Random Forest-Assisted Widely Targeted Lipidomic Reveals Differences in Tan Lamb Meat Quality in Different Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Meat Quality Determination
2.4. Fatty Acid Determination
2.5. Volatile Compound Determination
2.6. Lipid Extraction and Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Tan Lamb Quality from Different Regions
3.2. Comparison of Tan Lamb Fatty Acid from Different Regions
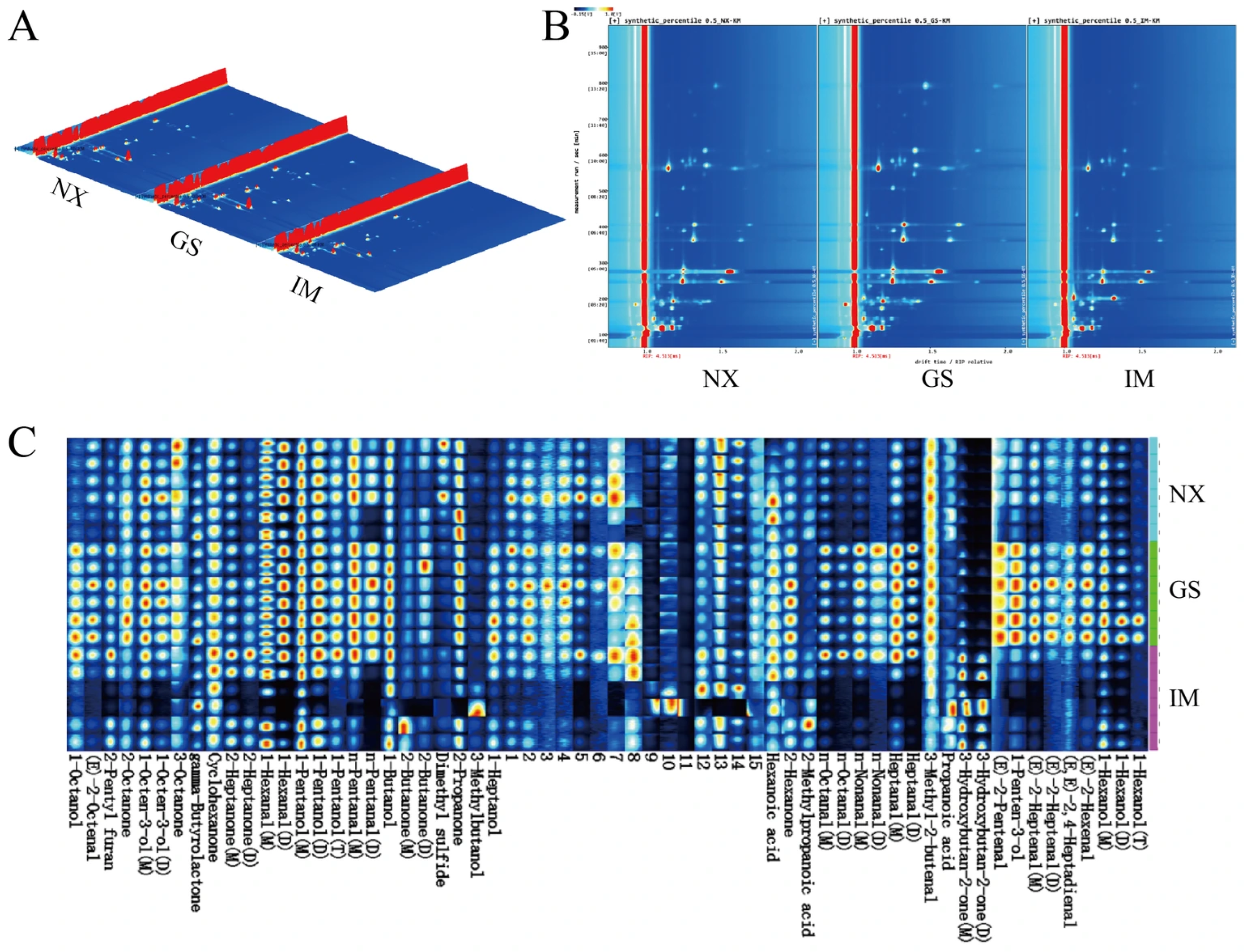
3.3. GC-IMS Analysis of Tan Lamb from Different Regions
3.4. Reliability Validation of Lipidomic Data
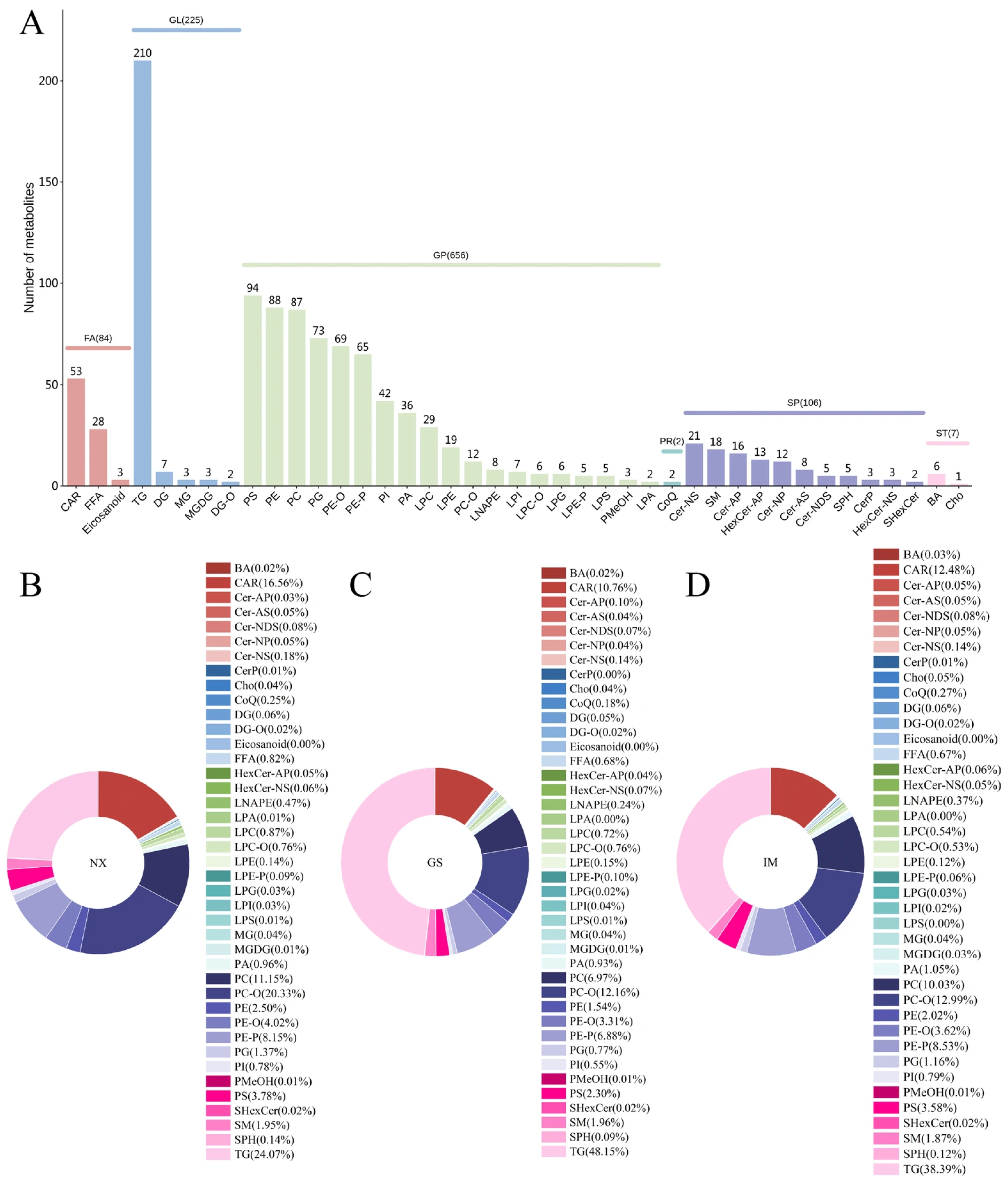
3.5. Comparison of Tan Lamb Lipids from Different Regions
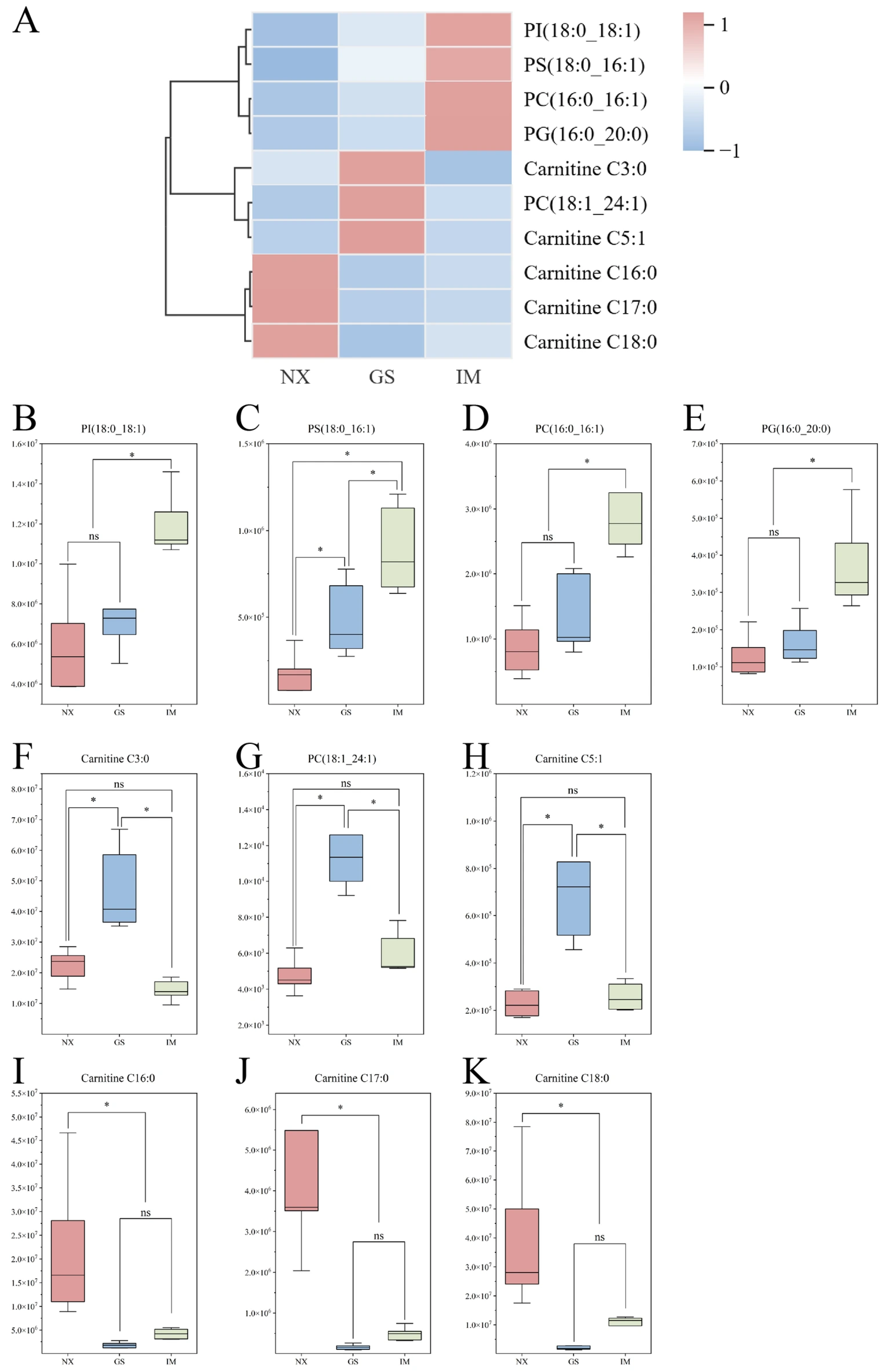
3.6. Screening of Differential Lipids in Tan Lamb from Different Regions
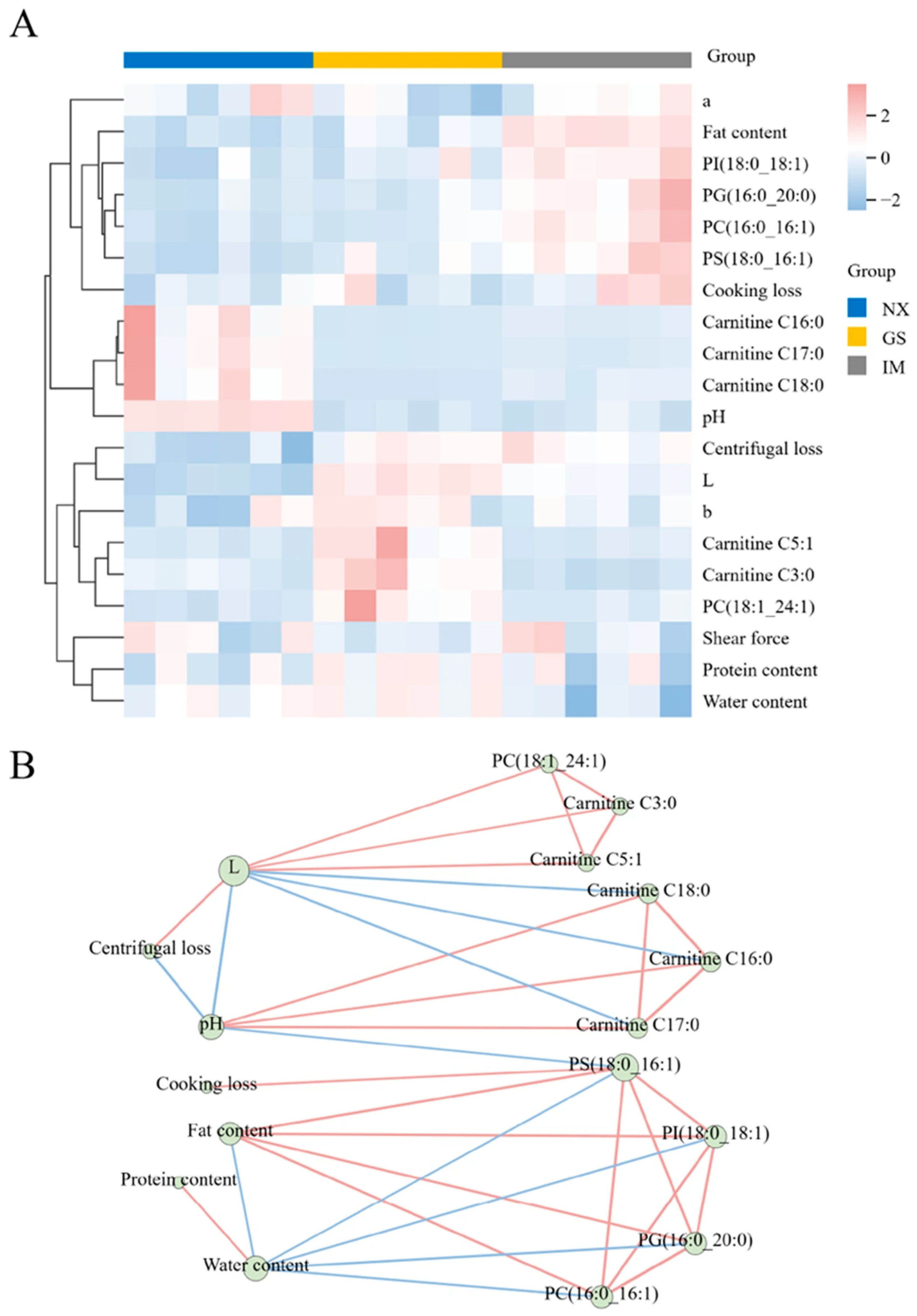
3.7. Correlation Analysis Between Differential Lipids and Meat Quality of Tan Lamb from Different Regions
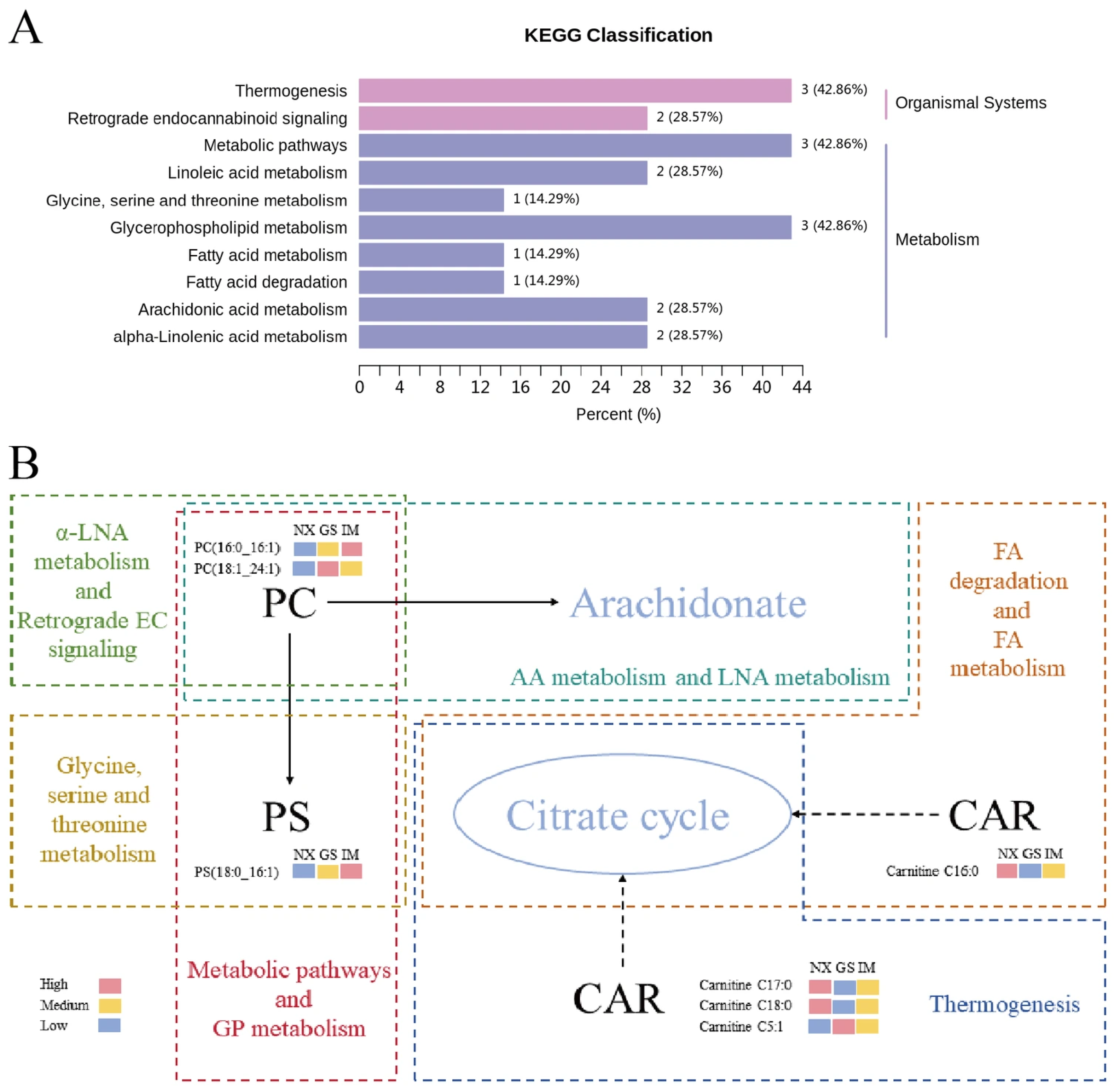
3.8. Analysis of Characteristic Lipid Metabolic Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zuo, S.; Guo, Y.; Zhang, C.; Wang, Y.; Peng, S.; Liu, M.; Wang, B.; Zhang, H.; Luo, H. Carcass meat quality, volatile compound profile, and gene expression in tan sheep under different feeding regimes. Food Biosci. 2023, 56, 103213. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Ma, Q.; Zhao, Q.; Guo, X.; Ma, L.; Gou, C.; Xia, Y.; Gan, R.; Zhang, J. Discrimination the geographical origin of Yanchi tan lamb with different muscle sections by stable isotopic ratios and elemental profiles. Int. J. Food Sci. Amp; Technol. 2020, 56, 2604–2611. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Yang, M.; Niu, N.; Wu, J.; Shu, Z.; Zhang, P.; Shi, L.; Zhao, F.; Wang, L.; et al. Metabolomics and lipidomics profiles related to intramuscular fat content and flavor precursors between Laiwu and Yorkshire Pigs. Food Chem. 2023, 404, 134699. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Wong, E.; Nixon, L.N.; Johnson, C.B. Volatile medium chain fatty acids and mutton flavor. J. Agric. Food Chem. 1975, 23, 495–498. [Google Scholar] [CrossRef]
- Ye, Y.; Eyres, G.T.; Reis, M.G.; Schreurs, N.M.; Silcock, P.; Agnew, M.P.; Johnson, P.L.; Maclean, P.; Realini, C.E. Fatty acid composition and volatile profile of M. longissimus thoracis from commercial lambs reared in different forage systems. Foods 2020, 9, 1885. [Google Scholar] [CrossRef] [PubMed]
- Haridas, P.C.; Ravichandran, R.; Shaikh, N.; Kishore, P.; Panda, S.K.; Banerjee, K.; Chatterjee, N.S. Authentication of the species identity of squid rings using UHPLC-Q-orbitrap MS/MS-based lipidome fingerprinting and Chemoinformatics. Food Chem. 2024, 442, 138525. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, D.; Wang, D.; Xu, W.; Zhang, C.; Li, P.; Sun, C. Lipidomic profile changes of yellow-feathered chicken meat during thermal processing based on UPLC-ESI-MS approach. Food Chem. 2023, 399, 133977. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, D.; Li, S.; Dunne, P.; Brunton, N.P.; Grasso, S.; Liu, C.; Zheng, X.; Li, C.; Chen, L. Combined quantitative lipidomics and back-propagation neural network approach to discriminate the breed and part source of lamb. Food Chem. 2024, 437, 137940. [Google Scholar] [CrossRef]
- Chen, G.; Qi, L.; Zhang, S.; Peng, H.; Lin, Z.; Zhang, X.; Nie, Q.; Luo, W. Metabolomic, lipidomic, and proteomic profiles provide insights on meat quality differences between Shitou and Wuzong Geese. Food Chem. 2024, 438, 137967. [Google Scholar] [CrossRef]
- Phan, Q.; Tomasino, E. Untargeted lipidomic approach in studying Pinot noir wine lipids and predicting wine origin. Food Chem. 2021, 355, 129409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, D.; Liu, C.; Xu, L.; Li, S.; Zheng, X.; Chen, L. The authentication of Yanchi Tan Lamb based on lipidomic combined with particle swarm optimization-back propagation neural network. Food Chem. X 2024, 24, 102031. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.; Li, S.; Xu, J.; Liu, H.; Zheng, X.; Zhang, D.; Chen, L. Association of lipidome evolution with the corresponding volatile characteristics of postmortem lamb during chilled storage. Food Res. Int. 2023, 169, 112916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Liu, Y.; Pei, S.; Kong, Y.; Li, F.; Wang, W.; Yue, X. Preliminary genetic parameter estimates of meat quality traits in Hu sheep. Meat Sci. 2024, 212, 109476. [Google Scholar] [CrossRef]
- Ran, Z.; Xu, J.; Liao, K.; Li, S.; Chen, S.; Yan, X. Biosynthesis of polyunsaturated fatty acids in the razor clam Sinonovacula constricta: Characterization of 5 and 6 fatty acid Desaturases. J. Agric. Food Chem. 2018, 66, 4592–4601. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Jin, J.; Li, H.; Chen, F.; Fei, Y.; Wang, Y. Characterization of the flavor profile and dynamic changes in Chinese traditional fish sauce (Yu-lu) based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2024, 192, 114772. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Liu, C.; Grasso, S.; Brunton, N.P.; Yang, Q.; Li, S.; Chen, L.; Zhang, D. Metabolomics for origin traceability of lamb: An ensemble learning approach based on random forest recursive feature elimination. Food Chem. X 2025, 29, 102856. [Google Scholar] [CrossRef]
- Mungure, T.E.; Bekhit, A.E.-D.; Birch, E.J.; Stewart, I. Effect of rigor temperature, ageing and display time on the meat quality and lipid oxidative stability of hot boned beef semimembranosus muscle. Meat Sci. 2016, 114, 146–153. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Vahmani, P.; Muchenje, V.; Dugan, M.E.R.; Mapiye, C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res. Int. 2018, 104, 25–38. [Google Scholar] [CrossRef]
- Sañudo, C.; Enser, M.E.; Campo, M.M.; Nute, G.R.; Marıía, G.; Sierra, I.; Wood, J.D. Fatty acid composition and sensory characteristics of lamb carcasses from Britain and Spain. Meat Sci. 2000, 54, 339–346. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, Y.; Wang, T.; Li, F. Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tan sheep. Bioengineered 2020, 11, 1159–1169. [Google Scholar] [CrossRef]
- Cheng, C.; Xie, X.; Li, S.; Chen, P.; Huang, C.; Zheng, X.; Chen, L.; Zhang, D. Analysis of bioactive substances in mutton and their effects on the quality of minced mutton. Food Res. Int. 2025, 200, 115474. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Ma, S.; Wu, H.; Liu, D.; Liu, J.; Zhang, M. Flavor profile analysis of grilled lamb seasoned with classic salt, chili pepper, and cumin (Cuminum cyminum) through Hs-Spme-GC-MS, HS-GC-IMS, e-nose techniques, and sensory evaluation on Sonit Sheep. Food Chem. 2024, 454, 139514. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.S.; El-Sayed, E.M. Dietary conjugated linoleic acid and medium-chain triglycerides for Obesity Management. J. Biosci. 2021, 46, 12. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Cheng, X.; Yang, H.; She, Z.-G.; Cai, J.; Li, H.; Zhang, X.-J. Emerging roles and therapeutic applications of arachidonic acid pathways in cardiometabolic diseases. Circ. Res. 2024, 135, 222–260. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Yang, R.; Dong, Y.; Lin, S. Mechanism of aroma compounds changes from sea cucumber peptide powders (scpps) under different storage conditions. Food Res. Int. 2020, 128, 108757. [Google Scholar] [CrossRef]
- Jiang, Q.; Zheng, X.; Xu, T.; Chen, M.; Chen, S.; Zhang, D.; Cai, B.; Gu, L. Comparative volatile profiles of plain poached (PP) and steamed over water (SW) Wenchang Chicken analyzed by GC-MS, GC-IMS, and E-nose. Foods 2025, 14, 3778. [Google Scholar] [CrossRef]
- Ma, Y.; Lan, Q.; Wang, C.; Laghi, L.; Zhu, C.; Picone, G. Analysis of changes in flavor profile and bacterial succession during pork fermentation using multi-omics-based analysis. Foods 2025, 14, 3804. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Tang, C.; Yue, S.; Zhao, Q.; Li, F.; Zhang, J. Changes in lipids and aroma compounds in intramuscular fat from Hu Sheep. Food Chem. 2022, 383, 132611. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, X.; Hu, C.; Fu, T.; Zhang, X.; Liu, Z.; Wang, Y.; Zhang, F.; He, X.; Yu, J.-H. Functional, transcriptomic, and lipidomic studies of the choC gene encoding a phospholipid methyltransferase in Aspergillus fumigatus. Microbiol. Spectr. 2024, 12, e02168-23. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Caroprese, M.; della Malva, A.; Santillo, A.; Sevi, A.; Albenzio, M. Role of whole linseed and sunflower seed on the nutritional and organoleptic properties of Podolian × limousine meat. Ital. J. Anim. Sci. 2024, 23, 868–879. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, H.; Yu, Y.; Gao, H.; Fan, X.; Li, Q.; Zhou, Z. Dietary salidroside supplementation improves meat quality and antioxidant capacity and regulates lipid metabolism in broilers. Food Chem. X 2024, 22, 101406. [Google Scholar] [CrossRef]
- Cui, X.; Yang, Y.; Zhang, M.; Bao, L.; Jiao, F.; Liu, S.; Wang, H.; Wei, X.; Qian, W.; Shi, X.; et al. Mulberry leaves supplementation alters lipid metabolism and promotes fatty acid β oxidation in growing mutton sheep. J. Anim. Sci. 2024, 102, skae076. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Wang, C.; Zhang, C.; Li, X.; Tang, C.; Wei, X. Effect of dietary phosphorus levels on meat quality and lipid metabolism in broiler chickens. Food Chem. 2016, 205, 289–296. [Google Scholar] [CrossRef]
- Xiong, L.; Pei, J.; Wang, X.; Guo, S.; Guo, X.; Yan, P. Effect of lipids in yak muscle under different feeding systems on meat quality based on untargeted lipidomics. Animals 2022, 12, 2814. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Yang, X.; Yang, X.; Zhang, H.; Wang, P.; Song, J.; Wang, L.; Zhang, W.; Wen, P. Pseudo-targeted lipidomics insights into lipid discrepancies between yak colostrum and mature milk based on UHPLC-Qtrap-MS. Food Chem. 2024, 442, 138462. [Google Scholar] [CrossRef]
- Yu, Q.; Gu, X.; Liu, Q.; Wen, R.; Sun, C. Effect of wet-aging on meat quality and exudate metabolome changes in different beef muscles. Food Res. Int. 2024, 184, 114260. [Google Scholar] [CrossRef]
- Adachi, S.; Yamaguchi, S.; Ozeki, T.; Kose, K. Application of a magnetic resonance imaging method for nondestructive, three-dimensional, high-resolution measurement of the water content of wet snow samples. Front. Earth Sci. 2020, 8, 179. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S.L. Structure and functionality of almond proteins as a function of pH. Food Struct. 2021, 30, 100229. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Lai, B.; Liu, R.; Wu, M.; Ge, Q.; Yu, H. Effects of ultrasound-assisted cooking on the physicochemical properties and microstructure of Pork Meatballs. Meat Sci. 2024, 208, 109382. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, J.; Zhou, H.; Lu, A.; Xu, B. A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Sci. 2021, 172, 108359. [Google Scholar] [CrossRef]
- Xie, G.; Chen, M.; Yang, Y.; Xie, Y.; Deng, K.; Xie, L. Comprehensive untargeted lipidomics study of Black Morel (Morchella sextelata) at different growth stages. Food Chem. 2024, 451, 139431. [Google Scholar] [CrossRef]
- Jia, W.; Shi, Q.; Shi, L. Effect of irradiation treatment on the lipid composition and nutritional quality of goat meat. Food Chem. 2021, 351, 129295. [Google Scholar] [CrossRef]
- Xie, P.; Wu, Y.; Lee, Y.-Y.; Wang, Y.; Zhang, Z. Asterias Rolleston Starfish gonad lipids: A novel source of omega-3 fatty acids—Assessment of major components and their antioxidant activities. Food Chem. 2024, 456, 140005. [Google Scholar] [CrossRef]
- Chen, J.; Singh, T.K.; Al Nemri, S.; Zaidi, M.; Billingsley, K.L.; Park, J.M. Hyperpolarized [1-13C]Acetyl-l-Carnitine Probes Tricarboxylic Acid Cycle Activity In Vivo. ACS Sens. 2023, 8, 2927–2932. [Google Scholar] [CrossRef]

| NX | GS | IM | |
|---|---|---|---|
| C10:0 | 0.004 ± 0.001 b | 0.008 ± 0.002 a | 0.004 ± 0.001 b |
| C12:0 | 0.008 ± 0.003 b | 0.030 ± 0.002 a | 0.008 ± 0.003 b |
| C14:0 | 0.081 ± 0.005 c | 0.251 ± 0.007 a | 0.089 ± 0.005 b |
| C15:0 | 0.020 ± 0.004 b | 0.025 ± 0.004 a | 0.023 ± 0.003 ab |
| C16:0 | 0.711 ± 0.009 c | 0.959 ± 0.010 a | 0.912 ± 0.009 b |
| C17:0 | 0.085 ± 0.008 b | 0.053 ± 0.008 c | 0.101 ± 0.008 a |
| C18:0 | 0.609 ± 0.011 b | 0.533 ± 0.010 c | 0.727 ± 0.010 a |
| C24:0 | 0.005 ± 0.002 | 0.006 ± 0.002 | 0.005 ± 0.002 |
| C14:1 | 0.003 ± 0.001 b | 0.009 ± 0.001 a | 0.003 ± 0.001 b |
| C16:1 | 0.078 ± 0.004 c | 0.115 ± 0.002 a | 0.085 ± 0.006 b |
| C18:1n9c | 1.181 ± 0.011 c | 1.279 ± 0.003 b | 1.429 ± 0.013 a |
| C20:1n9 | 0.003 ± 0.001 | 0.004 ± 0.002 | 0.004 ± 0.001 |
| C18:2n6c | 0.313 ± 0.002 a | 0.247 ± 0.002 c | 0.308 ± 0.002 b |
| C18:3n3 | 0.004 ± 0.002 b | 0.015 ± 0.002 a | 0.006 ± 0.002 b |
| C20:2 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0.001 |
| C20:3n6 | 0.010 ± 0.001 a | 0.005 ± 0.001 b | 0.011 ± 0.001 a |
| C20:4n6 | 0.132 ± 0.003 a | 0.089 ± 0.004 b | 0.135 ± 0.003 a |
| SFA | 1.523 ± 0.042 b | 1.864 ± 0.042 a | 1.869 ± 0.038 a |
| MUFA | 1.265 ± 0.014 c | 1.407 ± 0.004 b | 1.521 ± 0.006 a |
| PUFA | 0.460 ± 0.008 a | 0.358 ± 0.006 b | 0.462 ± 0.006 a |
| 9 | Formula | Molecular Weight | Ionization Model | Lipid Category |
|---|---|---|---|---|
| Carnitine C16:0 | C23H45NO4 | 399.33 | [M+H]+ | Fatty Acyls |
| Carnitine C17:0 | C24H47NO4 | 413.35 | [M+H]+ | Fatty Acyls |
| Carnitine C18:0 | C25H49NO4 | 427.37 | [M+H]+ | Fatty Acyls |
| Carnitine C3:0 | C10H19NO4 | 217.13 | [M+H]+ | Fatty Acyls |
| Carnitine C5:1 | C12H21NO4 | 243.15 | [M+H]+ | Fatty Acyls |
| PC (16:0_16:1) | C40H78NO8P | 731.55 | [M+COOH]− | Glycerophospholipids |
| PC (18:1_24:1) | C50H96NO8P | 869.69 | [M+COOH]− | Glycerophospholipids |
| PG (16:0_20:0) | C42H83O10P | 778.57 | [M−H]− | Glycerophospholipids |
| PI (18:0_18:1) | C45H85O13P | 864.57 | [M−H]− | Glycerophospholipids |
| PS (18:0_16:1) | C40H76NO10P | 761.52 | [M−H]− | Glycerophospholipids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liu, C.; Xu, M.; Gu, M.; Xu, L.; Li, S.; Zheng, X.; Zhang, D.; Chen, L. Random Forest-Assisted Widely Targeted Lipidomic Reveals Differences in Tan Lamb Meat Quality in Different Regions. Foods 2025, 14, 4046. https://doi.org/10.3390/foods14234046
Yang Q, Liu C, Xu M, Gu M, Xu L, Li S, Zheng X, Zhang D, Chen L. Random Forest-Assisted Widely Targeted Lipidomic Reveals Differences in Tan Lamb Meat Quality in Different Regions. Foods. 2025; 14(23):4046. https://doi.org/10.3390/foods14234046
Chicago/Turabian StyleYang, Qi, Chongxin Liu, Muxuan Xu, Minghui Gu, Le Xu, Shaobo Li, Xiaochun Zheng, Dequan Zhang, and Li Chen. 2025. "Random Forest-Assisted Widely Targeted Lipidomic Reveals Differences in Tan Lamb Meat Quality in Different Regions" Foods 14, no. 23: 4046. https://doi.org/10.3390/foods14234046
APA StyleYang, Q., Liu, C., Xu, M., Gu, M., Xu, L., Li, S., Zheng, X., Zhang, D., & Chen, L. (2025). Random Forest-Assisted Widely Targeted Lipidomic Reveals Differences in Tan Lamb Meat Quality in Different Regions. Foods, 14(23), 4046. https://doi.org/10.3390/foods14234046







