Green Extraction of Lotus Leaf (Nelumbo nucifera Gaertn) Polyphenols: Unraveling the Mechanism of Ultrasound-Assisted Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Deep Eutectic Solvents
2.3. Determination of Physical Properties of Deep Eutectic Solvents
2.4. Determination of Total Phenolic Content and Antioxidant Capacity
2.5. Extraction Procedure
2.6. Optimization of Extraction Process
2.7. Fourier Transform Infrared Spectrometer
2.8. Scanning Electron Microscope
2.9. Statistical Analysis
3. Results and Discussion
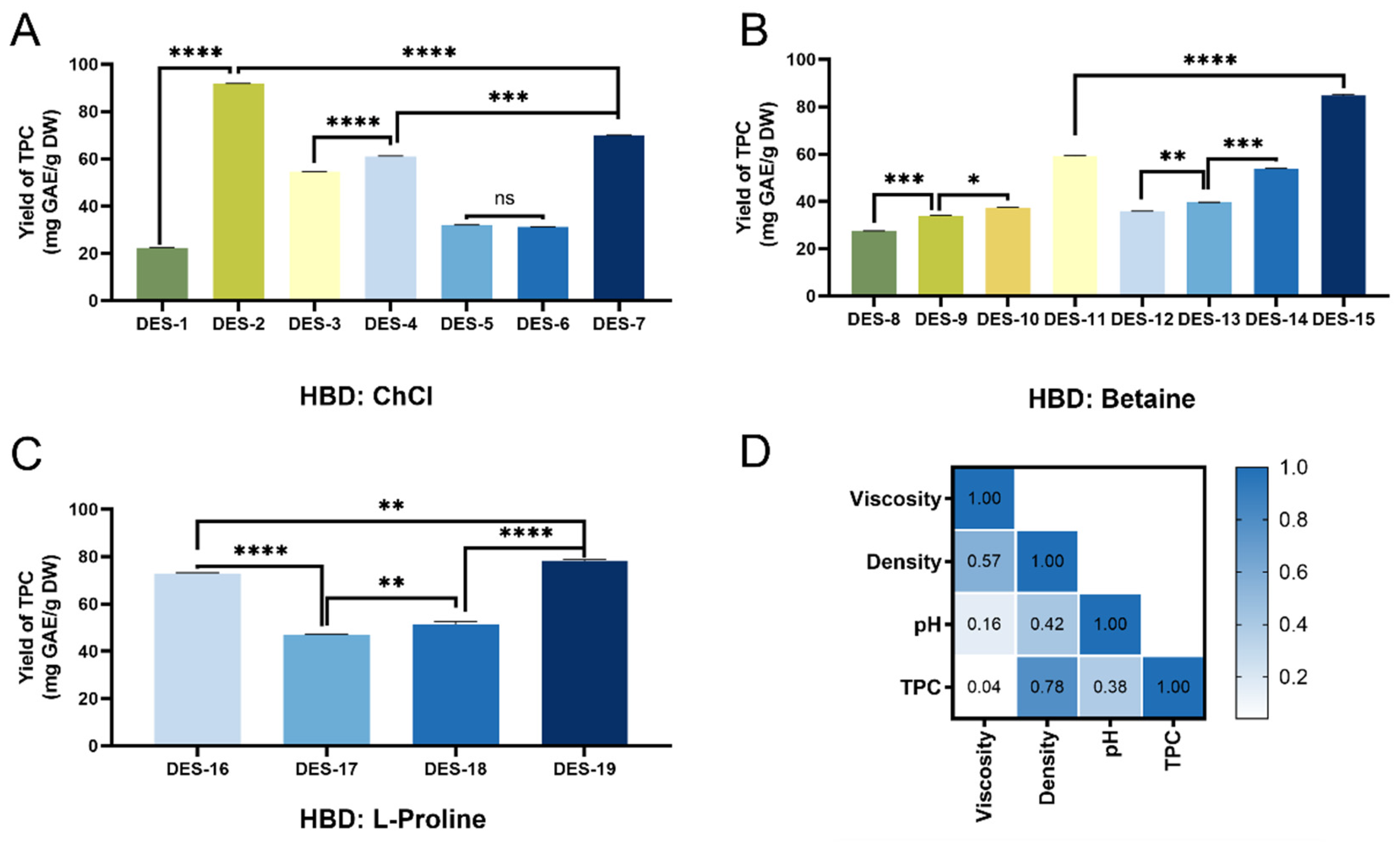
3.1. Characterization of Different DESs
3.2. Determination of the Optimal DES for Polyphenol Extraction
3.2.1. Screening of DESs for Enhanced Polyphenol Extraction
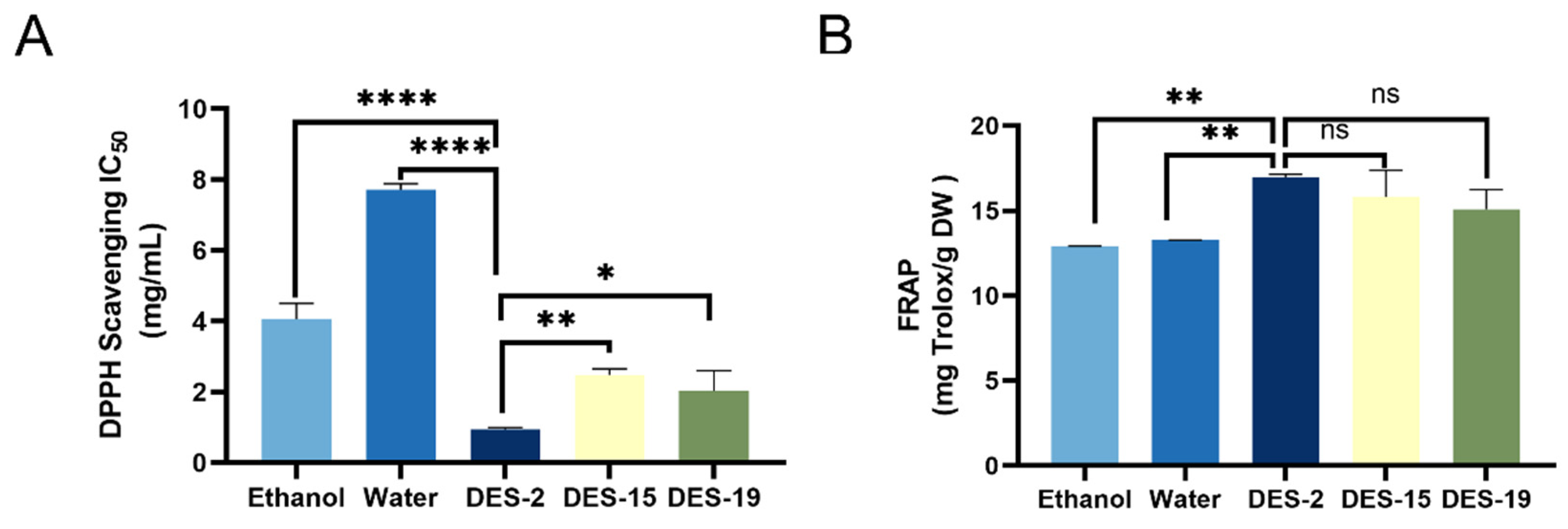
3.2.2. Comparison of the Effects of Different Extraction Methods on Antioxidant Activity
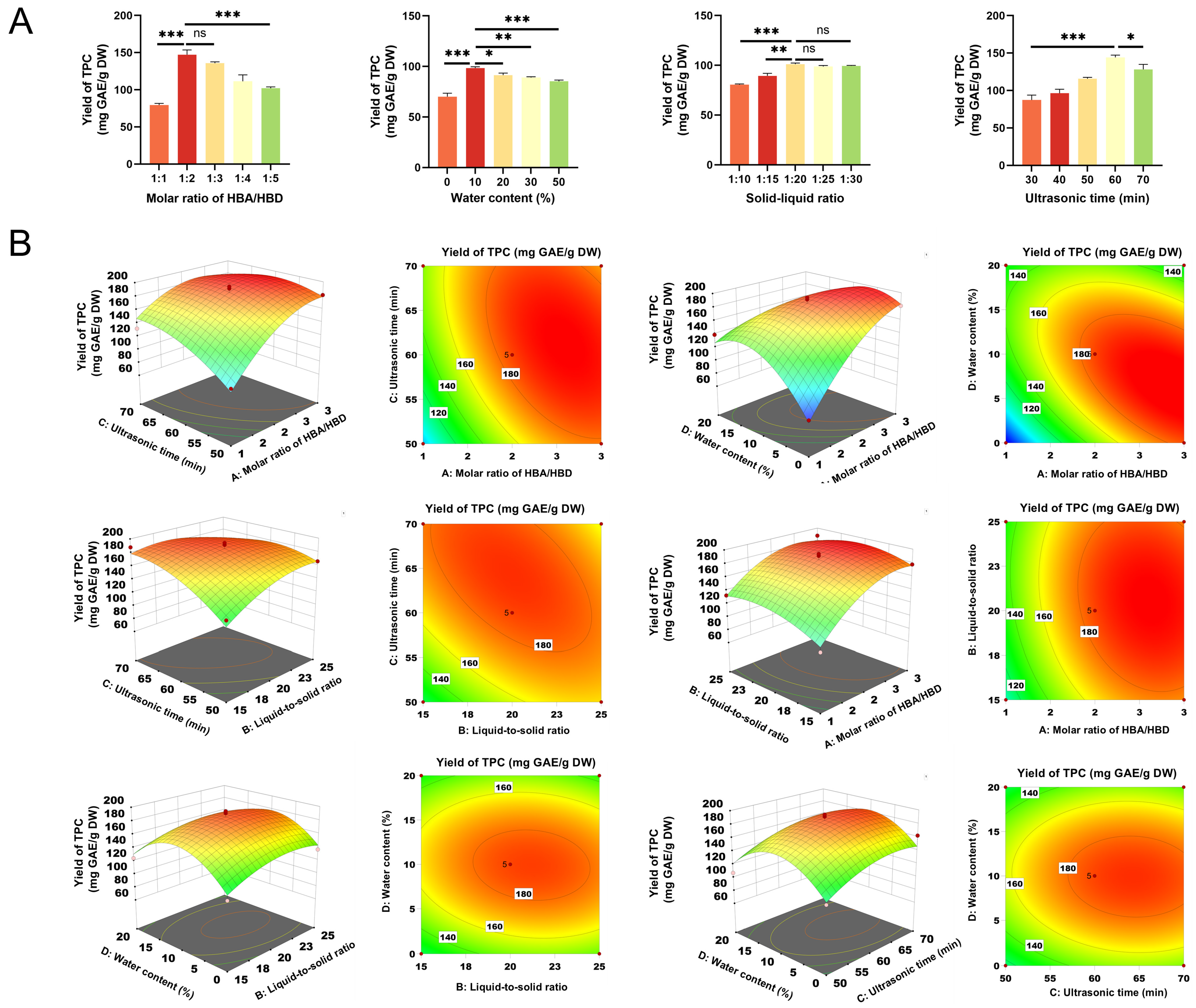
3.3. Optimization of DES Extraction Conditions
3.3.1. Single-Factor Test
3.3.2. BBD Experimental Design
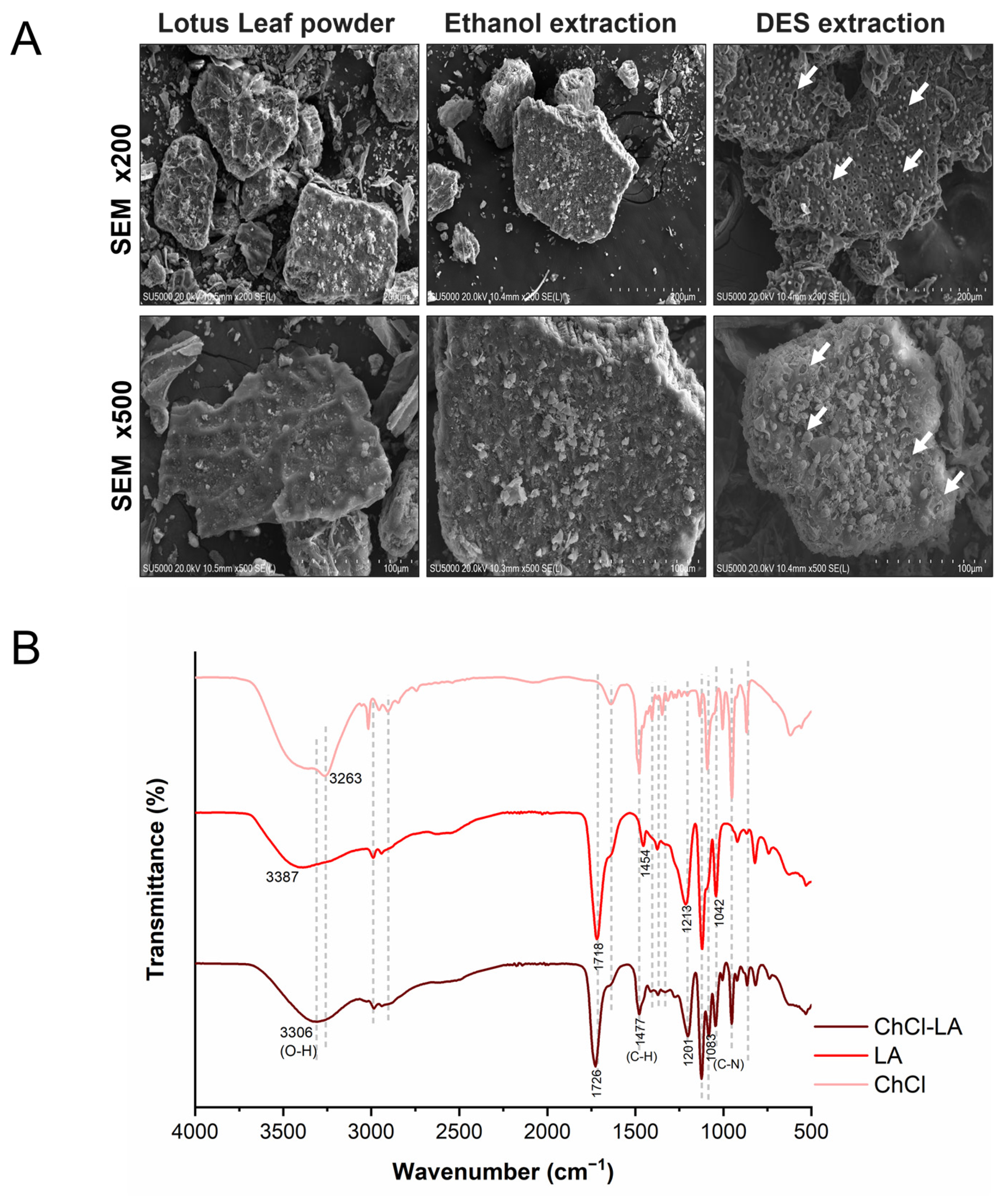
3.4. Chemical Interactions and Morphological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 4867–4900. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, N.; Liu, C.; Xue, J.; Chu, J.; Yao, X. Qualities and antioxidant activities of lotus leaf affected by different drying methods. Acta Physiol. Plant. 2020, 42, 14. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Liu, Z.; Huang, Z.; Xu, C.; Liu, Y.; Haran, Y.; Nisar, W.; Yan, S.; Li, J. Ultrasound-assisted extraction of polyphenols from lotus rhizome epidermis by alcohol/salt-based aqueous two-phase system: Optimization, extraction mechanism and antioxidant activities. Food Chem. 2024, 453, 139620. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guan, Y.; Song, X.; He, J.; Xie, Z.; Zhang, Y.; Zhang, H.; Tang, D. Polyphenols extract from lotus seedpod (Nelumbo nucifera Gaertn.): Phenolic compositions, antioxidant, and antiproliferative activities. Food Sci. Nutr. 2019, 7, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qiu, L.; Deng, H.; Wang, J.; Yao, L.; Deng, L. Ultrahigh surface area carbon nanosheets derived from lotus leaf with super capacities for capacitive deionization and dye adsorption. Appl. Surf. Sci. 2020, 524, 146485. [Google Scholar] [CrossRef]
- Atiya, A.; Majrashi, T.A.; Begum, M.Y.; Abdul Qadir, S.F.; Alqahtani, A.S.; Ali Alosman, A.S.; Alahmari, A.A.; Mesfer Al Aldabsh, A.N.; Alshahrani, A.T.; Alshahrani, R.R.M. Influence of solvent selection and extraction methods on the determination of polyphenols, antioxidant, lipoxygenase and tyrosinase inhibition activities of Opuntia ficus-indica fruits peel and pulp collected from the Kingdom of Saudi Arabia (KSA). Nat. Prod. Res. 2023, 37, 514–521. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Hanula, M.; Pogorzelski, G. Extraction of polyphenols and essential oils from herbs with green extraction methods—An insightful review. Food Chem. 2024, 460, 140456. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Yenduri, S.; Venkatesh, K.G.; Naga Prashant, K. Recent Advances in Extraction of Polyphenols by Advanced Extraction Methods. Phytochem. Anal. 2025, 36, 1875–1892. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Ultrasound-Assisted Extraction of Polyphenols from Maritime Pine Residues with Deep Eutectic Solvents. Foods 2022, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, Y.; Zhang, F.; Lin, S.; Zou, P.; Farag, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J. UPLC-ESI-MS/MS-based chemometric approach for investigating the effect of conventional versus modern extraction methods on polyphenols recovery from grape seed wastes. Food Chem. 2025, 487, 144619. [Google Scholar] [CrossRef] [PubMed]
- Souadia, A.; Djemoui, A.; Souli, L.; Haiouani, K.; Atoki, A.V.; Djemoui, D.; Messaoudi, M.; Hegazy, S.; Alsaeedi, H.; Barhoum, A. Ultrasound-assisted extraction for maximizing total phenolics, flavonoid content, and antioxidant activity in Thymus algeriensis: Box-Behnken experimental design. Biomass Convers. Biorefinery 2025, 15, 12979–12994. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Cheng, Q.; Wang, L.; Zhang, L. Deep Eutectic Solvent-Based Ultrahigh Pressure Extraction of Baicalin from Scutellaria baicalensis Georgi. Molecules 2018, 23, 3233. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New deep eutectic solvent assisted extraction of highly pure lignin from maritime pine sawdust (Pinus pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Ozbek Yazici, S.; Ozmen, İ. Ultrasound assisted extraction of phenolic compounds from Capparis Ovata var canescens fruit using deep eutectic solvents. J. Food Process. Preserv. 2022, 46, e16286. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, Z.; Li, L.; Zhang, L.; Zhao, M.; Yi, H.; Wang, Z.; Li, G.; Wang, Z.; Li, M.; et al. Green Extraction of Phenolic Compounds from Lotus (Nelumbo nucifera Gaertn) Leaf Using Deep Eutectic Solvents: Process Optimization and Antioxidant Activity. Separations 2023, 10, 272. [Google Scholar] [CrossRef]
- Benítez-Correa, E.; Bastías-Montes, J.M.; Acuña-Nelson, S.; Muñoz-Fariña, O. Effect of choline chloride-based deep eutectic solvents on polyphenols extraction from cocoa (Theobroma cacao L.) bean shells and antioxidant activity of extracts. Curr. Res. Food Sci. 2023, 7, 100614. [Google Scholar] [CrossRef]
- Nam, Y.H.; Ahn, S.M.; Seo, G.J.; Kim, N.W.; Shin, S.W.; Nuankaew, W.; Murughanantham, N.; Pandian, S.; Hwang, J.S.; Hong, B.N.; et al. Optimization of NADES-based green extraction of ellagitannins from rambutan peel with enhanced antioxidant activity. Food Chem. 2025, 475, 143308. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of deep eutectic solvents in the extraction of polyphenolic antioxidants from New Zealand Manuka leaves (Leptospermum Scoparium): Optimization and antioxidant activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, M.; Cheng, X.; Liu, X.; Yang, H.; Ma, W.; Guo, M.; Li, L. Deep eutectic solvent: Synthesis, classification, properties and application in macromolecular substances. Int. J. Biol. Macromol. 2024, 278, 134593. [Google Scholar] [CrossRef]
- Boateng, I.D. Evaluating the status quo of deep eutectic solvent in food chemistry. Potentials and limitations. Food Chem. 2023, 406, 135079. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Dey, S.; Chakraborty, R. Effect of choline chloride-oxalic acid based deep eutectic solvent on the ultrasonic assisted extraction of polyphenols from Aegle marmelos. J. Mol. Liq. 2019, 287, 110956. [Google Scholar] [CrossRef]
- Soukaina, K.; Safa, Z.; Soukaina, H.; Hicham, C.; Bouchra, C. Choline chloride-based deep eutectic solvents (NADES): Potential use as green extraction media for polyphenols from Mentha pulegium, antioxidant activity, and antifungal activity. Microchem. J. 2024, 199, 110174. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules extraction from coffee and cocoa by- and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef]
- Yu, P.; Li, Q.; Feng, Y.; Ma, S.; Chen, Y.; Li, G. Extraction and Analysis of Six Effective Components in Glycyrrhiza uralensis Fisch by Deep Eutectic Solvents (DES) Combined with Quantitative Analysis of Multi-Components by Single Marker (QAMS) Method. Molecules 2021, 26, 1310. [Google Scholar] [CrossRef]
- Tomas, M.; Wen, Y.; Liao, W.; Zhang, L.; Zhao, C.; McClements, D.J.; Nemli, E.; Bener, M.; Apak, R.; Capanoglu, E. Recent progress in promoting the bioavailability of polyphenols in plant-based foods. Crit. Rev. Food Sci. Nutr. 2025, 65, 2343–2364. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Shi, L.; Cheng, Y.; Hu, H.; Zeng, B.; Zhao, F. Water based-deep eutectic solvent for ultrasound-assisted liquid–liquid microextraction of parabens in edible oil. Food Chem. 2022, 383, 132586. [Google Scholar] [CrossRef]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Schmitt-Kopplin, P.; Malik, A.K. Green and efficient extraction of phenolic compounds from Neem leaves using deep eutectic solvents based ultrasonic-assisted extraction. Food Chem. 2024, 451, 139500. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-T.; Zhou, S.-X.; Sun, Z.-P.; Cao, M.-Y.; Zhou, T.; Zhao, L.-Y.; Chen, G.-T. Deep eutectic solvent-based ultrasonic-assisted extraction of polyphenol from Chenopodium quinoa Willd.: Optimization and lipid-lowering activity. Food Chem. 2025, 464, 141733. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction and Bioactivities of Flavonoids in Ampelopsis grossedentata Leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef]
- Milani, G.; Vian, M.; Cavalluzzi, M.M.; Franchini, C.; Corbo, F.; Lentini, G.; Chemat, F. Ultrasound and deep eutectic solvents: An efficient combination to tune the mechanism of steviol glycosides extraction. Ultrason. Sonochemistry 2020, 69, 105255. [Google Scholar] [CrossRef]
- Ling, J.K.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Song, X.; Zhang, R.; Xie, T.; Wang, S.; Cao, J. Deep Eutectic Solvent Micro-Functionalized Graphene Assisted Dispersive Micro Solid-Phase Extraction of Pyrethroid Insecticides in Natural Products. Front. Chem. 2019, 7, 594. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, F.; Ren, M.; Chen, X.; Liu, C.; Li, G.; Gao, Q.; Qiao, L.; Jiang, Y.; Zhu, L.; et al. Eco-friendly and efficient extraction of polyphenols from Ligustrum robustum by deep eutectic solvent assisted ultrasound. Food Chem. 2023, 429, 136828. [Google Scholar] [CrossRef]

| Plant Material | DESs Combination | Extraction Conditions | Extraction Yield | Results | Ref. |
|---|---|---|---|---|---|
| Lotus leaves | ChCl–Glycerol (1:2) ChCl–Propylene glycol (1:2) ChCl–Lactic acid (1:2) ChCl–Citric acid (1:2) ChCl–Malic acid (1:2) Bet–Glycerol (1:2) Bet–Propylene glycol (1:2) Bet–Citric acid (1:2) Bet–Malic acid (1:2) Lactic acid–Glycerol (1:2) Lactic acid–Propylene glycol (1:2) Citric acid–Glycerol (1:2) Citric acid–Propylene glycol (1:2) | 50 °C/60 min (140 r/min) | Flavonoids: 126.10 mg/g Polyphenols: 126.10 mg/g | More efficient extraction yields were demonstrated by DES made of lactic acid and glycerol in a 1:2 ratio, 29% of water content, 37:1 mL/g of liquid–solid ratio, 61 min of extraction time, and 53 °C of extraction temperature. | [25] |
| Capparis ovata var canescens fruit | ChCl–Urea (1:2) ChCl–Glycerol (1:3) ChCl–Lactic acid (1:3) | 30 °C/25 min, assisted by ultrasound extraction | Flavonoids: 13.04 mg QE/g DW Polyphenols: 30.55 mg GAE/g DW | The DES with a molar ratio of 1:2 between ChCl and lactic acid performed better for extraction under the ideal circumstances of 20% water, 20 mL/g, 50 °C and 30 min. | [19] |
| Moringa oleifera L. leaves | ChCl–Glycerol (1:1) ChCl–Levulinic acid (1:2) ChCl–Ethylene glycol (1:2) Bet–Levulinic acid (1:2) Bet–Glycerol (1:2) Pro–Levulinic acid (1:2) Pro–Glycerol (2:5) Pro–Lactic acid (1:1) | 40 °C/30 min, assisted by ultrasound extraction | Vicenin-2: 17.6 mg/g Orientin: 23.6 mg/g | The combination of DES L-Proline and glycerol produced the highest total phenolic content. 37% water content, 144 W ultrasonic power, and 40 °C were the ideal extraction conditions. | [20] |
| Mulberry leaves | ChCl–Urea (1:2) ChCl–Glycerol (1:2) ChCl–Citric acid (2:1) Bet–Lactic acid (1:1) Bet–Glycerol (1:2) Pro–Glycerol (2:5) Pro–Lactic acid (1:1) | 40 °C/30 min, assisted by ultrasound extraction | Polyphenols: 22.66 mg/g | The ideal circumstances for obtaining the high level of total phenolic content were ChCl: citric acid (2:1) DES with a 75% water content, 50 mg/mL of solid/liquid ratios, 40 °C and 30 min of extraction time. | [21] |
| Pollen Typhae | ChCl–Glycerol (1:4) ChCl-D–glucose (1:4) ChCl–Lactic acid (1:4) ChCl:1,2–propanediol (1:4) Pro–Glycerol (4:11) | Room temperature/35 min, assisted by ultrasound extraction | Quercetin: 0.383 μg /mg Naringenin: 0.048 μg /mg Kaempferol: 0.391 μg /mg Isorhamnetin: 3.149 μg /mg | ChCl:1,2-propanediol (1:4) produced the best polyphenols production under optimal circumstances: 30% of water content, 50 mg/mL of solid/liquid ratios. | [22] |
| Lycium barbarum L. fruits | ChCl–Glycerol (1:2) ChCl–Urea (1:2) ChCl-p-Toluenesulfonic acid (1:2) | Room temperature/1.5 h, assisted by ultrasound extraction | Myricetin: 57.2 mg/g Morin:12.7 mg/g Rutin:9.1 mg/g | The highest polyphenols production was detected in the ChCl-p-Toluenesulfonic acid (1:2) extracts over a period of 90 min and a solid/liquid ratio of 50 mg/mL. | [23] |
| L. citriodora leaves | ChCl–Lactic acid (1:2) ChCl–Ethylene Glycol (1:2) ChCl–Maltose (3:1) ChCl–Urea (1:2) | Microwaved irradiated at 65 °C for 20 min | Flavonoids: 9.02 mg/g Iridoids: 7.25 mg/g Phenylpropanoids: 17.23 mg/g | The maximum phenol extraction was obtained after 17.08 min of microwave irradiation time, 63.68 °C, 32.19% of water content with a ChCl: lactic acid (1:2) mixture. | [24] |
| Parameters Factor | Symbol | Unit | Low | Medium | High |
|---|---|---|---|---|---|
| Molar ratio of HBA/HBD | A | 1 | 2 | 3 | |
| Liquid–solid ratio | B | 15 | 20 | 25 | |
| Ultrasonic time | C | min | 50 | 60 | 70 |
| Water content | D | % | 0 | 10 | 20 |
| No. | HBA | HBD | Mole Ratio (HBA/HBD) | Water Content (%) | Heating Time (min) | Viscosity/mPa·s | Density/g/cm3 | pH |
|---|---|---|---|---|---|---|---|---|
| DES-1 | Choline chloride | Levulinic acid | 1:2 | 20% | 6 | 219.1 ± 3.56 | 1.13 ± 0.12 | 1.63 ± 0.02 |
| DES-2 | Lactic acid | 1:2 | 3.5 | 167.4 ± 2.04 | 1.14 ± 0.13 | 0.72 ± 0.02 | ||
| DES-3 | D-glucose | 2:1 | 11.5 | 618.2 ± 3.42 | 1.20 ± 0.02 | 3.88 ± 0.01 | ||
| DES-4 | Maltose | 4:1 | 1 | 23.4 ± 1.98 | 1.15 ± 0.04 | 3.71 ± 0.02 | ||
| DES-5 | Glycerol | 1:2 | 2 | 347.7 ± 1.23 | 1.19 ± 0.10 | 4.14 ± 0.04 | ||
| DES-6 | Glycerol | 1:3 | 2 | 357.3 ± 5.78 | 1.20 ± 0.10 | 3.95 ± 0.03 | ||
| DES-7 | Urea | 1:2 | 1.5 | 13.2 ± 0.68 | 1.16 ± 0.12 | 8.51 ± 0.05 | ||
| DES-8 | Betaine | Levulinic acid | 1:2 | 20% | 23 | 1055.1 ± 5.44 | 1.16 ± 0.15 | 4.88 ± 0.03 |
| DES-9 | Lactic acid | 1:2 | 13 | 754.2 ± 4.32 | 1.18 ± 0.05 | 3.72 ± 0.02 | ||
| DES-10 | D-glucose | 2:1 | 40 | 52 ± 9.78 | 1.27 ± 0.13 | 6.94 ± 0.03 | ||
| DES-11 | Maltose | 4:1 | 16 | 95.8 ± 2.07 | 1.19 ± 0.14 | 7.52 ± 0.01 | ||
| DES-12 | Ethylene glycol | 1:2 | 3 | 56 ± 1.22 | 1.12 ± 0.08 | 8.77 ± 0.04 | ||
| DES-13 | Glycerol | 1:2 | 13 | 78.7 ± 1.26 | 1.18 ± 0.03 | 7.94 ± 0.02 | ||
| DES-14 | Glycerol | 1:3 | 13 | 41.3 ± 0.74 | 1.20 ± 0.02 | 6.07 ± 0.02 | ||
| DES-15 | Urea | 1:2 | 2 | 4.8 ± 1.66 | 1.13 ± 0.04 | 9.87 ± 0.03 | ||
| DES-16 | L-Proline | Maltose | 4:1 | 20% | 16 | 61.6 ± 1.56 | 1.25 ± 0.04 | 6.48 ± 0.05 |
| DES-17 | Glycerol | 1:2 | 22 | 744.8 ± 7.87 | 1.29 ± 0.10 | 7.64 ± 0.04 | ||
| DES-18 | Glycerol | 1:3 | 17 | 145.5 ± 4.67 | 1.26 ± 0.12 | 6.55 ± 0.02 | ||
| DES-19 | Urea | 1:2 | 9 | 20.8 ± 5.79 | 1.20 ± 0.16 | 8.07 ± 0.01 |
| Run | BBD Experiments | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C (min) | D (%) | Y (mg GAE/g DW) | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | ||
| 1 | 1 | 15 | 60 | 10 | 100.43 | model | 30,556.63 | 14 | 2182.62 | 16.07 | <0.0001 |
| 2 | 3 | 15 | 60 | 10 | 177.64 | A. Molar ratio of HBA/HBD | 9914.46 | 1 | 9914.46 | 73.01 | <0.0001 |
| 3 | 1 | 25 | 60 | 10 | 132.78 | B. Liquid–solid ratio | 547.56 | 1 | 547.56 | 4.03 | 0.0643 |
| 4 | 3 | 25 | 60 | 10 | 187.80 | C. Ultrasonic time | 2071.94 | 1 | 2071.94 | 15.26 | 0.0016 |
| 5 | 2 | 20 | 50 | 0 | 111.48 | D. Water contents | 2.86 | 1 | 2.86 | 0.0211 | 0.8866 |
| 6 | 2 | 20 | 70 | 0 | 162.37 | AB | 123.03 | 1 | 123.03 | 0.906 | 0.3573 |
| 7 | 2 | 20 | 50 | 20 | 107.31 | AC | 940.28 | 1 | 940.28 | 6.92 | 0.0197 |
| 8 | 2 | 20 | 70 | 20 | 148.10 | AD | 4023.09 | 1 | 4023.09 | 29.63 | <0.0001 |
| 9 | 1 | 20 | 60 | 0 | 66.19 | BC | 1046.35 | 1 | 1046.35 | 7.71 | 0.0149 |
| 10 | 3 | 20 | 60 | 0 | 181.01 | BD | 89.7 | 1 | 89.7 | 0.6606 | 0.43 |
| 11 | 1 | 20 | 60 | 20 | 139.30 | CD | 25.47 | 1 | 25.47 | 0.1875 | 0.6716 |
| 12 | 3 | 20 | 60 | 20 | 127.27 | A2 | 3342.37 | 1 | 3342.37 | 24.61 | 0.0002 |
| 13 | 2 | 15 | 50 | 10 | 128.79 | B2 | 1314.7 | 1 | 1314.7 | 9.68 | 0.0077 |
| 14 | 2 | 25 | 50 | 10 | 166.05 | C2 | 1502.13 | 1 | 1502.13 | 11.06 | 0.005 |
| 15 | 2 | 15 | 70 | 10 | 187.80 | D2 | 9899.97 | 1 | 9899.97 | 72.9 | <0.0001 |
| 16 | 2 | 25 | 70 | 10 | 160.37 | Residual | 1901.11 | 14 | 135.79 | ||
| 17 | 1 | 20 | 50 | 10 | 95.16 | Lack of fit | 1733.97 | 10 | 173.4 | 4.15 | 0.0913 |
| 18 | 3 | 20 | 50 | 10 | 180.78 | Pure error | 167.14 | 4 | 41.78 | ||
| 19 | 1 | 20 | 70 | 10 | 132.15 | Cor total | 32,457.74 | 28 | |||
| 20 | 3 | 20 | 70 | 10 | 156.44 | ||||||
| 21 | 2 | 15 | 60 | 0 | 112.60 | ||||||
| 22 | 2 | 25 | 60 | 0 | 136.43 | ||||||
| 23 | 2 | 15 | 60 | 20 | 124.54 | ||||||
| 24 | 2 | 25 | 60 | 20 | 129.43 | ||||||
| 25 | 2 | 20 | 60 | 10 | 190.76 | ||||||
| 26 | 2 | 20 | 60 | 10 | 193.68 | ||||||
| 27 | 2 | 20 | 60 | 10 | 181.56 | ||||||
| 28 | 2 | 20 | 60 | 10 | 181.05 | ||||||
| 29 | 2 | 20 | 60 | 10 | 179.38 | ||||||
| Water | Ethanol | DES-2 | |
|---|---|---|---|
| TPC (mg GAE/g DW) | 19.92 ± 3.50 a | 31.13 ± 9.41 a | 187.23 ± 14.67 b |
| DPPH-IC50 (mg/mL) | 7.36 ± 1.08 a | 4.38 ± 0.83 b | 0.92 ± 0.23 c |
| FRAP (mg Trolox/g DW) | 13.27 ± 0.10 a | 14.92 ± 1.00 a | 21.56 ± 3.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Qin, M.; Chen, L.; Li, X.; Wu, X.; Ye, G.; Deng, J.; Yang, H. Green Extraction of Lotus Leaf (Nelumbo nucifera Gaertn) Polyphenols: Unraveling the Mechanism of Ultrasound-Assisted Deep Eutectic Solvents. Foods 2025, 14, 4045. https://doi.org/10.3390/foods14234045
Sun J, Qin M, Chen L, Li X, Wu X, Ye G, Deng J, Yang H. Green Extraction of Lotus Leaf (Nelumbo nucifera Gaertn) Polyphenols: Unraveling the Mechanism of Ultrasound-Assisted Deep Eutectic Solvents. Foods. 2025; 14(23):4045. https://doi.org/10.3390/foods14234045
Chicago/Turabian StyleSun, Jing, Mengqi Qin, Luyang Chen, Xin Li, Xinyan Wu, Gang Ye, Jianjun Deng, and Haixia Yang. 2025. "Green Extraction of Lotus Leaf (Nelumbo nucifera Gaertn) Polyphenols: Unraveling the Mechanism of Ultrasound-Assisted Deep Eutectic Solvents" Foods 14, no. 23: 4045. https://doi.org/10.3390/foods14234045
APA StyleSun, J., Qin, M., Chen, L., Li, X., Wu, X., Ye, G., Deng, J., & Yang, H. (2025). Green Extraction of Lotus Leaf (Nelumbo nucifera Gaertn) Polyphenols: Unraveling the Mechanism of Ultrasound-Assisted Deep Eutectic Solvents. Foods, 14(23), 4045. https://doi.org/10.3390/foods14234045









