Preparation and Application of Magnetic Microporous Organic Networks for Rapid Adsorption Enrichment of Multiple Mycotoxins in Complex Food Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthesis Procedures
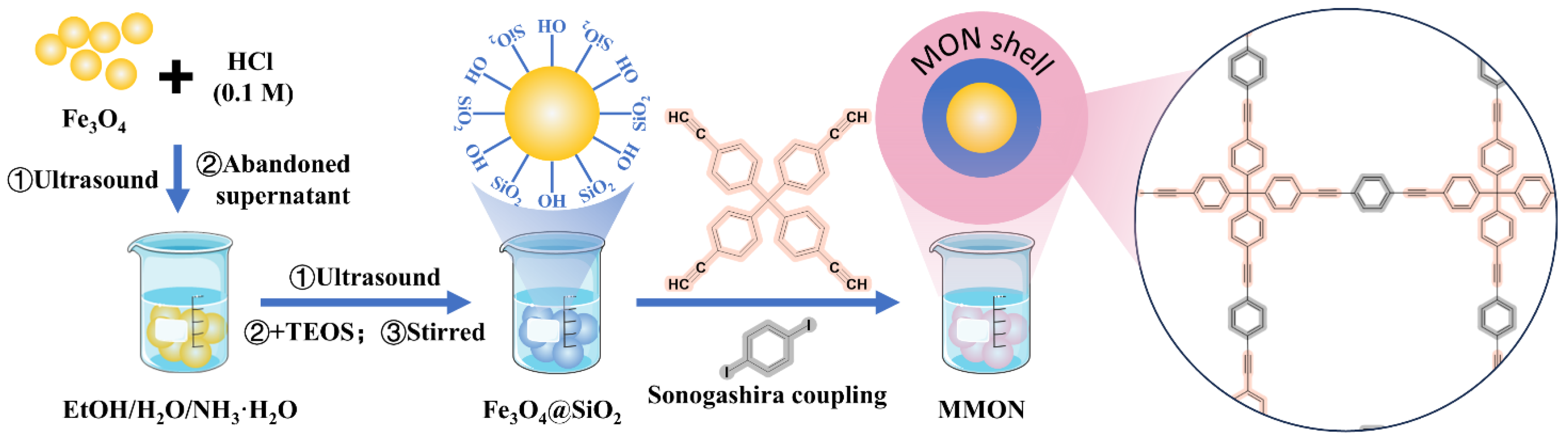
2.2.1. Synthesis of MMON
2.2.2. Synthesis of MOFs
2.3. MSPE Procedure and Batch Experiments
2.4. Optimization of MSPE Parameters
2.5. Preparation of Standard and Real Samples
2.6. Mechanism Analysis
2.7. UPLC-MS/MS Conditions
2.8. Data Analysis and Statistical Methods
3. Results
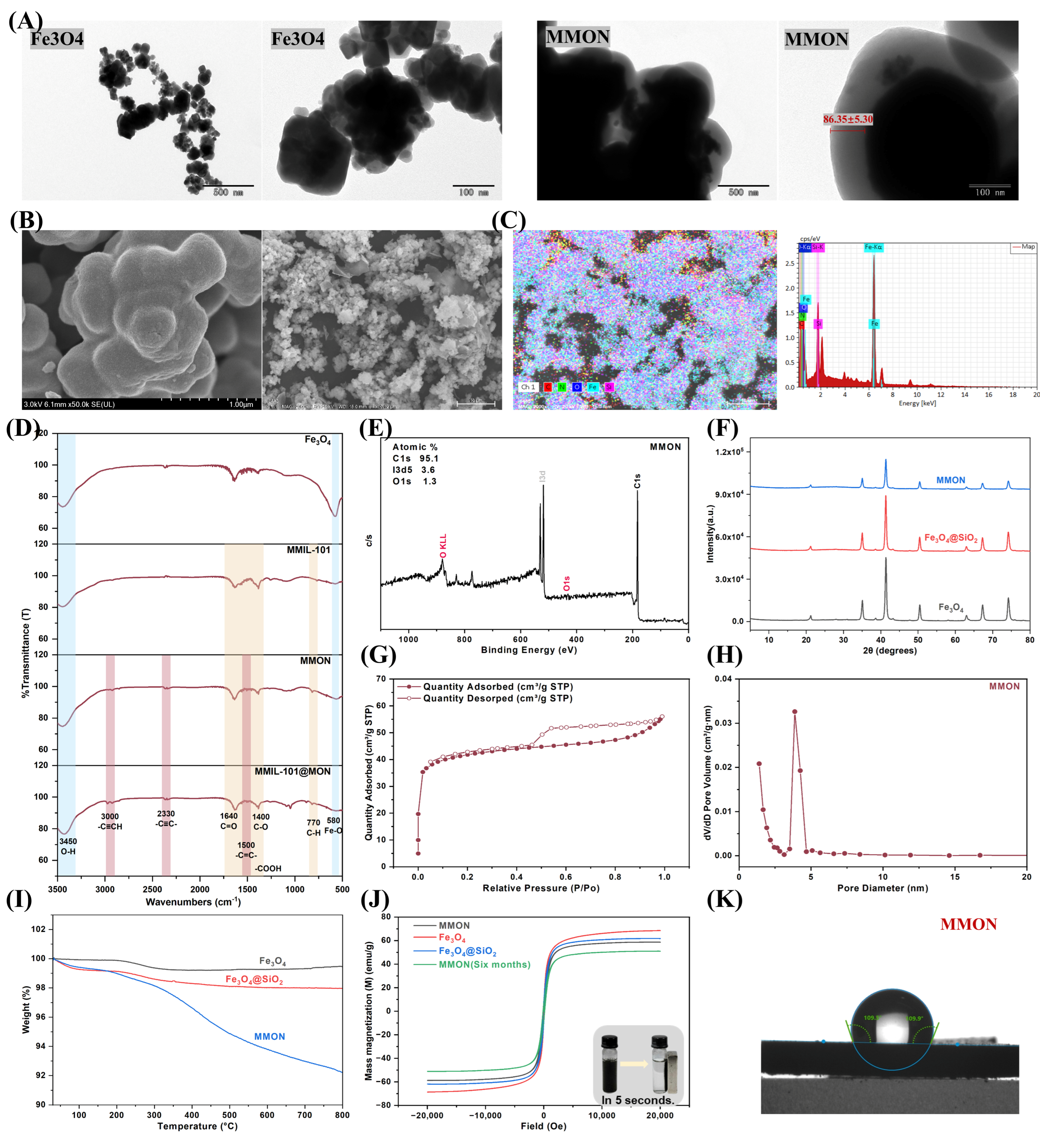
3.1. Synthesis and Characterization of MONs
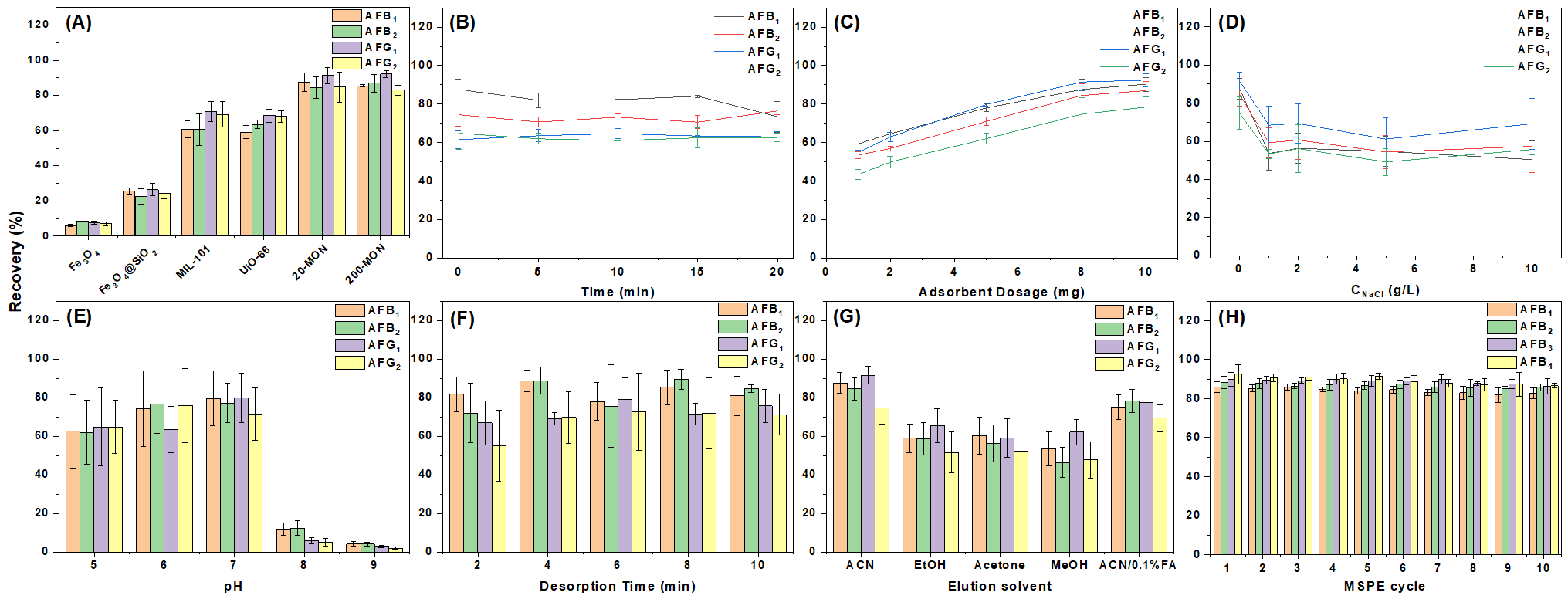
3.2. Adsorption Performance
3.3. Optimization of MSPE Parameters
3.3.1. Adsorption Time
3.3.2. Adsorbent Dosage
3.3.3. Ionic Strength
3.3.4. pH
3.3.5. Desorption Conditions
3.4. Method Validation
3.5. Reusability and Stability
3.6. Real Sample Analysis
3.7. Comparison with Other Methods
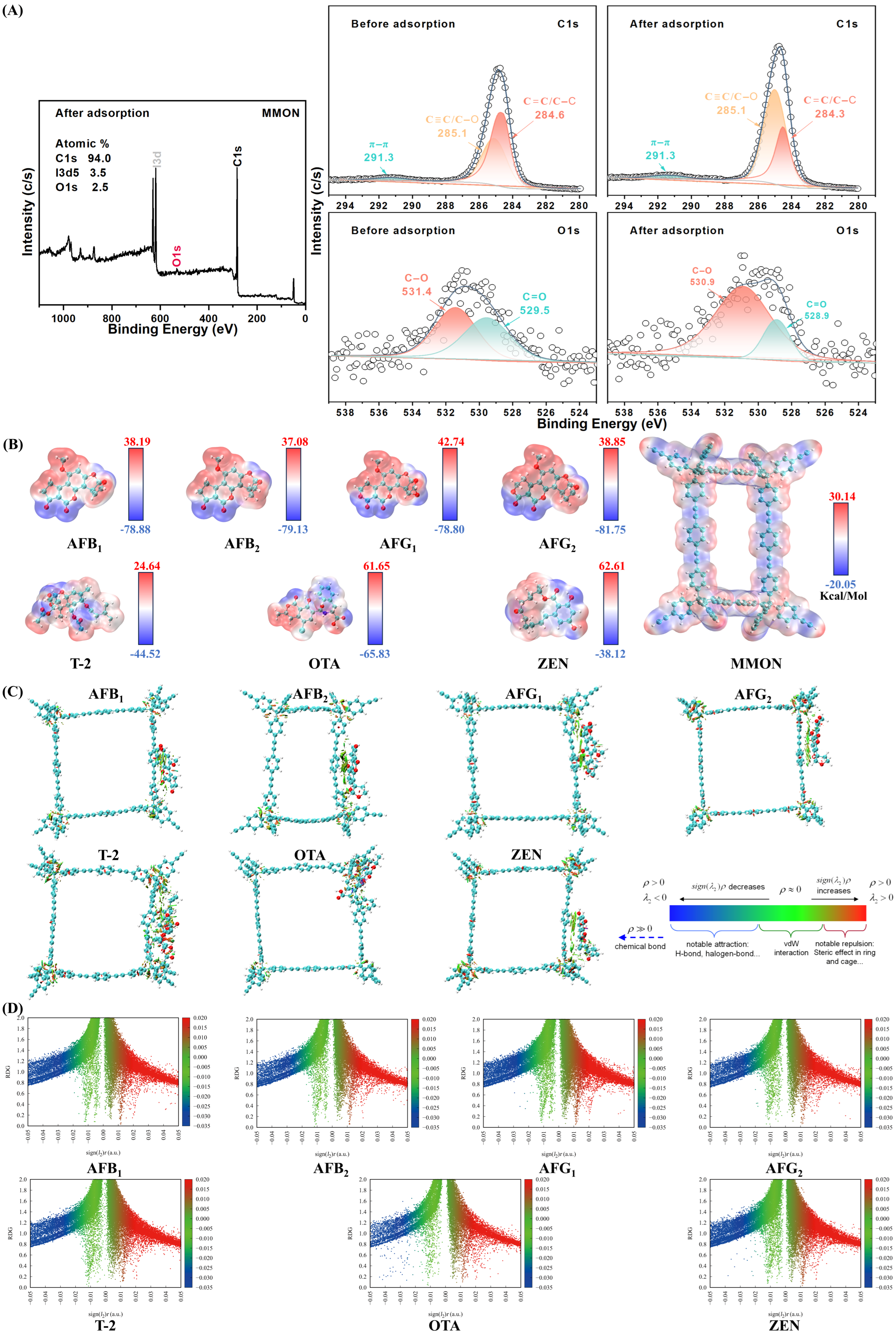
3.8. Adsorption Mechanism Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dellafiora, L.; Dall’Asta, C. Forthcoming challenges in mycotoxins toxicology research for safer food—A need for multi-omics approach. Toxins 2017, 9, 18. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, P.; Zhang, B.; Kong, L.; Xiao, C.; Song, Z. Progress on gut health maintenance and antibiotic alternatives in broiler chicken production. Front. Nutr. 2021, 8, 692839. [Google Scholar] [CrossRef]
- Shrestha, N.; Thomas, C.A.; Richtsmeier, D.; Bogard, A.; Hermann, R.; Walker, M.; Abatchev, G.; Brown, R.J.; Fologea, D. Temporary membrane permeabilization via the pore-forming toxin lysenin. Toxins 2020, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-derived polyphenols as nrf2 activators to counteract oxidative stress and intestinal toxicity induced by deoxynivalenol in swine: An emerging research direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, X.; Liu, K.; Sun, J.; Li, B.; Malyar, R.M.; Liu, D.; Pan, C.; Gan, F.; Liu, Y.; et al. Ochratoxin A induces nephrotoxicity in vitro and in vivo via pyroptosis. Arch. Toxicol. 2021, 95, 1489–1502. [Google Scholar] [CrossRef]
- Pinto, A.C.S.M.; De Pierri, C.R.; Evangelista, A.G.; de Gomes, A.S.L.P.B.; Luciano, F.B. Deoxynivalenol: Toxicology, degradation by bacteria, and phylogenetic analysis. Toxins 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Qu, Z.; Moretti, A.; Logrieco, A.F.; Chu, H.; Zhang, Q.; Sun, C.; Ren, X.; Cui, L.; Chen, Q.; et al. Aspergillus mycotoxins: The major food contaminants. Adv. Sci. 2025, 12, 2412757. [Google Scholar] [CrossRef]
- Köhler, A.; Job, L.; Worek, F.; Skerra, A. Inhibition of an organophosphate-detoxifying bacterial phosphotriesterase by albumin and plasma thiol components. Toxicol. Lett. 2021, 350, 194–201. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Yu, Z.; Tang, X.; Zhou, M.; Guo, Y.; Xiong, B. A disposable immunosensor array using cellulose paper assembled chemiresistive biosensor for simultaneous monitoring of mycotoxins AFB1 and FB1. Talanta 2024, 276, 126145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.-S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.; Feng, J.; Sun, M.; Liu, Y. Online solid-phase extraction on customized pyridyl conjugated microporous polymer for efficient enrichment and sensitive determination of bisphenols in water. Microchem. J. 2025, 214, 114084. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Bhogal, S.; Gupta, K. A review of the advances in magnetic solid phase extraction for phthalate analysis: Improved materials and applications in complex matrices. Talanta 2026, 296, 128519. [Google Scholar] [CrossRef]
- Yazdizadeh, S.; Ebrahimipour, S.Y.; Fatemi, S.J.; Khaleghi, M.; Ramezanpour, S. Mesoporous Fe3O4@SiO2@La nanocomposite for efficient methylene blue removal from wastewater. J. Mol. Struct. 2025, 1322, 140337. [Google Scholar] [CrossRef]
- Xie, Z.; Lin, J.; Hu, Y.; Li, G.; Zhong, Q. Rapid construction of double macrocycles, hierarchical covalent organic framework with size-sieving and host-guest recognition for selective adsorption and targeted analysis of mycotoxins in cereals. Chem. Eng. J. 2024, 493, 152464. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Hu, Y.; Zhong, Q. Host-guest mediated recognition and rapid extraction of fusarium mycotoxins in cereals by nickel ferrite magnetic calix[4]arene-derived covalent organic framework fabricated in room-temperature. Food Chem. 2025, 464, 141887. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Deng, Q.; Li, J.; Xu, J.; Guo, M.; Wu, H.; Zhang, J.; Jin, J.; Wang, X. Carboxyl-functionalized magnetic amide-linked covalent organic framework for efficient extraction of multi-class mycotoxins from soybeans prior to HPLC-MS/MS analysis. LWT 2024, 193, 115753. [Google Scholar] [CrossRef]
- Guo, D.; Huang, Q.; Zhao, R.; Guo, W.; Fan, K.; Han, Z.; Zhao, Z.; Nie, D. MIL-101(Cr)@Fe3O4 nanocomposites as magnetic solid-phase extraction adsorbent for the determination of multiple mycotoxins in agricultural products by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Control 2023, 146, 109540. [Google Scholar] [CrossRef]
- del Pérez-Álvarez, M.C.; Arroyo-Manzanares, N.; Campillo, N.; Viñas, P. Magnetic molecularly imprinted polymers for selective extraction of aflatoxins from feeds. Toxins 2024, 16, 120. [Google Scholar] [CrossRef]
- Jia, Y.; Su, H.; Wang, Z.; Wong, Y.-L.E.; Chen, X.; Wang, M.; Chan, T.-W.D. Metal–organic framework@microporous organic network as adsorbent for solid-phase microextraction. Anal. Chem. 2016, 88, 9364–9367. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Liu, J.-M.; Wang, Z.-H.; Lv, S.-W.; Zhao, N.; Wang, S. Integration of Fe3O4@UiO-66-NH2@MON core-shell structured adsorbents for specific preconcentration and sensitive determination of aflatoxins against complex sample matrix. J. Hazard. Mater. 2020, 384, 121348. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; He, X.-Q.; Yang, C.-X.; Yan, X.-P. Application of microporous organic networks in separation science. TrAC Trends Anal. Chem. 2021, 139, 116268. [Google Scholar] [CrossRef]
- Zeng, H.; Peng, J.; Peng, H.; Yang, H.; Wang, X.; Xu, Z.; Chen, W. Post-cationic modification of a pyridyl-based conjugated microporous polymer stationary phase for mixed mode liquid chromatographic separation and food additive detection. Microchem. J. 2025, 211, 113120. [Google Scholar] [CrossRef]
- de Jesus, J.R.; de Sousa Pereira, M.V.; Ribeiro, I.S. Advances in chemical analysis: Microporous metal–organic frameworks as eco-friendly solutions for selective separations of (bio)molecules. Microchem. J. 2024, 203, 110951. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, Z.; Zhang, X.; Lin, S.; Liang, B.; Liang, S.-X. Ultra-fast ultrasound-assisted synthesis of pyridine-based microporous organic network for pipette-tip solid-phase extraction of triazine herbicides in water: An experimental and adsorption mechanism study. Microchem. J. 2025, 212, 113243. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; Ren, H.-B.; Yang, C.-X.; Yan, X.-P. Facile synthesis of hydroxyl enriched microporous organic networks for enhanced adsorption and removal of tetrabromobisphenol a from aqueous solution. Chem. Eng. J. 2019, 373, 606–615. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; Ren, H.-B.; Yang, C.-X.; Yan, X.-P. Room-temperature synthesis of microporous organic network for efficient adsorption and removal of tetrabromobisphenol A from aqueous solution. Chem. Eng. J. 2019, 368, 589–597. [Google Scholar] [CrossRef]
- He, X.-Q.; Cui, Y.-Y.; Yang, C.-X. Engineering of amino microporous organic network on zeolitic imidazolate framework-67 derived nitrogen-doped carbon for efficient magnetic extraction of plant growth regulators. Talanta 2021, 224, 121876. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Ma, H.; Wang, Z.-H.; Zhao, N.; Wang, S. Fabrication of Fe3O4@UiO-66-SO3H core–shell functional adsorbents for highly selective and efficient removal of organic dyes. New J. Chem. 2019, 43, 7770–7777. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, multiwfn. J. Chem. Phys. 2024, 161, 82503. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Gao, S.-W.; Chen, L.-H.; Cui, Y.-Y.; Yang, C.-X. Sacrificial template synthesis of hollow sulfonate group functionalized microporous organic network for efficient solid phase extraction of sulfonamide antibiotics from milk and honey samples. J. Chromatogr. A 2024, 1721, 464844. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Lv, H.; Ji, X.-M.; Liu, J.-M.; Wang, S. Hollow-structured microporous organic networks adsorbents enabled specific and sensitive identification and determination of aflatoxins. Toxins 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.-Y.; Wang, Y.; Yang, W.-Z.; Yang, C.-X. Fabrication of amino- and hydroxyl dual-functionalized magnetic microporous organic network for extraction of zearalenone from traditional Chinese medicine prior to the HPLC determination. J. Chromatogr. A 2024, 1724, 464915. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.-C.; Bai, X.-L.; Liu, Y.-M.; Wu, G.-F.; Yang, F.-S.; Liao, X. Multi-mycotoxins analysis in liquid milk by UHPLC-Q-exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes. Food Chem. 2020, 305, 125429. [Google Scholar] [CrossRef]
- Huang, Z.; He, J.; Li, H.; Zhang, M.; Wang, H.; Zhang, Y.; Li, Y.; You, L.; Zhang, S. Synthesis and application of magnetic-surfaced pseudo molecularly imprinted polymers for zearalenone pretreatment in cereal samples. Food Chem. 2020, 308, 125696. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Yang, Y.; Zhu, K.; Guo, L.; Zhu, X.; Hao, N.; Ma, X.; Feng, L.; Chen, Y. Nanochannel array gating dual-mode sensor for T-2 toxin detection via photoelectrochemical and colorimetric approaches. Sens. Actuators B Chem. 2025, 431, 137446. [Google Scholar] [CrossRef]
- Hou, Y.; Long, N.; Xu, Q.; Li, Y.; Song, P.; Yang, M.; Wang, J.; Zhou, L.; Sheng, P.; Kong, W. Development of a nafion-MWCNTs and in-situ generated Au nanopopcorns dual-amplification electrochemical aptasensor for ultrasensitive detection of OTA. Food Chem. 2023, 403, 134375. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shao, D.; Wang, J.; Zheng, X.; Yang, Z.; Wang, A.; Chen, Z.; Gao, Y. Pre-ligand-induced porous MOF as a peroxidase mimic for electrochemical analysis of deoxynivalenol (DON). Food Chem. 2025, 480, 143860. [Google Scholar] [CrossRef]
- Yang, Y.; Du, K.; Liu, M.; He, X.; Li, H.; Li, H.; Li, X. A computer-assisted design of poly(deep eutectic solvent)@MIL-101-NH2(Cr) imprinting strategy: Selective removal of zearalenone from coix seeds. Food Chem. 2025, 491, 145219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Q.; Guo, W.; Guo, D.; Han, Z.; Nie, D. Fe3O4@COF(TAPT–DHTA) nanocomposites as magnetic solid-phase extraction adsorbents for simultaneous determination of 9 mycotoxins in fruits by UHPLC–MS/MS. Toxins 2023, 15, 117. [Google Scholar] [CrossRef]
- Tang, Z.T.; Han, Q.R.; Yu, G.; Liu, F.; Tan, Y.Z.; Peng, C. Fe3O4@PDA/MIL-101(Cr) as magnetic solid-phase extraction sorbent for mycotoxins in licorice prior to ultrahigh-performance liquid chromatography-tandem mass spectrometry analysis. Food Sci. Nutr. 2022, 10, 2224–2235. [Google Scholar] [CrossRef]
- García-Nicolás, M.; Arroyo-Manzanares, N.; Viñas, P. Dispersive magnetic solid-phase extraction as a novelty sample treatment for the determination of the main aflatoxins in paprika. Toxins 2023, 15, 160. [Google Scholar] [CrossRef]
- Cavaliere, C.; Antonelli, M.; Cerrato, A.; La Barbera, G.; Laganà, A.; Laus, M.; Piovesana, S.; Capriotti, A.L. A novel magnetic molecular imprinted polymer for selective extraction of zearalenone from cereal flours before liquid chromatography-tandem mass spectrometry determination. Toxins 2019, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, J.; Zheng, S.; Guo, M.; Xu, J.; Deng, Q.; Wang, X. Effective extraction and detection of aflatoxins in cereals using nitrogen-rich benzodiimidazole linkage magnetic covalent organic framework based solid phase extraction and HPLC-MS/MS analysis. Food Chem. X 2024, 24, 101797. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Mi, X.; Qian, J.; Ma, N.; Dai, W. Modular construction of an MIL-101(Fe)@MIL-100(Fe) dual-compartment nanoreactor and its boosted photocatalytic activity toward tetracycline. ACS Appl. Mater. Interfaces 2022, 14, 48285–48295. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, L.-Q.; Liu, F.; Sun, Q.-Y.; Zhao, Y.-F.; Wang, X.-L.; Li, N.; Jiang, H.-L.; Chen, X.-F.; Wang, M.-L.; et al. Facile synthesis of hydroxylated triazine-based magnetic microporous organic network for ultrahigh adsorption of phenylurea herbicides: An experimental and density-functional theory study. J. Hazard. Mater. 2024, 465, 133468. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Linear Range (µg/L) | Correlation Coefficient (R2) | Linear Fit Equation | LOD (µg/L) | LOQ (µg/L) | EFs | RSD(%) (n = 5) | |
|---|---|---|---|---|---|---|---|---|
| Intraday | Interday | |||||||
| AFB1 | 0.05–20 | 0.9989 | y = −1100.5 + 297596.9x | 0.005 | 0.015 | 21.3 | 3.0 | 3.3 |
| AFB2 | 0.01–20 | 0.9983 | y = 142.3 + 216831.1x | 0.002 | 0.007 | 20.8 | 4.5 | 4.2 |
| AFG1 | 0.02–50 | 0.9985 | y = −591.0 + 146631.9x | 0.005 | 0.016 | 21.8 | 3.9 | 3.6 |
| AFG2 | 0.05–50 | 0.9981 | y = 958.1 + 80828.9x | 0.010 | 0.031 | 20.9 | 3.3 | 3.7 |
| T-2 | 0.10–50 | 0.9956 | y = 188.8 + 221829.9x | 0.020 | 0.060 | 21.6 | 1.9 | 2.6 |
| OTA | 0.20–100 | 0.9996 | y = 3428.8 + 9770.5x | 0.050 | 0.150 | 21.7 | 5.6 | 5.8 |
| ZEN | 0.05–100 | 0.9969 | y = −231.5 + 62135.4x | 0.010 | 0.030 | 20.1 | 2.3 | 2.1 |
| Mycotoxins | Found (μg/kg) ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| Arecae Nut | Coix Seed | Platycladi Seed | Spine Date Seed | Barley | Malt | Peanut | Corn | |
| AFB1 | 0.41 ± 0.13 | 0.40 ± 0.15 | ND | ND | ND | ND | 0.54 ± 0.21 | ND |
| AFB2 | ND | ND | 0.37 ± 0.14 | ND | 0.04 ± 0.03 | ND | ND | ND |
| AFG1 | ND | ND | ND | ND | ND | ND | ND | ND |
| AFG2 | 0.28 ± 0.07 | ND | ND | ND | ND | ND | ND | ND |
| T-2 | ND | ND | ND | ND | ND | ND | ND | ND |
| OTA | 0.12 ± 0.04 | ND | ND | ND | ND | ND | ND | ND |
| ZEN | ND | ND | ND | ND | ND | ND | ND | 0.87 ± 0.27 |
| Mycotoxins | Spiked (μg/L) | Recovery ± SD (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Areca Nut | Coix Seed | Platycladi Seed | Spine Date Seed | Barley | Malt | Peanut | Corn | ||
| AFB1 | 0.5 | 106.33 ± 4.55 | 105.59 ± 8.16 | 90.89 ± 2.56 | 81.32 ± 6.49 | 82.55 ± 3.37 | 82.53 ± 6.56 | 85.01 ± 7.82 | 88.17 ± 8.7 |
| 5 | 102.07 ± 0.80 | 100.72 ± 2.79 | 95.42 ± 2.20 | 91.61 ± 3.36 | 91.59 ± 8.28 | 85.36 ± 5.43 | 93.16 ± 9.98 | 86.31 ± 4.39 | |
| 50 | 87.15 ± 1.62 | 89.69 ± 1.68 | 98.19 ± 2.14 | 94.52 ± 2.06 | 92.07 ± 4.28 | 90.88 ± 1.72 | 93.44 ± 3.25 | 92.48 ± 13.78 | |
| AFB2 | 0.5 | 105.30 ± 5.13 | 105.59 ± 8.16 | 87.53 ± 3.93 | 81.07 ± 2.07 | 92.94 ± 1.73 | 91.42 ± 1.57 | 94.61 ± 1.48 | 86.25 ± 6.61 |
| 5 | 98.07 ± 0.95 | 96.82 ± 1.76 | 96.52 ± 2.11 | 90.04 ± 2.07 | 91.48 ± 2.35 | 83.66 ± 5.22 | 89.69 ± 9.96 | 83.72 ± 4.45 | |
| 50 | 86.53 ± 2.57 | 85.57 ± 1.73 | 105.24 ± 3.37 | 96.25 ± 10.45 | 99.91 ± 4.61 | 90.74 ± 1.91 | 90.56 ± 10.02 | 98.4 ± 11.35 | |
| AFG1 | 0.5 | 108.58 ± 6.77 | 104.29 ± 2.69 | 87.89 ± 3.65 | 81.15 ± 7.12 | 89.75 ± 9.37 | 90.3 ± 11.53 | 91.79 ± 12.63 | 94.73 ± 10.13 |
| 5 | 103.01 ± 3.49 | 102.45 ± 2.05 | 101.38 ± 2.42 | 99.92 ± 1.62 | 88.96 ± 1.47 | 91.43 ± 4.34 | 86.76 ± 6.62 | 81.77 ± 3.35 | |
| 50 | 90.63 ± 2.55 | 90.07 ± 2.24 | 97.30 ± 2.69 | 101.52 ± 3.19 | 89.03 ± 4.24 | 88.35 ± 1.59 | 87.18 ± 7.72 | 94.98 ± 10.11 | |
| AFG2 | 0.5 | 92.57 ± 5.74 | 84.72 ± 8.05 | 85.08 ± 4.64 | 86.81 ± 10.98 | 90.57 ± 11.87 | 92.92 ± 8.89 | 88.65 ± 12.52 | 91.22 ± 9.78 |
| 5 | 97.11 ± 2.73 | 92.22 ± 4.02 | 95.13 ± 3.30 | 96.5 ± 2.32 | 88.41 ± 9.83 | 90.18 ± 3.33 | 84.76 ± 1.18 | 90.53 ± 3.55 | |
| 50 | 96.16 ± 3.02 | 94.21 ± 1.43 | 94.44 ± 3.27 | 98.15 ± 2.7 | 88.71 ± 3.22 | 87.13 ± 1.98 | 95.46 ± 8.89 | 83.09 ± 9.39 | |
| T-2 | 0.5 | 87.04 ± 11.96 | 99.66 ± 9.53 | 86.35 ± 2.71 | 95.32 ± 3.34 | 84.34 ± 2.43 | 93.99 ± 7.76 | 96.4 ± 4.91 | 86.7 ± 7.31 |
| 5 | 96.01 ± 6.33 | 111.81 ± 4.01 | 86.74 ± 2.65 | 96.47 ± 1.12 | 93.95 ± 10.72 | 87.49 ± 2.12 | 88.06 ± 1.19 | 96.2 ± 3.51 | |
| 50 | 100.69 ± 1.45 | 97.06 ± 2.17 | 91.24 ± 5.20 | 94.27 ± 6.35 | 94.56 ± 1.98 | 94.66 ± 6.34 | 98.54 ± 2.21 | 86.37 ± 6.01 | |
| OTA | 0.5 | 89.74 ± 12.58 | 96.55 ± 18.38 | 87.76 ± 7.63 | 89.17 ± 8.83 | 86.12 ± 4.13 | 84.08 ± 4.07 | 86.4 ± 4.80 | 93.85 ± 6.13 |
| 5 | 103.86 ± 9.06 | 105.94 ± 1.88 | 90.17 ± 1.35 | 86.63 ± 3.99 | 87.78 ± 5.22 | 91.75 ± 2.79 | 90.56 ± 1.41 | 89.42 ± 2.34 | |
| 50 | 87.72 ± 4.90 | 88.15 ± 3.30 | 103.66 ± 2.86 | 101.94 ± 10.03 | 95.83 ± 2.13 | 86.02 ± 4.30 | 96.39 ± 2.74 | 88.25 ± 4.5 | |
| ZEN | 0.5 | 99.25 ± 18.19 | 116.10 ± 15.58 | 93.97 ± 4.57 | 93.42 ± 5.77 | 105.1 ± 12.21 | 92.99 ± 10.32 | 91.78 ± 4.08 | 91.42 ± 12.93 |
| 5 | 93.24 ± 5.70 | 111.62 ± 6.01 | 107.32 ± 4.06 | 98.61 ± 3.49 | 89.94 ± 10.01 | 84.76 ± 6.37 | 91.22 ± 9.39 | 96.02 ± 6.11 | |
| 50 | 103.17 ± 4.15 | 103.57 ± 4.35 | 91.63 ± 3.67 | 94.77 ± 9.01 | 89.90 ± 3.56 | 98.94 ± 1.18 | 88.65 ± 7.78 | 87.21 ± 11.05 | |
| Adsorbent | Method | Matrix | Analytes | Linear Range (µg/L) | Recovery (%) | LOD (µg/L) | Equilibrium Time(s) | Reusability (Cycle) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4@COF | MSPE-UHPLC-MS/MS | tomato, strawberry, watermelon, melon, hawthorn | AFB1, AFB2, AFG1, AFG2, OTA, OTB, ZEN | 0.05–200 | 74.25–111.75 | 0.01–0.5 | 480 | 1 | [47] |
| MIL-101(Cr)@Fe3O4 | MSPE-UHPLC-MS/MS | Maize, wheat, watermelon, melon | AFB1, AFB2, AFG1, AFG2, OTA, OTB, T-2, HT-2, DAS | 0.2–100 | 83.50–108.50 | 0.02–0.06 | 240 | Not mentioned | [19] |
| Fe3O4@PDA/MIL-101(Cr) | MSPE-UHPLC-MS/MS | Licorice | AFB1, AFG1, STE, ZEN, OTA | 0.5–50 | 78.53–116.28 | 0.01–0.09 | 1800 | 5 | [48] |
| Fe3O4@PPy | DMSPE- UHPLC-HRMS | Paprika | AFG1, AFG2, AFB1, AFB2 | 3.5–50 | 81.90–99.40 | 1.0–1.4 | 1800 | 5 | [49] |
| mMIP | SPE-HPLC-MS/MS | wheat, maize | ZEN | 5–300 | 76.00–98.00 | 0.044 | 900 | Not mentioned | [50] |
| Fe3O4@BB-COF | MSPE-UHPLC-MS/MS | soybean, rice, Corn, brown rice and buckwheat | AFB1, AFB2, AFG1, AFG2, AFM1 | 0.05–20 | 76.80–97.10 | 0.01–0.45 | 60 | 6 | [51] |
| MMON | MSPE-UPLC-MS/MS | Arecae nut, Coix seed, Platycladi Seed, Spine Date Seed, Barley, Malt, Peanut, Corn | AFB1 AFB2 AFG1 AFG2 T-2 ZEN OTA | 0.01–100 | 81.32–116.10 | 0.002–0.15 | 10 | 10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, J.; Wang, Y.-X.; Kong, D.-D.; Lv, J.-X.; Zhang, Y.-Y.; Li, X.-L.; Kang, X.-X.; Guo, M.-Y.; Luo, J.-Y.; et al. Preparation and Application of Magnetic Microporous Organic Networks for Rapid Adsorption Enrichment of Multiple Mycotoxins in Complex Food Matrices. Foods 2025, 14, 3984. https://doi.org/10.3390/foods14233984
Wang C, Zhang J, Wang Y-X, Kong D-D, Lv J-X, Zhang Y-Y, Li X-L, Kang X-X, Guo M-Y, Luo J-Y, et al. Preparation and Application of Magnetic Microporous Organic Networks for Rapid Adsorption Enrichment of Multiple Mycotoxins in Complex Food Matrices. Foods. 2025; 14(23):3984. https://doi.org/10.3390/foods14233984
Chicago/Turabian StyleWang, Chuang, Jing Zhang, Yu-Xin Wang, Dan-Dan Kong, Jian-Xin Lv, Yuan-Yuan Zhang, Xue-Li Li, Xin-Xin Kang, Meng-Yue Guo, Jiao-Yang Luo, and et al. 2025. "Preparation and Application of Magnetic Microporous Organic Networks for Rapid Adsorption Enrichment of Multiple Mycotoxins in Complex Food Matrices" Foods 14, no. 23: 3984. https://doi.org/10.3390/foods14233984
APA StyleWang, C., Zhang, J., Wang, Y.-X., Kong, D.-D., Lv, J.-X., Zhang, Y.-Y., Li, X.-L., Kang, X.-X., Guo, M.-Y., Luo, J.-Y., & Yang, M.-H. (2025). Preparation and Application of Magnetic Microporous Organic Networks for Rapid Adsorption Enrichment of Multiple Mycotoxins in Complex Food Matrices. Foods, 14(23), 3984. https://doi.org/10.3390/foods14233984







