66 ± 5114.72 | 51,194.04 ± 7442.51 | 54,924.78 ± 9474.24 | 69,476.59 ± 18,431.33 | 0.392 |
| 37 | 1-Octen-3-ol | mushroom | 4862.46 ± 395.34 b | nd | 2952.10 ± 718.85 b | 24,036.53 ± 1988.79 a | <0.001 |
| 38 | 1-Heptanol | chemical, green | 1653.27 ± 477.39 c | 4495.62 ± 618.97 ab | 3834.73 ± 785.54 b | 5096.65 ± 746.87 a | <0.001 |
| 39 | Octyl acetate | coconut, vegetable oil, aromatic | 896.32 ± 188.04 c | 3779.71 ± 1143.82 a | 4064.55 ± 423.03 a | 2071.07 ± 328.49 b | <0.001 |
| 40 | 2-Ethylhexanol | rose, green | 1001.08 ± 135.45 b | nd | nd | 1559.82 ± 21.17 a | <0.001 |
| 41 | Benzaldehyde | almond, burnt sugar | 455.72 ± 91.87 b | 638.17 ± 97.94 a | 461.83 ± 86.99 b | 387.99 ± 79.60 b | <0.001 |
| 42 | (E)-2-Nonenal | cucumber, fat, green | nd | nd | 21,343.19 ± 1255.49 | nd | - |
| 43 | 1-Octanol | chemical, metal, burnt | 2328.84 ± 945.05 c | 5081.59 ± 831.94 b | 5815.75 ± 1007.00 b | 8053.98 ± 1307.92 a | <0.001 |
| 44 | Nonyl acetate | sweet, fruit | 1349.61 ± 145.91 | nd | nd | nd | - |
| 45 | Terpinen-4-ol | turpentine, nutmeg, must | nd | nd | 9789.53 ± 1989.03 | nd | - |
| 46 | 2-Undecanone | orange, fresh, green | nd | nd | 15,376.01 ± 2169.577 b | 31,326.94 ± 4791.55 a | 0.039 |
| 47 | Phenylacetaldehyde | hawthorn, honey, sweet | nd | 198.17 ± 3.99 | nd | nd | - |
| 48 | (Z)-2-Decenal | tallow | 7277.38 ± 819.50 a | 6566.06 ± 285.38 b | nd | nd | 0.010 |
| 49 | 1-Nonanol | fat, green | 7750.55 ± 1210.23 bc | 12,293.87 ± 1826.74 a | 7515.57 ± 1013.92 c | 9343.18 ± 1700.22 b | <0.001 |
| 50 | (Z)-3-Hexenyl hexanoate | fruit, prune | 11,557.08 ± 65.46 b | 10,511.71 ± 1026.11 b | 16,287.84 ± 2349.83 a | nd | <0.001 |
| 51 | Isoamyl octanoate | fruity, orange, pear, melon | 4657.59 ± 770.07 | 4804.05 ± 1049.69 | 4285.41 ± 1689.11 | 5112.82 ± 830.36 | 0.375 |
| 52 | Decyl acetate | orange, oil | 7370.33 ± 2229.59 c | 9875.28 ± 1892.89 b | 12,007.74 ± 554.22 a | nd | <0.001 |
| 53 | Neral | lemon | 9459.33 ± 1310.74 a | 7782.53 ± 2466.56 b | 6933.11 ± 1442.44 b | nd | 0.007 |
| 54 | Linalyl butyrate | pear, sweet | 1455.47 ± 563.34 b | 4044.05 ± 859.18 a | nd | nd | <0.001 |
| 55 | (E,E)-2,4-nonadienal | fat, wax, green | nd | nd | 2912.11 ± 945.50 | nd | - |
| 56 | gamma-Caprolactone | coumarin, sweet | 8912.09 ± 1932.89 b | nd | 20,724.54 ± 6754.67 a | 23,929.92 ± 6131.51 a | <0.001 |
| 57 | 3-Methyl-2,4-nonanedione | straw, fruit | 5277.06 ± 174.49 c | 7107.94 ± 644.46 c | 40,609.36 ± 4491.47 a | 23,809.75 ± 7967.48 b | <0.001 |
| 58 | Neodihydrocarveol | - | nd | 4031.13 ± 623.33 | nd | nd | - |
| 59 | Neryl acetate | fruit | nd | 7017.12 ± 1107.90 | nd | nd | - |
| 60 | Geranial | lemon, mint | 4214.65 ± 643.91 b | 6370.90 ± 1584.24 a | 4342.29 ± 204.12 b | nd | <0.001 |
| 61 | Naphthalene | tar | 47.75 ± 5.85 | nd | nd | nd | - |
| 62 | trans-2-Undecenal | soap, fat, green | 2604.32 ± 230.76 c | 17,725.50 ± 2194.52 a | 9059.91 ± 2283.96 b | 9994.97 ± 944.88 b | <0.001 |
| 63 | Methyl 2-nonynoate | green, floral, violet | nd | nd | 5886.43 ± 970.74 | 6028.42 ± 493.02 | 0.659 |
| 64 | 1-Decanol | fat | 4595.91 ± 296.38 b | nd | 22,501.71 ± 8511.43 a | 27,981.03 ± 7502.72 a | <0.001 |
| 65 | Citronellol | rose | 5806.27 ± 894.07 c | 10,203.10 ± 3212.73 a | 7767.29 ± 2214.03 b | 8400.31 ± 1294.11 b | <0.001 |
| 66 | gamma-Heptalactone | nut, fat, fruit | 6497.89 ± 2079.67 c | 12,191.69 ± 5505.41 b | 19,015.56 ± 5830.05 a | 15,490.80 ± 3568.64 ab | <0.001 |
| 67 | Geranyl isobutyrate | floral | nd | 126.18 ± 19.36 | nd | nd | - |
| 68 | cis-Geranylacetone | - | nd | nd | 138.64 ± 25.82 a | 96.15 ± 14.28 b | <0.001 |
| 69 | Capronic acid | sweat | 32.12 ± 9.69 b | 41.15 ± 11.41 b | 44.37 ± 12.10 b | 69.40 ± 17.83 a | <0.001 |
| 70 | trans-p-Methane-8-thiol-3-one | - | nd | nd | nd | 62.02 ± 7.99 | - |
| 71 | alpha-Ionone | wood, violet | nd | 5.86 ± 0.61 | nd | nd | - |
| 72 | Neryl butyrate | - | nd | 205.35 ± 66.16 | nd | nd | - |
| 73 | 1-Undecanol | mandarin | 116.97 ± 40.46 b | 181.21 ± 27.70 a | 169.20 ± 5.39 a | nd | <0.001 |
| 74 | cis-p-Methane-8-thiol-3-one | - | 20.03 ± 0.67 b | nd | 42.97 ± 5.42 a | 54.05 ± 4.01 a | <0.001 |
| 75 | 12-Methyltridecanal | cooked meat, tallow, fat, meat broth, sweat | 40.44 ± 4.52 | nd | nd | nd | - |
| 76 | (E)-Whiskey lactone | flower, lactone | 17.34 ± 8.42 c | 51.82 ± 18.26 b | 57.20 ± 14.29 b | 70.38 ± 8.32 a | <0.001 |
| 77 | gamma-Octalactone | coconut | nd | nd | 1480.07 ± 153.22 a | 998.90 ± 439.13 b | 0.002 |
| 78 | Butylated hydroxytoluene | musty | 7.13 ± 0.96 | nd | nd | nd | - |
| 79 | Tetradecanal | flower, wax | 58.20 ± 14.98 | nd | nd | nd | - |
| 80 | beta-Ionone | seaweed, violet, flower, raspberry | nd | nd | 73.80 ± 10.41 b | 84.57 ± 13.67 a | 0.047 |
| 81 | Maltol | caramel | 464.57 ± 125.92 | nd | nd | nd | - |
| 82 | Benzothiazole | gasoline, rubber | 10.73 ± 2.10 b | 13.91 ± 1.93 a | 14.19 ± 2.92 a | 14.26 ± 4.07 a | 0.011 |
| 83 | (E)-2-Hexenoic acid | must, fat | 377.65 ± 50.00 | nd | nd | nd | - |
| 84 | 1-Dodecanol | fat, wax | 25.46 ± 3.32 b | 97.55 ± 32.45 a | nd | nd | <0.001 |
| 85 | Caryophyllene oxide | herbal, sweet, spice | nd | nd | nd | 1204.91 ± 117.96 | - |
| 86 | (Z)-Nerolidol | wax | 91.41 ± 42.53 | 124.94 ± 39.11 | 98.31 ± 34.53 | 103.19 ± 37.60 | 0.186 |
| 87 | (E)-2-dodecen-1-ol | oil | nd | nd | 112.46 ± 28.36 b | 157.11 ± 35.07 a | 0.003 |
| 88 | 4-Methoxybenzaldehyde | mint, sweet | 7.03 ± 0.99 b | nd | 22.67 ± 5.98 a | 22.71 ± 4.27 a | <0.001 |
| 89 | gamma-Nonalactone | coconut, peach | 5.21 ± 1.56 c | 44.24 ± 16.03 b | 118.76 ± 37.66 a | 140.20 ± 22.12 a | <0.001 |
| 90 | Pentadecanal | fresh | nd | nd | 72.28 ± 6.84 b | 87.62 ± 12.03 a | 0.001 |
| 91 | Caprylic acid | sweat, cheese | nd | 7.96 ± 2.00 | 8.16 ± 0.89 | nd | 0.768 |
| 92 | Hexadecanal | cardboard | 17.20 ± 2.94 b | 19.94 ± 4.97 b | 39.50 ± 9.46 a | 45.37 ± 16.32 a | <0.001 |
| 93 | gamma-Decalactone | peach, fat | nd | nd | 53.33 ± 8.33 | 61.40 ± 11.63 | 0.072 |
| 94 | Nonanoic acid | green, fat | 31.13 ± 1.73 a | 15.01 ± 2.84 b | 14.46 ± 1.70 b | 11.08 ± 0.88 c | <0.001 |
| 95 | 1-Tetradecanol | coconut | 14.83 ± 2.50 | nd | nd | nd | - |
| 96 | Massoia lactone | peach | nd | nd | 9.15 ± 4.05 | 11.16 ± 4.32 | 0.264 |
| 97 | Capric acid | fat | nd | 13.54 ± 3.14 b | 11.48 ± 4.21 b | 19.30 ± 3.56 a | <0.001 |
| 98 | 1-Hexadecanol | wax, flower | 8.70 ± 1.53 a | nd | 5.21 ± 1.12 b | nd | <0.001 |
| NO. | Compound | OT * (ng/g) | OAV | |||
|---|---|---|---|---|---|---|
| DX-Se | DX | JF-Se | JF | |||
| 1 | Butyl acetate | 58 | 31.21 | 36.84 | 25.64 | 27.51 |
| 2 | Hexanal | 5 | 244.20 | 218.40 | nd | 689.27 |
| 4 | p-Xylene | 490 | 0.25 | 0.29 | 0.20 | 0.21 |
| 6 | 1-Butanol | 459.2 | 1.58 | 1.09 | 0.81 | 0.65 |
| 14 | Isoamyl alcohol | 8100 | 0.11 | 0.21 | 0.06 | 0.06 |
| 17 | 1-Pentanol | 1 | 1366.88 | 2040.63 | 722.07 | 2192.49 |
| 22 | Octanal | 0.7 | 24,382.24 | nd | nd | 9328.27 |
| 25 | 1-Hexanol | 1 | 2195.81 | 2862.71 | 3429.51 | 3628.86 |
| 28 | cis-3-Hexen-1-ol | 200 | 1.53 | 2.51 | 4.09 | nd |
| 29 | 2-Nonanone | 38.9 | nd | nd | 269.08 | nd |
| 31 | Nonanal | 1.1 | 695.21 | 670.91 | 1340.78 | 4153.58 |
| 37 | 1-Octen-3-ol | 1 | 4862.46 | nd | 2952.10 | 24,036.53 |
| 38 | 1-Heptanol | 1 | 1653.27 | 4495.62 | 3834.73 | 5096.65 |
| 41 | Benzaldehyde | 350 | 1.30 | 1.82 | 1.32 | 1.11 |
| 43 | 1-Octanol | 1 | 2328.84 | 5081.59 | 5815.75 | 8053.98 |
| 47 | Phenylacetaldehyde | 4 | nd | 49.54 | nd | nd |
| 49 | 1-Nonanol | 45.5 | 170.34 | 270.19 | 165.18 | 205.34 |
| 60 | Geranial | 32 | 131.71 | 199.09 | 135.70 | nd |
| 80 | beta-Ionone | 6 | nd | nd | 12.30 | 14.10 |
| 82 | Benzothiazole | 8 | 1.34 | 1.74 | 1.77 | 1.78 |
| 84 | 1-Dodecanol | 82 | 0.31 | 1.19 | nd | nd |
| Fatty Acids | DX-Se | DX | JF-Se | JF | p-Value |
|---|---|---|---|---|---|
| C4:0 | 0.31 ± 0.04 b | 0.27 ± 0.06 b | 0.34 ± 0.19 b | 1.18 ± 0.62 a | 0.048 |
| C6:0 | 1.30 ± 0.76 a | 1.41 ± 1.23 a | 1.37 ± 0.83 a | 1.78 ± 0.88 a | 0.650 |
| C8:0 | 0.76 ± 0.43 a | 0.98 ± 0.87 a | 0.93 ± 0.54 a | 1.28 ± 0.48 a | 0.485 |
| C10:0 | 0.54 ± 0.47 a | 0.87 ± 0.80 a | 0.41 ± 0.25 a | 1.10 ± 0.54 a | 0.287 |
| C12:0 | 0.61 ± 0.19 a | 1.38 ± 0.35 a | 0.72 ± 0.18 a | 1.13 ± 0.60 a | 0.138 |
| C13:0 | 0.62 ± 0.23 a | 0.81 ± 0.50 a | 0.59 ± 0.25 a | 1.31 ± 0.86 a | 0.072 |
| C14:0 | 0.56 ± 0.12 a | 0.59 ± 0.16 a | 0.85 ± 0.59 a | 0.51 ± 0.16 a | 0.060 |
| C15:0 | 1.31 ± 0.56 a | 1.96 ± 0.65 a | 1.34 ± 0.58 a | 2.05 ± 1.20 a | 0.387 |
| C16:0 | 22.41 ± 2.26 a | 22.89 ± 2.55 a | 24.42 ± 1.21 a | 24.26 ± 3.34 a | 0.331 |
| C17:0 | 0.32 ± 0.20 a | 0.33 ± 0.21 a | 0.35 ± 0.26 a | 0.44 ± 0.25 a | 0.703 |
| C18:0 | 8.60 ± 1.30 a | 9.22 ± 1.56 a | 9.03 ± 3.63 a | 9.60 ± 1.03 a | 0.793 |
| C20:0 | 0.15 ± 0.08 a | 0.17 ± 0.11 a | 0.16 ± 0.10 a | 0.29 ± 0.22 a | 0.116 |
| C21:0 | 0.20 ± 0.07 a | 0.16 ± 0.05 a | 0.16 ± 0.10 a | 0.29 ± 0.22 a | 0.129 |
| Σ SFA | 37.69 | 41.04 | 40.67 | 45.22 | |
| C14:1 | 1.04 ± 0.03 b | 1.76 ± 0.88 ab | 1.04 ± 0.55 b | 2.27 ± 1.09 a | 0.011 |
| C15:1 | 0.14 ± 0.03 a | 0.28 ± 0.12 a | 0.21 ± 0.15 a | 0.39 ± 0.11 a | 0.203 |
| C16:1 | 3.56 ± 0.79 a | 3.13 ± 0.77 a | 3.14 ± 0.55 a | 3.00 ± 0.73 a | 0.290 |
| C17:1 | 0.30 ± 0.07 ab | 0.16 ± 0.08 b | 0.13 ± 0.08 b | 0.44 ± 0.31 a | 0.043 |
| C18:1 | 39.58 ± 4.44 a | 37.55 ± 3.99 a | 39.25 ± 3.06 a | 33.70 ± 5.59 a | 0.369 |
| C20:1 | 0.28 ± 0.11 a | 0.26 ± 0.06 a | 0.28 ± 0.09 a | 0.33 ± 0.06 a | 0.276 |
| C22:1 | 0.15 ± 0.02 a | 0.14 ± 0.03 a | 0.14 ± 0.02 a | 0.11 ± 0.04 a | 0.235 |
| C24:1 | 0.13 ± 0.03 b | 0.16 ± 0.04 b | 0.14 ± 0.05 b | 0.29 ± 0.17 a | 0.049 |
| Σ MUFA | 45.18 | 42.90 | 44.33 | 40.53 | |
| C18:2 | 15.53 ± 3.17 a | 14.42 ± 1.56 a | 13.28 ± 4.14 a | 12.16 ± 1.69 a | 0.621 |
| C20:2 | 0.12 ± 0.04 a | 0.13 ± 0.03 a | 0.17 ± 0.04 a | 0.13 ± 0.05 a | 0.146 |
| C22:2 | 0.20 ± 0.10 b | 0.20 ± 0.07 b | 0.18 ± 0.11 b | 0.35 ± 0.14 a | 0.028 |
| C18:3 | 0.49 ± 0.15 a | 0.53 ± 0.13 a | 0.51 ± 0.25 a | 0.57 ± 0.16 a | 0.747 |
| C20:4 | 0.56 ± 0.37 b | 0.63 ± 0.32 b | 0.67 ± 0.35 b | 0.81 ± 0.43 b | 0.501 |
| C22:6 | 0.23 ± 0.11 b | 0.21 ± 0.18 b | 0.19 ± 0.14 b | 0.45 ± 0.29 a | 0.035 |
| Σ PUFA | 17.13 | 16.05 | 14.99 | 14.25 |
| Amino Acids (g/100 g) | DX-Se | DX | JF-Se | JF | p-Value | |
|---|---|---|---|---|---|---|
| Bitter amino acids | His | 0.85 ± 0.08 a | 0.88 ± 0.20 a | 0.83 ± 0.08 a | 0.75 ± 0.10 a | 0.126 |
| Arg | 1.42 ± 0.11 a | 1.40 ± 0.08 a | 1.49 ± 0.13 a | 1.39 ± 0.13 a | 0.218 | |
| Tyr | 0.78 ± 0.06 a | 0.78 ± 0.05 a | 0.82 ± 0.07 a | 0.77 ± 0.07 a | 0.175 | |
| Val | 1.19 ± 0.08 a | 1.15 ± 0.09 a | 1.22 ± 0.11 a | 1.13 ± 0.10 a | 0.242 | |
| Lys | 1.94 ± 0.15 a | 1.93 ± 0.13 a | 2.02 ± 0.16 a | 1.90 ± 0.15 a | 0.271 | |
| Ile | 1.13 ± 0.09 a | 1.09 ± 0.07 a | 1.17 ± 0.11 a | 1.09 ± 0.10 a | 0.188 | |
| Leu | 1.78 ± 0.14 a | 1.75 ± 0.11 a | 1.85 ± 0.16 a | 1.73 ± 0.15 a | 0.260 | |
| Phe | 1.12 ± 0.07 ab | 1.10 ± 0.08 ab | 1.16 ± 0.10 a | 1.05 ± 0.09 b | 0.049 | |
| Sweet amino acids | Ser | 0.76 ± 0.06 ab | 0.78 ± 0.06 ab | 0.79 ± 0.07 a | 0.72 ± 0.06 b | 0.044 |
| Gly | 0.96 ± 0.06 a | 0.95 ± 0.07 a | 1.00 ± 0.08 a | 0.94 ± 0.09 a | 0.302 | |
| Thr | 0.94 ± 0.07 a | 0.95 ± 0.06 a | 0.98 ± 0.08 a | 0.90 ± 0.07 a | 0.162 | |
| Ala | 1.34 ± 0.10 a | 1.32 ± 0.10 a | 1.40 ± 0.13 a | 1.30 ± 0.12 a | 0.221 | |
| Pro | 0.80 ± 0.09 a | 0.76 ± 0.06 a | 0.79 ± 0.08 a | 0.75 ± 0.08 a | 0.437 | |
| Umami amino acids | Glu | 3.23 ± 0.28 a | 3.21 ± 0.21 a | 3.39 ± 0.29 a | 3.20 ± 0.28 a | 0.349 |
| Asp | 2.01 ± 0.15 a | 1.99 ± 0.15 a | 2.09 ± 0.17 a | 1.95 ± 0.16 a | 0.267 | |
| Sulfur-containing amino acids | Met | 0.69 ± 0.08 a | 0.66 ± 0.04 a | 0.70 ± 0.08 a | 0.65 ± 0.07 a | 0.325 |
| EAA (g/100 g) | 8.79 | 8.63 | 9.1 | 8.44 | ||
| TAA (g/100 g) | 20.94 | 20.7 | 21.7 | 20.21 | ||
| EAA/TAA/% | 41.98 | 41.69 | 41.94 | 41.76 | ||
| EAA/NEAA/% | 72.35 | 71.50 | 72.22 | 71.71 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, D.; Xie, M.; Li, D.; Guang, Y.; Zhou, Y. Metabolic Pathway Analysis in Chicken Induced by Selenium-Enriched Yeast: Insights from Flavoromics and Metabolomics. Foods 2025, 14, 4060. https://doi.org/10.3390/foods14234060
Fei D, Xie M, Li D, Guang Y, Zhou Y. Metabolic Pathway Analysis in Chicken Induced by Selenium-Enriched Yeast: Insights from Flavoromics and Metabolomics. Foods. 2025; 14(23):4060. https://doi.org/10.3390/foods14234060
Chicago/Turabian StyleFei, Dan, Min Xie, Daojie Li, Yelan Guang, and Yaomin Zhou. 2025. "Metabolic Pathway Analysis in Chicken Induced by Selenium-Enriched Yeast: Insights from Flavoromics and Metabolomics" Foods 14, no. 23: 4060. https://doi.org/10.3390/foods14234060
APA StyleFei, D., Xie, M., Li, D., Guang, Y., & Zhou, Y. (2025). Metabolic Pathway Analysis in Chicken Induced by Selenium-Enriched Yeast: Insights from Flavoromics and Metabolomics. Foods, 14(23), 4060. https://doi.org/10.3390/foods14234060





