Ultrasound-Assisted Fibril Formation Enhances Complexation of Oat Globulin with Quercetin: Mechanism, Structure Evolution, Delivery Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of OG Fibrils
2.3. Characteristics of OG Fibrils
2.3.1. Analysis of Fluorescence Quenching Binding Mechanism
2.3.2. Synchronous Fluorescence Spectroscopy
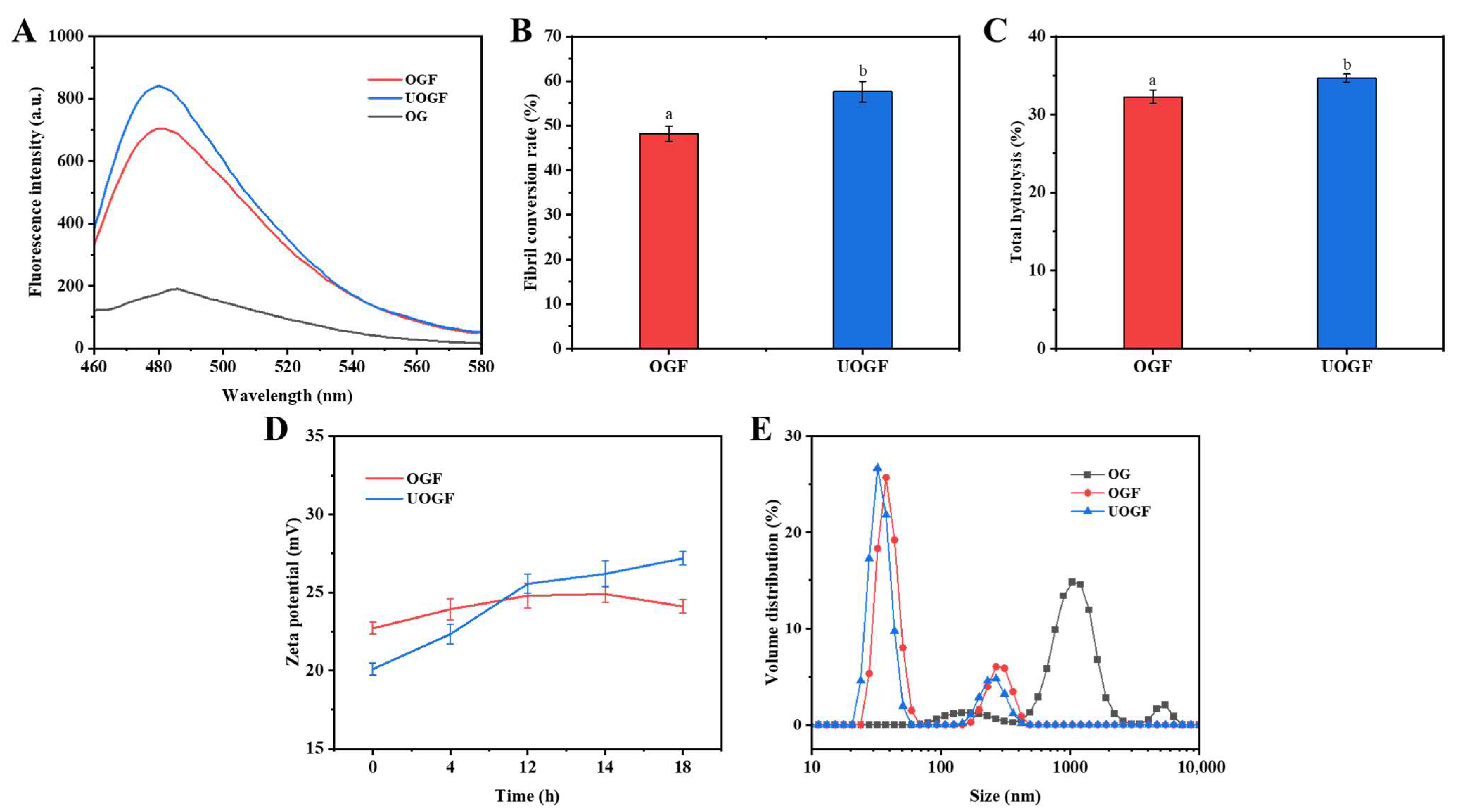
2.3.3. Determination of Fibril Conversion Rate
2.3.4. Determination of Hydrolysis
2.4. Preparation of Oat Protein/Fibril Complexes with Quercetin
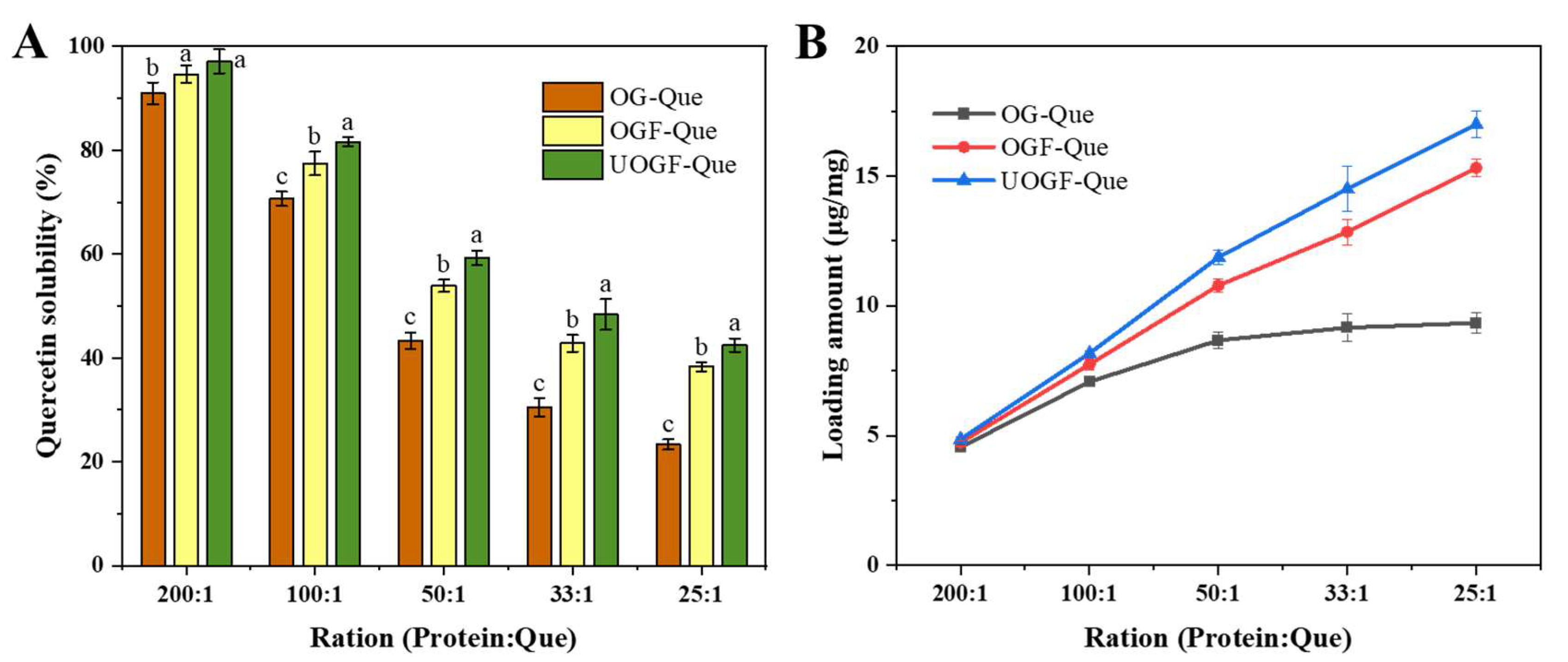
2.5. Determination of Encapsulation Efficiency and Loading Capacity
2.6. Characterization of Cross-β Structural Changes in Fibrils
2.7. ATR-FTIR Spectroscopy
2.8. Size Distribution and Zeta-Potential Measurement
2.9. Transmission Electron Microscopy (TEM)
2.10. Stability Analysis of Quercetin
2.11. In Vitro Digestion Characteristics
2.12. Cytotoxicity Assessment
2.13. Statistical Analysis
3. Results and Discussion
3.1. Interaction Mechanism During Complex Formation
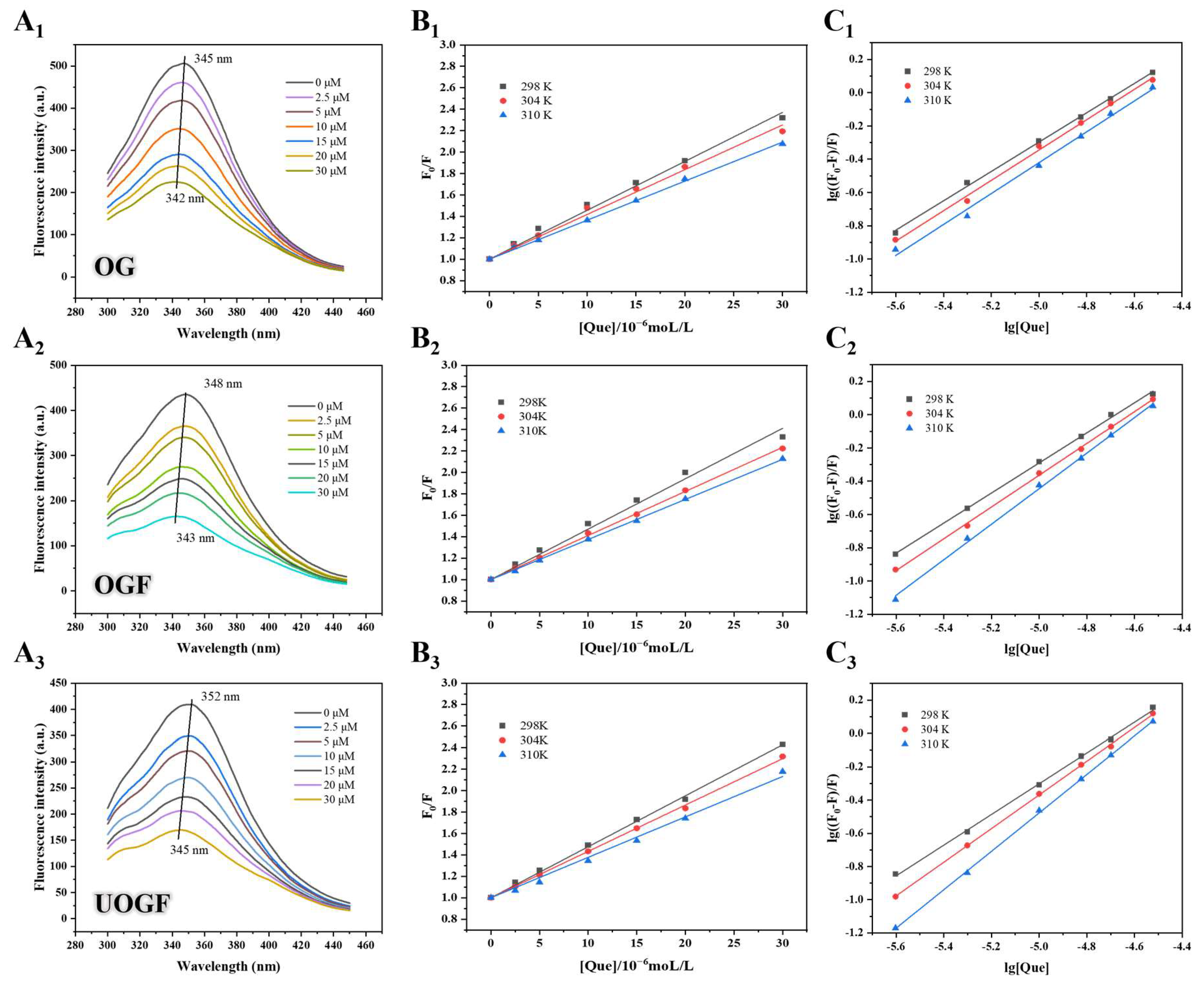
3.1.1. Intrinsic Fluorescence Spectra Analysis
3.1.2. Fluorescence Quenching Mechanisms
3.1.3. Binding Mechanism of the Complex
3.1.4. Thermodynamic Parameter Analysis
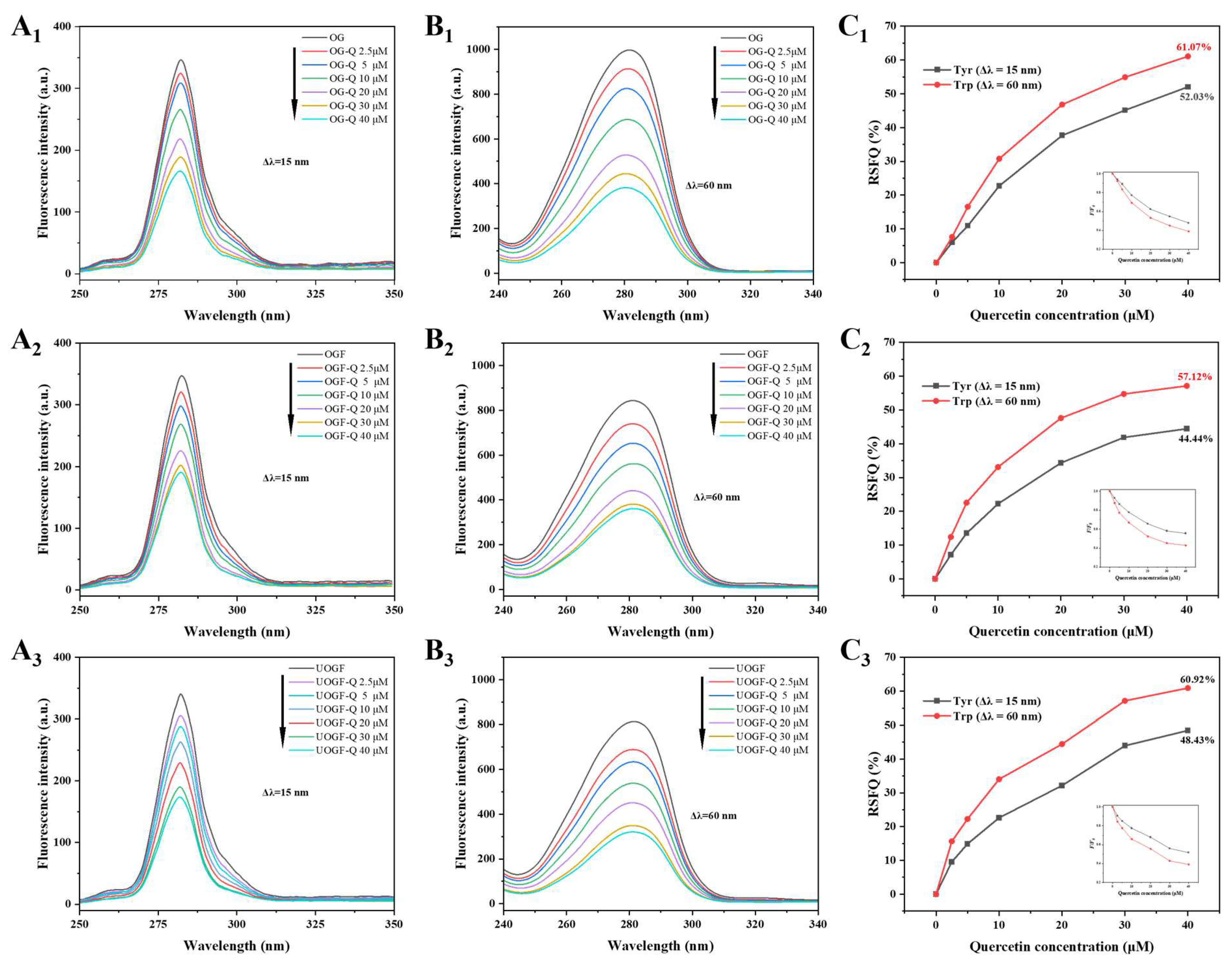
3.1.5. Synchronous Fluorescence Spectra
3.1.6. Differential Fibrillation of OG Induced by Ultrasound Pretreatment
3.2. Delivery Performance for Quercetin
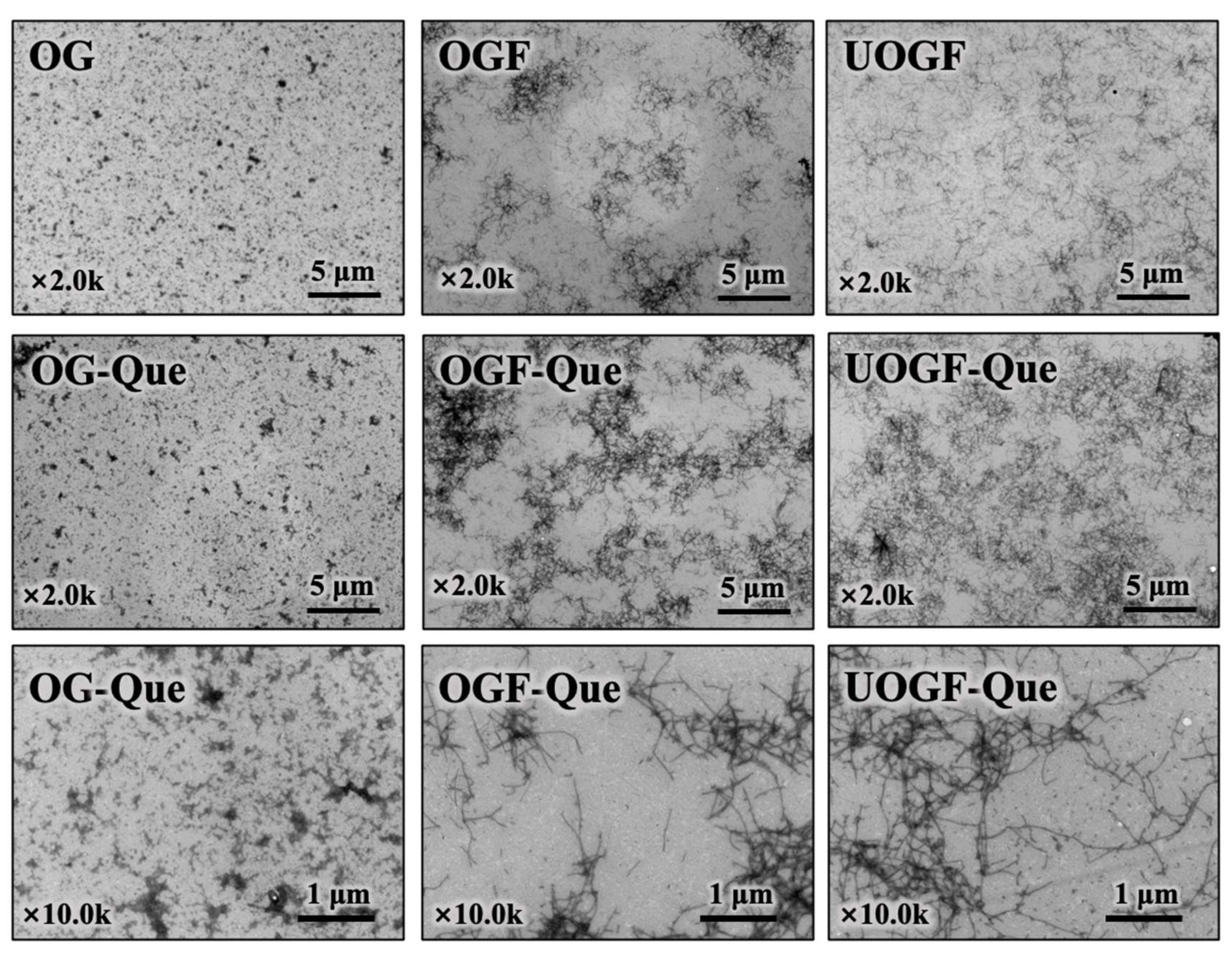
3.3. Transmission Electron Microscopy Analysis
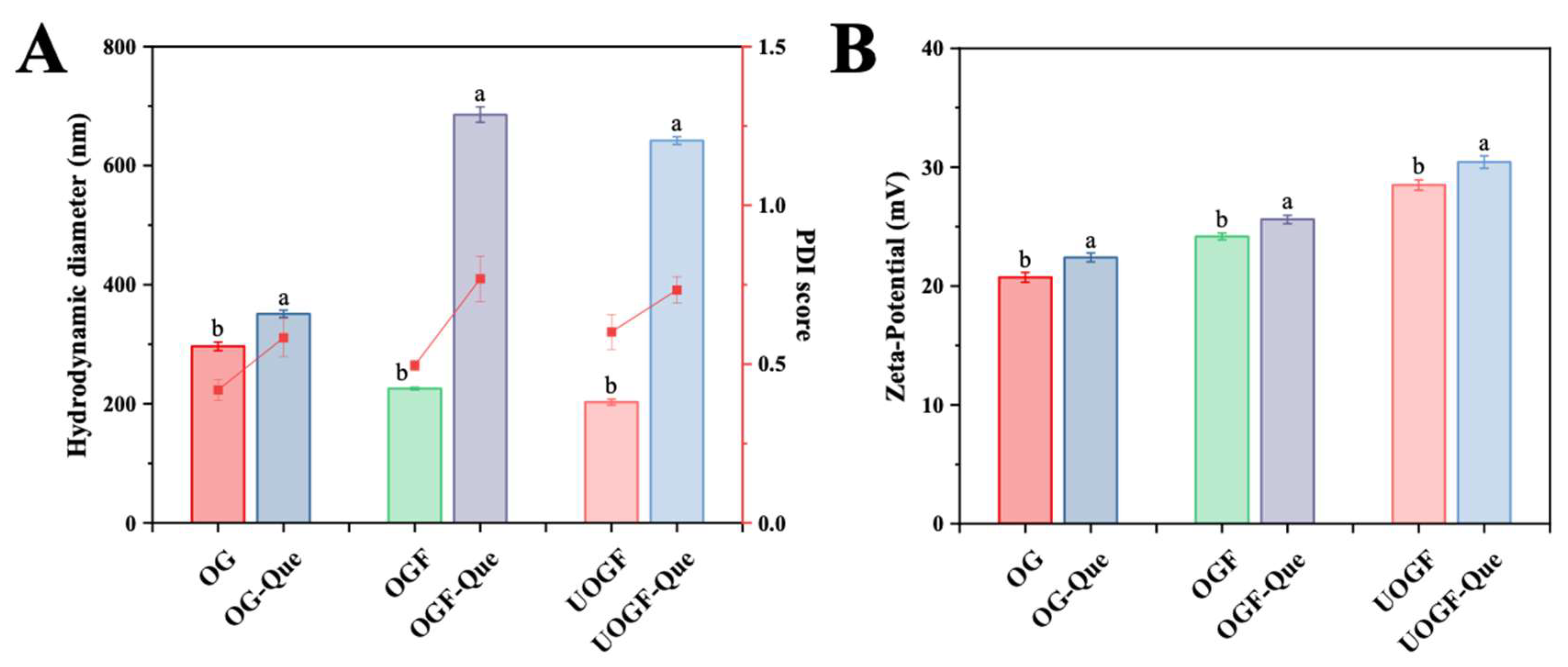
3.4. Hydrodynamic Diameter and Zeta-Potential Analysis
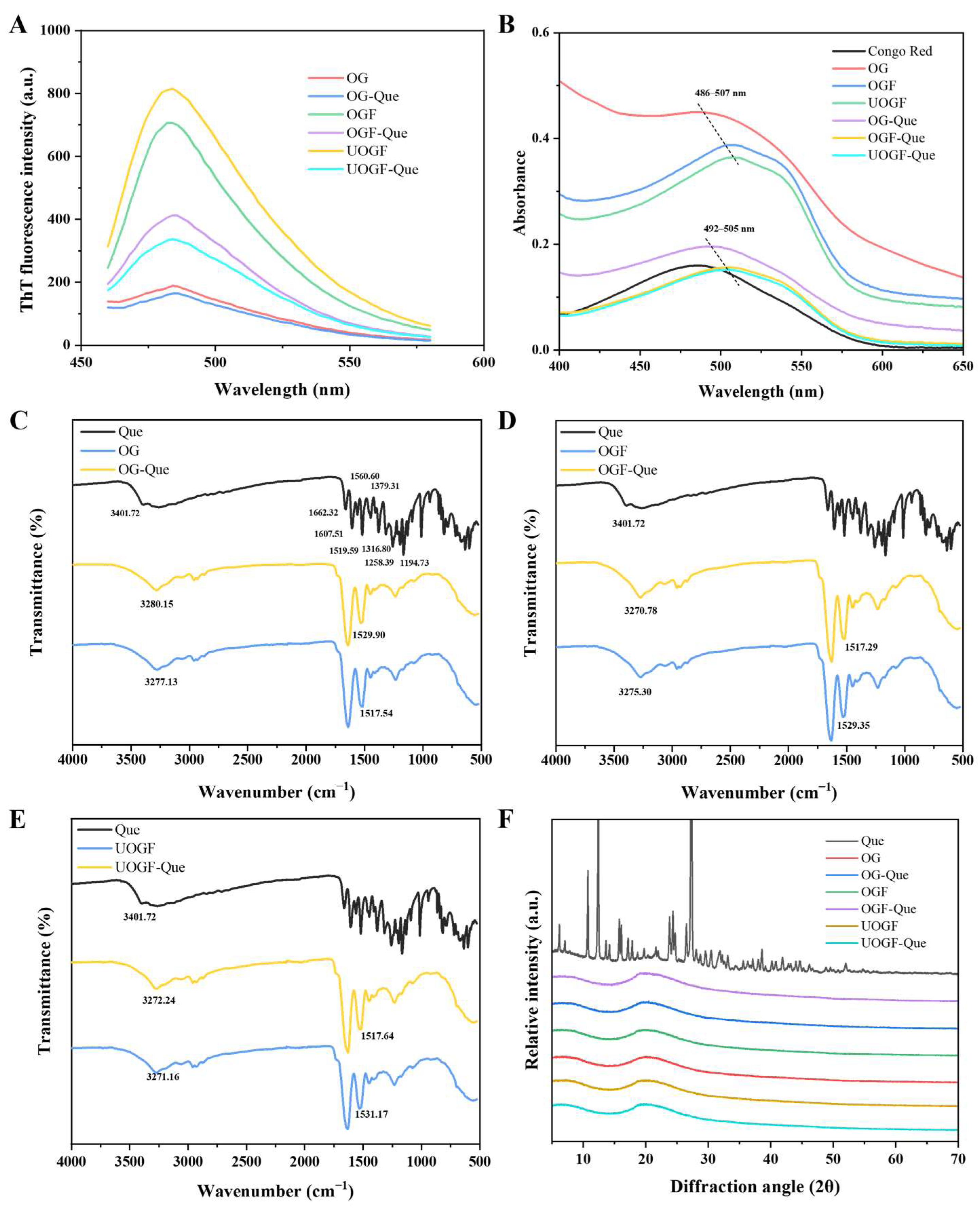
3.5. Changes in Fibril Cross-β Structures by Que
3.6. Fourier-Transform Infrared Spectroscopy Analysis
3.7. X-Ray Diffraction Analysis
3.8. Functional Characteristic Evaluation
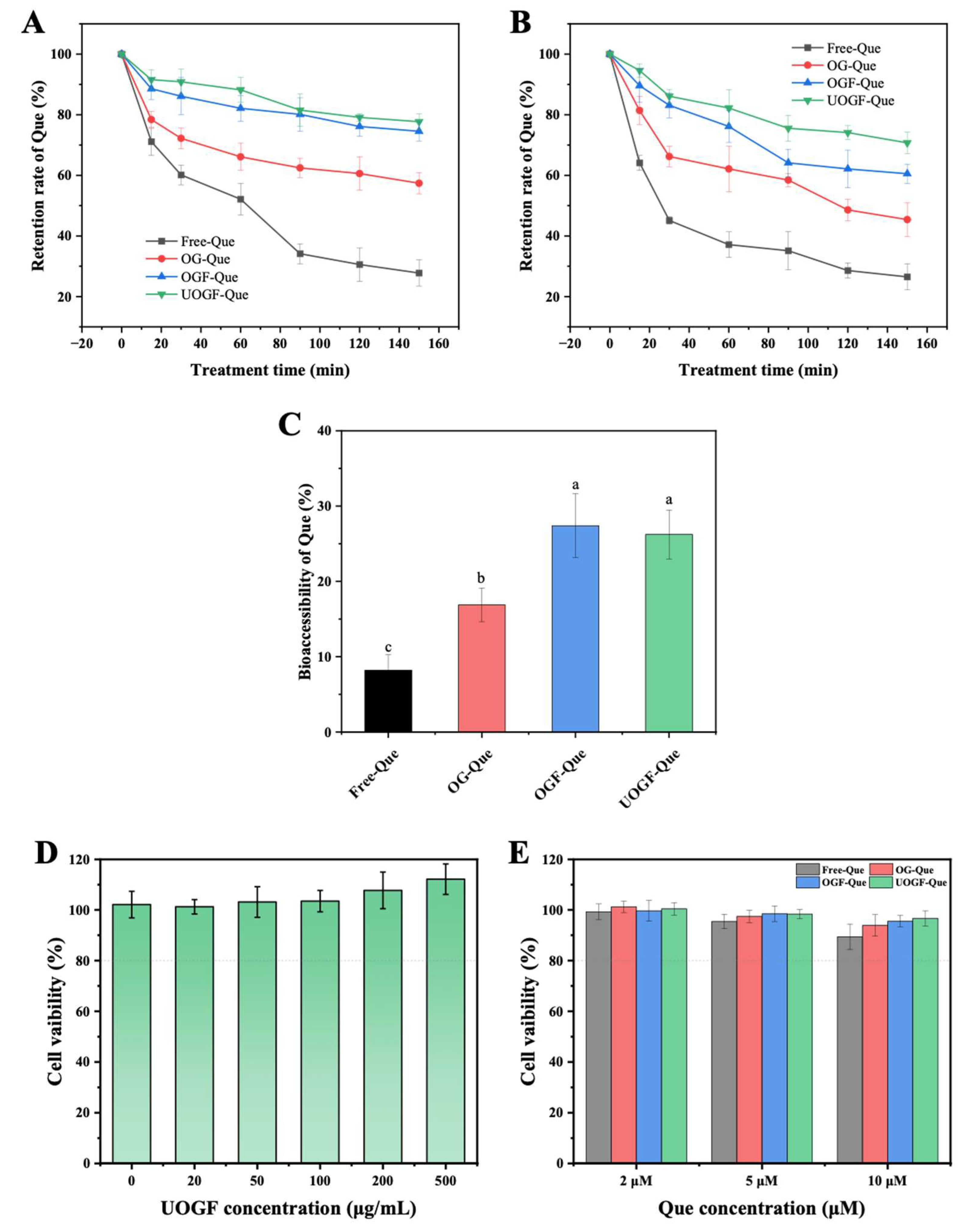
3.8.1. Environmental Stability Assessment
3.8.2. Bioaccessibility Performance of Que
3.8.3. Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, W.F.; Wong, W.T. Design and optimization of quercetin-based functional foods. Crit. Rev. Food Sci. 2022, 62, 7319–7335. [Google Scholar] [CrossRef]
- Najaf Najafi, N.; Armide, N.; Akbari, A.; Baradaran Rahimi, V.; Askari, V.R. Quercetin a promising functional food additive against allergic Diseases: A comprehensive and mechanistic review. J. Funct. Foods 2024, 116, 106152. [Google Scholar] [CrossRef]
- Rajesh, R.U.; Dhanaraj, S. A critical review on quercetin bioflavonoid and its derivatives: Scope, synthesis, and biological applications with future prospects. Arab. J. Chem. 2023, 16, 104881. [Google Scholar] [CrossRef]
- Vollmannová, A.; Bojnanská, T.; Lidiková, J.; Musilová, J.; Cifrová, M. Quercetin as one of the most abundant represented biological valuable plant components with remarkable chemoprotective effects—A review. Heliyon 2024, 10, e33342. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regue, J.; Garcia-Sala, X.; Boix Montanes, A.; Lamuela-Raventos, R.M. In Vivo Anti-inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. Evid. Based Complement. Alternat Med. 2020, 2020, 5926381. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, S.; Chen, T.; Li, Y.; Chi, X.; Yu, S.; Wang, W.; Liu, D.; Zhu, B.; Hu, J. Innovative delivery strategies for quercetin: A comprehensive review of advances and challenges. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70146. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kilic, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, P.; Ban, Q. Green development strategy for efficient quercetin- loaded whey protein complex: Focus on quercetin loading characteristics, component interactions, stability, antioxidant, and in vitro digestive properties. Food Chem. 2025, 472, 142939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, S.; Yang, Z.; Chen, T.; Chi, X.; Wu, D.; Wang, W.; Liu, D.; Zhu, B.; Hu, J. Targeted quercetin delivery nanoplatform via folic acid-functionalized metal-organic framework for alleviating ethanol-induced gastric ulcer. Chem. Eng. J. 2024, 498, 155700. [Google Scholar] [CrossRef]
- Mohammadian, M.; Moghaddam, A.D.; Sharifan, A.; Dabaghi, P.; Hadi, S. Nanocomplexes of whey protein fibrillar aggregates and quercetin as novel multi-functional biopolymeric ingredients: Interaction, chemical structure, and bio-functionality. J. Iran. Chem. Soc. 2020, 17, 2481–2492. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ma, Y.D.; Jiang, Y.; Zhou, F.; Wu, Y.L.; Jiang, H.T.; Wang, R.L.; Xu, Q.; Hua, C. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Lan, T.; Dong, Y.; Xu, Z.; Zhang, Y.; Jiang, L.; Zhou, W.; Sui, X. Quercetin directed transformation of calcium carbonate into porous calcite and their application as delivery system for future foods. Biomaterials 2023, 301, 122216. [Google Scholar] [CrossRef]
- Gao, J.; Tan, X.; Dai, H.; Wang, H.; Chen, H.; Zhang, Y. Properties regulation and mechanism on ferritin/chitooligosaccharide dual-compartmental emulsions and its application for co-encapsulation of curcumin and quercetin bioactive compounds. Food Chem. 2024, 458, 140243. [Google Scholar] [CrossRef]
- Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics 2022, 14, 1601. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Tang, M.; Ban, Q.; Zhao, R.; An, J.; Wang, M. Tannic acid-mediated reconfiguration of oat globulin fibril-based hydrogels for quercetin encapsulation: Construction, mechanism and performance. Food Chem. X 2025, 30, 102930. [Google Scholar] [CrossRef]
- Sun, Z.; Li, D.; Lin, P.; Zhao, Y.; Zhang, J.; Sergeeva, I.; Li, Y.; Zheng, H. Preparation, characterization, and binding mechanism of quercetin-loaded composite nanoparticles based on zein-soybean protein isolate. Food Chem. 2025, 463, 141359. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, T.; Hu, S.; Chi, X.; Yu, S.; Li, Y.; Wang, W.; Zhu, B.; Liu, D.; Hu, J. Orally administered metal–organic framework nanocubes for sequence-targeted quercetin delivery in colitis alleviation. Chem. Eng. J. 2025, 507, 160373. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Technological functionality and biological properties of food protein nanofibrils formed by heating at acidic condition. Trends Food Sci. Tech. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Guan, C.; Wang, C.Y.; Fu, S.X. Food Protein Nanofibril Gels: From Conditions, Types and Properties to Applications. Foods 2024, 13, 2173. [Google Scholar] [CrossRef]
- Peng, J.; Simon, J.R.; Venema, P.; van der Linden, E. Protein Fibrils Induce Emulsion Stabilization. Langmuir 2016, 32, 2164–2174. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Xu, X. Effect of ultrasound pretreatment on the fibrillization of oat globulins: Aggregation kinetics, structural evolution, and core composition. Food Hydrocoll. 2025, 165, 111233. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid. Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Yang, Q.; Jiang, Y.X.; Chen, H.Q. Enhanced solubility, thermal stability and antioxidant activity of resveratrol by complexation with ovalbumin amyloid-like fibrils: Effect of pH. Food Hydrocoll. 2024, 148, 109463. [Google Scholar] [CrossRef]
- Eisenberg, D.S.; Sawaya, M.R. Structural Studies of Amyloid Proteins at the Molecular Level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef]
- Wang, F.; Lin, L.; Wang, X.; Zhang, L.; Tao, N. Astaxanthin complexes of five plant protein fibrils: Aggregation behavior, interaction, and delivery properties. Food Hydrocoll. 2025, 162, 110995. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Wang, D.; Xie, Q.; Xu, X. Exploring the self-assembly journey of oat globulin fibrils: From structural evolution to modified functionality. Food Hydrocoll. 2024, 149, 109587. [Google Scholar] [CrossRef]
- Spaen, J.; Silva, J.V.C. Oat proteins: Review of extraction methods and techno-functionality for liquid and semi-solid applications. LWT 2021, 147, 111478. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Zhao, M.; Yan, B.; Zhang, Y.; Gao, X.; Fan, J.; Wang, M. Transition metal ion-catalyzed assembly of oat peptides into amyloid fibrils: Feasibility, physicochemical properties, and chitosan film reinforcement. Food Hydrocoll. 2025, 169, 111628. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Wang, Q.; Wang, M.; Everett, D.W.; Huang, M.; Zhai, Y.; Li, T.; Fu, Y. Amyloid fibrils for beta-carotene delivery—Influence of self-assembled structures on binding and in vitro release behavior. Food Chem. 2025, 464, 141849. [Google Scholar] [CrossRef]
- Ouyang, K.; Xie, H.; Wang, Y.; Woo, M.W.; Chen, Q.; Lai, S.; Xiong, H.; Zhao, Q. Whey protein isolate nanofibrils formed with phosphoric acid: Formation, structural characteristics, and emulsion stability. Food Hydrocoll. 2023, 135, 108170. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Guo, R.; Xu, H.; Jin, C. Structural modification of whey protein nanofibrils by a multiround induction pathway for enhancing the stability of Pickering emulsions. Food Hydrocoll. 2024, 150, 109703. [Google Scholar] [CrossRef]
- Ren, C.; Xiong, W.; Li, B. Binding interaction between β-conglycinin/glycinin and cyanidin-3-O-glucoside in acidic media assessed by multi-spectroscopic and thermodynamic techniques. Int. J. Biol. Macromol. 2019, 137, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, L.; Gilbert, E.P.; Loo, T.S.; Lee, S.J.; Harrison, J.; Yang, Z. Fibrillisation of faba bean protein isolate by thermosonication for process efficacy: Microstructural characteristics, assembly behaviour, and physicochemical properties. Food Hydrocoll. 2024, 154, 110127. [Google Scholar] [CrossRef]
- Zong, D.; Zhao, H.; Li, X.; Yi, S.; Li, J.; Xu, Y. Ultrasound-assisted heat treatment: Accelerating rice glutelin fibrils formation and enhancing emulsifying properties. Int. J. Biol. Macromol. 2025, 298, 139942. [Google Scholar] [CrossRef]
- Marinea, M.; Lopez-Sanchez, P.; Ortiz, D.; Larsson, K.; Ström, A. Oat protein in vitro digestion is not influenced by pectin in dispersion or gel systems. Food Hydrocoll. 2025, 163, 111108. [Google Scholar] [CrossRef]
- Ji, F.; Wang, Z.; Bai, X.; Zhao, Y.; Zhong, X.; Luo, S.; Shen, Y.; Jiang, S.; Zheng, Z. Ultrasound-treated soy protein fibrils: A potential vehicle for curcumin with improved water solubility, antioxidant activity and sustained-release property. Food Hydrocoll. 2023, 143, 108929. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Hosseini, S.M.H.; Nekoei, A.R. Efficient delivery of quercetin after binding to beta-lactoglobulin followed by formation soft-condensed core-shell nanostructures. Food Chem. 2017, 233, 282–289. [Google Scholar] [CrossRef]
- Li, N.; Cui, Y.F.; Liu, Y.A.; Zhang, M.Y.; Wang, Y.C.; Shi, J.H.; Wang, X.B.; Xu, N.; Chen, Q.S. Mechanism of interaction between astaxanthin and soy protein fibrils: Effects on complexes structure, rheological properties and bioaccessibility. Food Hydrocoll. 2024, 146, 109227. [Google Scholar] [CrossRef]
- Zhang, Y.; Dee, D.R. Morphology, Formation Kinetics and Core Composition of Pea and Soy 7S and 11S Globulin Amyloid Fibrils. J. Agric. Food Chem. 2023, 71, 4755–4765. [Google Scholar] [CrossRef]
- Zhou, J.; Li, T.; Peydayesh, M.; Usuelli, M.; Lutz-Bueno, V.; Teng, J.; Wang, L.; Mezzenga, R. Oat Plant Amyloids for Sustainable Functional Materials. Adv. Sci. 2022, 9, e2104445. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Bhutto, R.A.; Bhutto, N.U.A.H.; Fan, Y.; Yi, J. Formation, physiochemical stability, and bioaccessibility of quercetin-loaded α-lactalbumin amyloid-like fibril nanocomposite with ultrasound and its application in yogurt. Food Hydrocoll. 2024, 157, 110435. [Google Scholar] [CrossRef]
- Fan, Y.; Gan, C.; Zhang, H.; Yi, J. Characteristics, physicochemical stability and in vitro release of curcumin-loaded glycated bovine serum albumin nanofibrils: Effects of molecular weight of saccharide. Food Hydrocoll. 2024, 155, 110210. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.; Li, S.; Deng, N.; Zhang, Y.; Fang, Y. Fibrillization kinetics and rheological properties of panda bean (Vigna umbellata (Thunb.) Ohwi et Ohashi) protein isolate at pH 2.0. Int. J. Biol. Macromol. 2023, 228, 816–825. [Google Scholar] [CrossRef]
- Cui, Q.; Dong, Y.; Zhang, A.; Wang, X.; Zhao, X.-H. Multiple spectra analysis and calculation of the interaction between Anthocyanins and whey protein isolate. Food Biosci. 2021, 44, 101353. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.M.; Yang, M. Effect of change in pH, heat and ultrasound pre-treatments on binding interactions between quercetin and whey protein concentrate. Food Chem. 2022, 384, 132508. [Google Scholar] [CrossRef]
- Khan, S.N.; Islam, B.; Yennamalli, R.; Sultan, A.; Subbarao, N.; Khan, A.U. Interaction of mitoxantrone with human serum albumin: Spectroscopic and molecular modeling studies. Eur. J. Pharm. Sci. 2008, 35, 371–382. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.; Qi, W.; Su, R.; He, Z. Affinity of rosmarinic acid to human serum albumin and its effect on protein conformation stability. Food Chem. 2016, 192, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, Q. Binding between Bixin and Whey Protein at pH 7.4 Studied by Spectroscopy and Isothermal Titration Calorimetry. J. Agric. Food Chem. 2012, 60, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhou, Z. Ellagic acid can act as a chaperone and suppress the heat-induced amyloid-like aggregation of ovalbumin. Food Hydrocoll. 2020, 100, 105408. [Google Scholar] [CrossRef]
- Bose, A. Interaction of tea polyphenols with serum albumins: A fluorescence spectroscopic analysis. J. Lumin. 2016, 169, 220–226. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, L.; Pan, J. Probing the Binding of the Flavonoid Diosmetin to Human Serum Albumin by Multispectroscopic Techniques. J. Agric. Food Chem. 2012, 60, 2721–2729. [Google Scholar] [CrossRef]
- Tian, T.; Liu, S.; Li, L.; Wang, S.; Cheng, L.; Feng, J.; Wang, Z.; Tong, X.; Wang, H.; Jiang, L. Soy protein fibrils–β-carotene interaction mechanisms: Toward high nutrient plant-based mayonnaise. LWT 2023, 184, 114870. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Liao, L.; Julian McClements, D.; Chen, X.; Zhu, Y.; Liu, Y.; Liang, R.; Zou, L.; Liu, W. Dietary proteins as excipient ingredients for improving the solubility, stability, and bioaccessibility of quercetin: Role of intermolecular interactions. Food Res. Int. 2022, 161, 111806. [Google Scholar] [CrossRef]
- Bi, S.; Pang, B.; Wang, T.; Zhao, T.; Yu, W. Investigation on the interactions of clenbuterol to bovine serum albumin and lysozyme by molecular fluorescence technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Y.; Hu, X.; Pan, J.; Gong, D.; Zhang, G. New insights into the binding mechanism between osthole and β-lactoglobulin: Spectroscopic, chemometrics and docking studies. Food Res. Int. 2019, 120, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kozell, A.; Eliaz, D.; Solomonov, A.; Benyamin, D.; Shmul, G.; Brookstein, O.; Rosenhek-Goldian, I.; Raviv, U.; Shimanovich, U. Modulating amyloids’ formation path with sound energy. Proc. Natl. Acad. Sci. USA 2023, 120, e2212849120. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are Building Blocks of Heat-Induced Fibrillar Protein Aggregates of β-Lactoglobulin Formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Kroes-Nijboer, A.; Venema, P.; Bouman, J.; van der Linden, E. Influence of Protein Hydrolysis on the Growth Kinetics of β-lg Fibrils. Langmuir 2011, 27, 5753–5761. [Google Scholar] [CrossRef]
- Cai, M.; Cao, H.; Li, S.; Song, H.; Zhang, Y.; Huang, K.; Wang, M.; Sun, Z.; Guan, X. Enhancing the formation rate and level of oat globulin fibrils by microwave heating: Elucidation of potential mechanisms. Food Hydrocoll. 2025, 166, 111327. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Xu, D.; Sheng, B.; Qin, D.; Chen, J.; Li, B.; Zhang, X. Application of ultrasound pretreatment and glycation in regulating the heat-induced amyloid-like aggregation of β-lactoglobulin. Food Hydrocoll. 2018, 80, 122–129. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Z.; Yang, T.; Yang, H.; Li, L.; Li, B. Effects of ultrasonic pretreatment on fibrillation kinetics, morphologies, and functional properties of bovine serum albumin fibrils. Food Hydrocoll. 2024, 149, 109520. [Google Scholar] [CrossRef]
- Althans, D.; Schrader, P.; Enders, S. Solubilisation of quercetin: Comparison of hyperbranched polymer and hydrogel. J. Mol. Liq. 2014, 196, 86–93. [Google Scholar] [CrossRef]
- Yi, J.; He, Q.; Peng, G.; Fan, Y. Improved water solubility, chemical stability, antioxidant and anticancer activity of resveratrol via nanoencapsulation with pea protein nanofibrils. Food Chem. 2022, 377, 131942. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Han, X.E.; Tian, B.; Li, G.L.; Sun, L.A.; Tian, S.F.; Qin, L.X.; Wang, S. Effects of covalent binding of different polyphenols on structure, rheology and functional properties of whey protein isolate. LWT—Food Sci. Technol. 2023, 184, 114968. [Google Scholar] [CrossRef]
- Huang, X.; Xia, B.X.; Liu, Y.X.; Wang, C.N. Non-covalent interactions between rice protein and three polyphenols and potential application in emulsions. Food Chem. X 2024, 22, 101459. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; Mezzenga, R. Inhibiting, promoting, and preserving stability of functional protein fibrils. Soft Matter 2012, 8, 876–895. [Google Scholar] [CrossRef]
- Chen, F.P.; Li, B.S.; Tang, C.H. Nanocomplexation of soy protein isolate with curcumin: Influence of ultrasonic treatment. Food Res. Int. 2015, 75, 157–165. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Characterization of fibrillated antioxidant whey protein hydrolysate and comparison with fibrillated protein solution. Food Hydrocoll. 2016, 52, 221–230. [Google Scholar] [CrossRef]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef]
- Espargaro, A.; Llabres, S.; Saupe, S.J.; Curutchet, C.; Luque, F.J.; Sabate, R. On the Binding of Congo Red to Amyloid Fibrils. Angew. Chem. Int. Ed. Engl. 2020, 59, 8104–8107. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, X.; Yu, P.; Chen, Z. Complexation of rice glutelin fibrils with cyanidin-3-O-glucoside at acidic condition: Thermal stability, binding mechanism and structural characterization. Food Chem. 2021, 363, 130367. [Google Scholar] [CrossRef]
- Wu, Z.; Yan, J.; Zhou, Z.; Xu, Q.; Zhong, Q.; Fang, X.; Huang, C.; He, X.; Li, L.; Li, Q. Preparation of soybean protein isolate-quercetin particles and its application in curcumin-camellia oil Pickering emulsion. J. Food Meas. Charact. 2024, 18, 2086–2100. [Google Scholar] [CrossRef]
- Yan, L.; Wang, R.R.; Wang, H.M.; Sheng, K.L.; Liu, C.H.; Qu, H.; Ma, A.J.; Zheng, L. Formulation and characterization of chitosan hydrochloride and carboxymethyl chitosan encapsulated quercetin nanoparticles for controlled applications in foods system and simulated gastrointestinal condition. Food Hydrocoll. 2018, 84, 450–457. [Google Scholar] [CrossRef]
- Li, S.-F.; Wu, J.-H.; Hu, T.-G.; Wu, H. Encapsulation of quercetin into zein-ethyl cellulose coaxial nanofibers: Preparation, characterization and its anticancer activity. Int. J. Biol. Macromol. 2023, 248, 125797. [Google Scholar] [CrossRef]
- Moon, H.-J.; Park, J.Y. Factors Influencing Intentions to Care For Emerging Infectious Disease Patients among National and Public Hospitals Nurses. J. Korean Acad. Fundam. Nurs. 2021, 28, 11–22. [Google Scholar] [CrossRef]
- Pathak, R.; Bhangu, S.K.; Martin, G.J.O.; Separovic, F.; Ashokkumar, M. Ultrasound-induced protein restructuring and ordered aggregation to form amyloid crystals. Eur. Biophys. J. 2022, 51, 335–352. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Tao, N.; Wang, X. Ultrasound and enzymolysis pretreatment induce pea protein to form new fibrils for efficient delivery of astaxanthin. Food Hydrocoll. 2025, 170, 111691. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Ji, F.; Xu, J.; Liu, H.; Shao, D.; Wang, C.; Zhao, Y.; Luo, S.; Zhong, X.; Zheng, Z. Improved water solubility, antioxidant, and sustained-release properties of curcumin through the complexation with soy protein fibrils. LWT 2023, 180, 114723. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, J.; Soon, W.L.; Kutzli, I.; Molière, A.; Diedrich, S.; Radiom, M.; Handschin, S.; Li, B.; Li, L.; et al. Food amyloid fibrils are safe nutrition ingredients based on in-vitro and in-vivo assessment. Nat. Commun. 2023, 14, 6806. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.M.; Zhu, J.Y.; Peng, X.H.; Feng, J.L.; Dong, H.X.; Tong, X.H.; Wang, H.; Jiang, L.Z. Effects of CaCl2 concentration on fibrils formation and characteristics of soybean protein isolate and beta-conglycinin/glycinin. Food Hydrocoll. 2023, 142, 108769. [Google Scholar] [CrossRef]
| Sample | Temperature (K) | Ksv (×104 M−1) | kq (×10−12 M−1S−1) |
|---|---|---|---|
| OG-Que | 298 | 4.564 ± 0.104 | 4.564 ± 0.104 |
| 304 | 4.177 ± 0.101 | 4.177 ± 0.101 | |
| 310 | 3.642 ± 0.035 | 3.642 ± 0.035 | |
| OGF-Que | 298 | 4.705 ± 0.126 | 4.705 ± 0.126 |
| 304 | 4.113 ± 0.029 | 4.113 ± 0.029 | |
| 310 | 3.740 ± 0.024 | 3.740 ± 0.024 | |
| UOGF-Que | 298 | 4.757 ± 0.050 | 4.757 ± 0.050 |
| 304 | 4.326 ± 0.037 | 4.326 ± 0.037 | |
| 310 | 3.772 ± 0.085 | 3.772 ± 0.085 |
| Samples | T (K) | Binding Constants | Thermodynamic Parameters | ||||
|---|---|---|---|---|---|---|---|
| Ka (M−1) | n | R2 | ΔG (kJmol−1) | ΔH (kJmol−1) | ΔS (Jmol−1K−1) | ||
| OG-Que | 298 | 1.285 × 104 | 0.881 ± 0.016 | 0.9983 | −23.533 | 15.961 | 132.529 |
| 304 | 1.633 × 104 | 0.912 ± 0.025 | 0.9964 | −24.328 | |||
| 310 | 1.647 × 104 | 0.928 ± 0.035 | 0.9930 | −25.123 | |||
| OGF-Que | 298 | 1.714 × 104 | 0.905 ± 0.015 | 0.9987 | −23.859 | 98.042 | 409.064 |
| 304 | 2.606 × 104 | 0.956 ± 0.014 | 0.9989 | −26.313 | |||
| 310 | 7.967 × 104 | 1.069 ± 0.023 | 0.9977 | −28.768 | |||
| UOGF-Que | 298 | 2.091 × 104 | 0.924 ± 0.016 | 0.9985 | −24.413 | 145.104 | 568.848 |
| 304 | 4.987 × 104 | 1.013 ± 0.010 | 0.9996 | −27.826 | |||
| 310 | 2.026 × 105 | 1.157 ± 0.011 | 0.9995 | −31.239 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhao, X.; Ban, Q. Ultrasound-Assisted Fibril Formation Enhances Complexation of Oat Globulin with Quercetin: Mechanism, Structure Evolution, Delivery Performance. Foods 2025, 14, 3916. https://doi.org/10.3390/foods14223916
Xu J, Zhao X, Ban Q. Ultrasound-Assisted Fibril Formation Enhances Complexation of Oat Globulin with Quercetin: Mechanism, Structure Evolution, Delivery Performance. Foods. 2025; 14(22):3916. https://doi.org/10.3390/foods14223916
Chicago/Turabian StyleXu, Jinzhao, Xiao Zhao, and Qingfeng Ban. 2025. "Ultrasound-Assisted Fibril Formation Enhances Complexation of Oat Globulin with Quercetin: Mechanism, Structure Evolution, Delivery Performance" Foods 14, no. 22: 3916. https://doi.org/10.3390/foods14223916
APA StyleXu, J., Zhao, X., & Ban, Q. (2025). Ultrasound-Assisted Fibril Formation Enhances Complexation of Oat Globulin with Quercetin: Mechanism, Structure Evolution, Delivery Performance. Foods, 14(22), 3916. https://doi.org/10.3390/foods14223916






