Integrated Transcriptomic and Metabolomic Analysis Reveals VASH1 Influences Pork Quality by Regulating Skeletal Muscle Glycolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Sample Collection
2.3. Meat Trait Measurement
2.4. Glycolysis Flux Measurement
2.5. Protein Extraction and Western Blot
2.6. Quantitative Real-Time PCR
2.7. Transcriptome Sequencing and Analysis
2.8. Metabolomics Sequencing and Analysis
2.9. Weight Gene Co-Expression Network Analysis (WGCNA)
2.10. Multi-Omics Integration Analysis
2.11. Vash1-Knockdown C2C12 Cell Construction
2.12. Statistical Analysis
3. Results
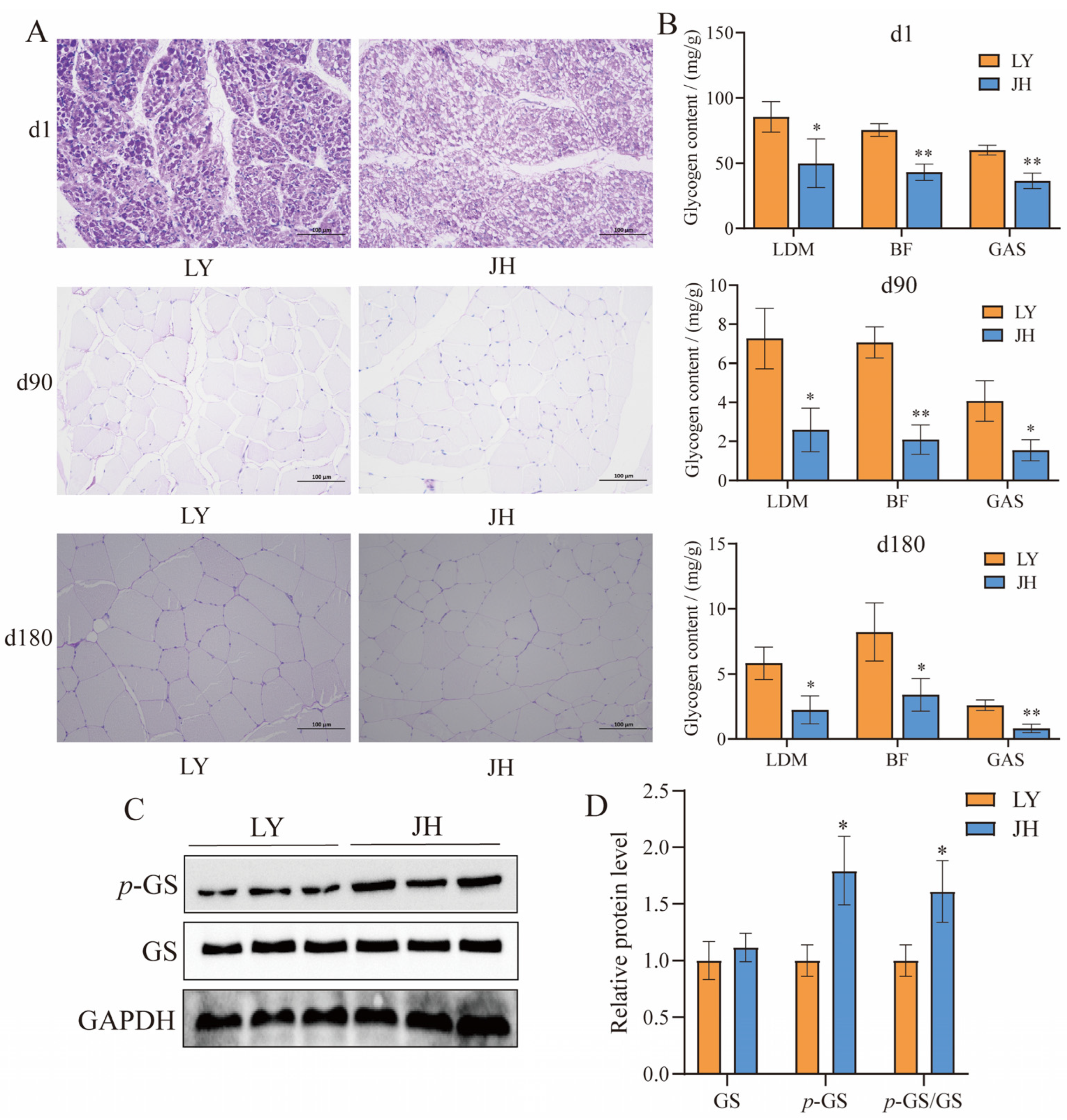
3.1. Meat Quality and Glycogen Differential Analysis
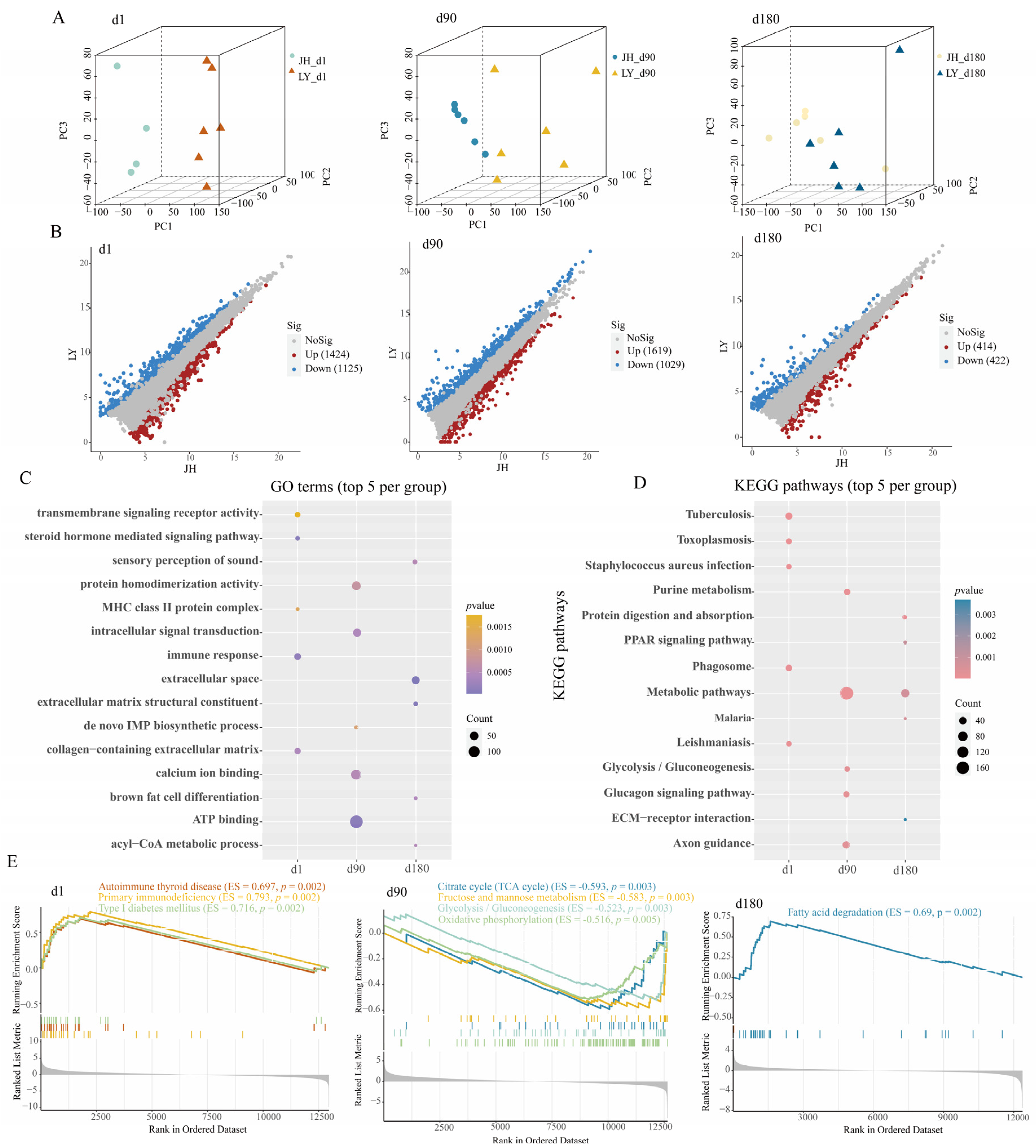
3.2. Transcriptional Specificity Analysis
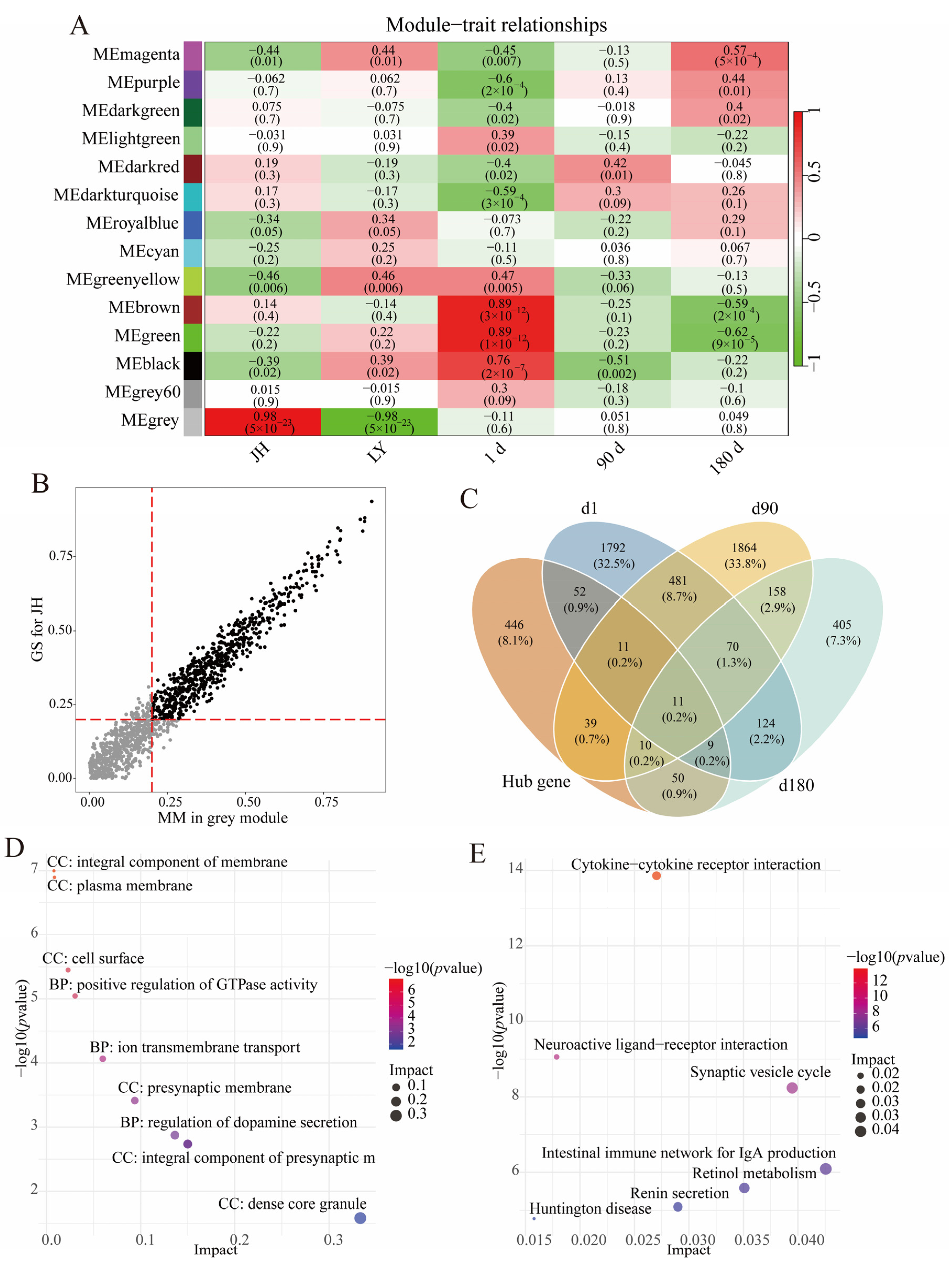
3.3. Hub Gene Selection in JH Pigs
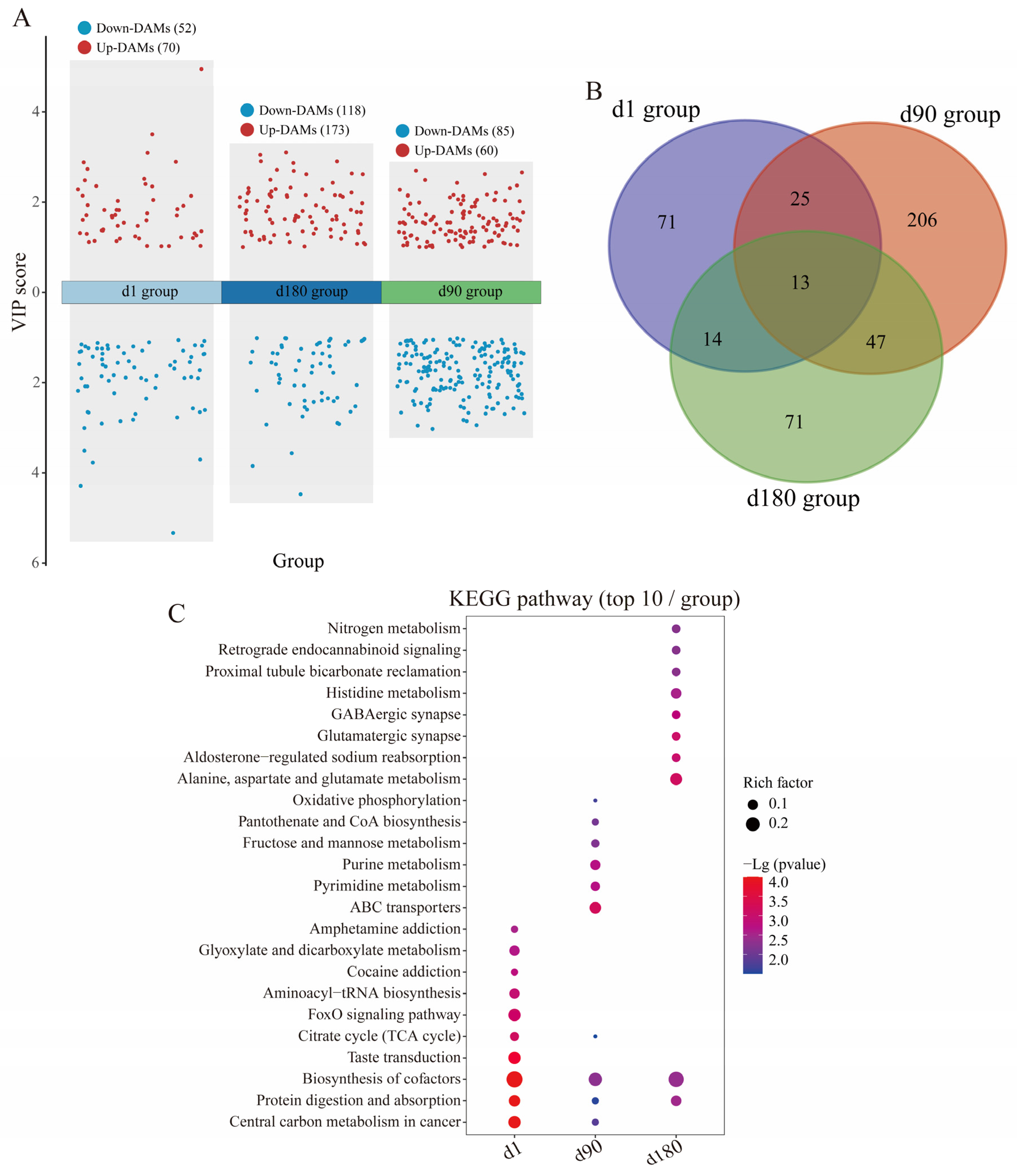
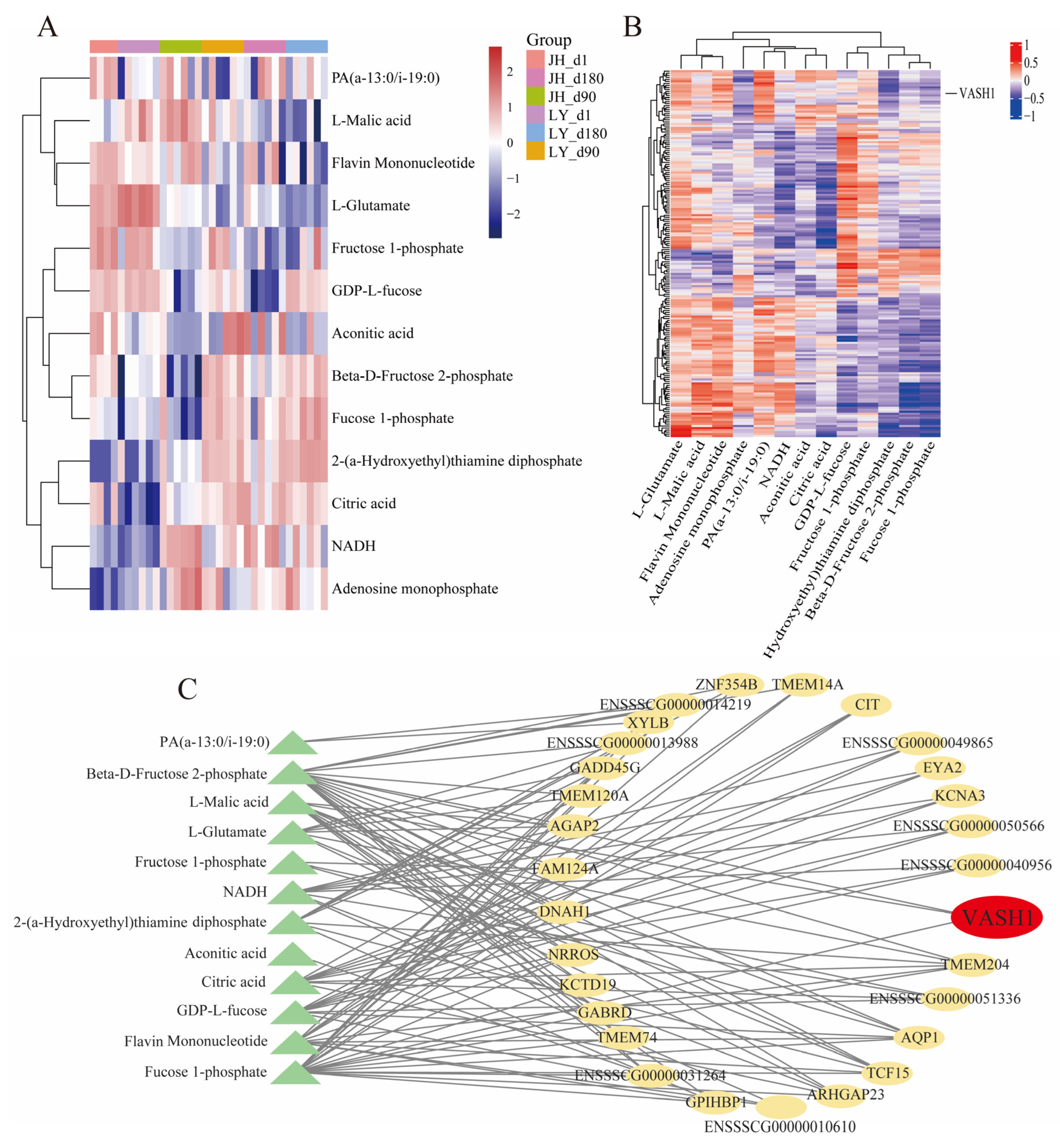
3.4. Metabolic Diversity and Composition Analysis
3.5. Key Candidate Gene Screening
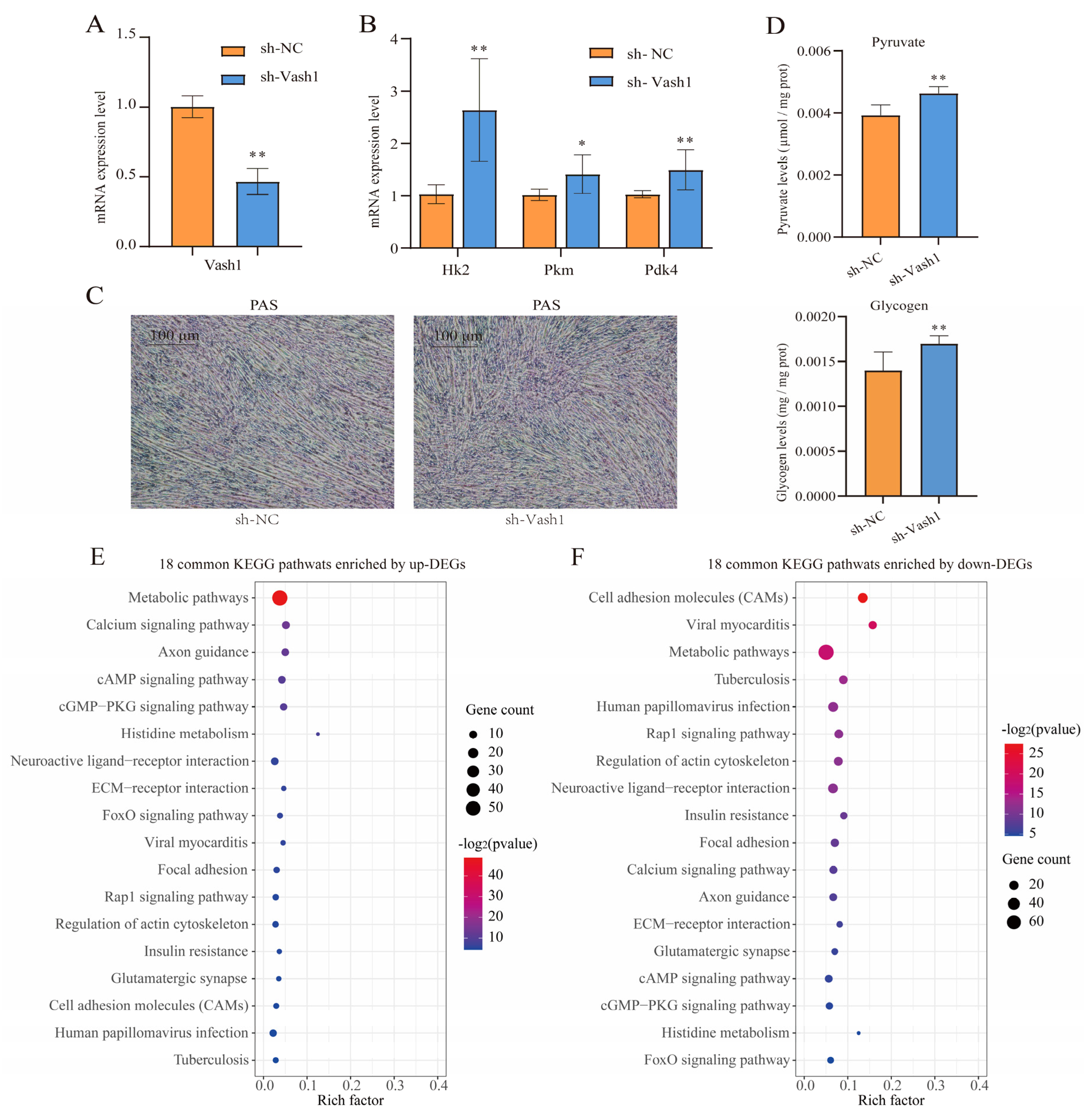
3.6. Vash1 Knockdown Promotes Glycolysis and Glycogenesis Within C2C12 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, J.M.; Fialho, R.; Albuquerque, A.; Neves, J.; Freitas, A.; Nunes, J.T.; Charneca, R. Growth, Blood, Carcass and Meat Quality Traits from Local Pig Breeds and Their Crosses. Animal 2020, 14, 636–647. [Google Scholar] [CrossRef]
- Liu, R.; Li, K.; Wu, G.; Qin, M.; Yu, H.; Wu, M.; Ge, Q.; Wu, S.; Bao, W.; Zhang, W. A Comparative Study of S-Nitrosylated Myofibrillar Proteins between Red, Firm and Non-Exudative (RFN) and Pale, Soft and Exudative (PSE) Pork by iodoTMT-Based Proteomics Assay. Food Chem. 2022, 395, 133577. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, E.; Eissen, J.J.; Nooijent, R.I.J.; Ducro, B.J.; Smits, C.H.M.; den Hartog, L.A.; Verstegen, M.W.A. Preslaughter Stress and Muscle Energy Largely Determine Pork Quality at Two Commercial Processing Plants. J. Anim. Sci. 2004, 82, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Nanni Costa, L.; Lo Fiego, D.P.; Dall’olio, S.; Davoli, R.; Russo, V. Influence of Loading Method and Stocking Density during Transport on Meat and Dry-Cured Ham Quality in Pigs with Different Halothane Genotypes. Meat Sci. 1999, 51, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Ma, T.; Long, H.; Niu, L.; Zhang, X.; Lei, Y.; Wang, L.; Chen, Y.; Wang, Q.; et al. A Splicing Mutation in PHKG1 Decreased Its Expression in Skeletal Muscle and Caused PSE Meat in Duroc × Luchuan Crossbred Pigs. Anim. Genet. 2019, 50, 395–398. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Zhou, L.; Ren, J.; Liu, X.; Zhang, H.; Yang, B.; Zhang, Z.; Ma, H.; Xie, X.; et al. A Splice Mutation in the PHKG1 Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle. PLoS Genet. 2014, 10, e1004710. [Google Scholar] [CrossRef]
- Ryan, M.T.; Hamill, R.M.; O’Halloran, A.M.; Davey, G.C.; McBryan, J.; Mullen, A.M.; McGee, C.; Gispert, M.; Southwood, O.I.; Sweeney, T. SNP Variation in the Promoter of the PRKAG3 Gene and Association with Meat Quality Traits in Pig. BMC Genet. 2012, 13, 66. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Yuan, Z.; Xie, K.; He, Z.; Zhou, X.; Wang, M.; He, J. Metabolomics Analysis Provides Novel Insights into the Difference in Meat Quality between Different Pig Breeds. Foods 2023, 12, 3476. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J.; et al. A Large-Scale Comparison of Meat Quality and Intramuscular Fatty Acid Composition among Three Chinese Indigenous Pig Breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, H.; Fu, Y.; Chen, Y.; Song, G.; Jin, Z.; Zhang, Y.; Yin, J.; Yin, Y.; Xu, K. Comprehensive Characterization of the Differences in Metabolites, Lipids, and Volatile Flavor Compounds between Ningxiang and Berkshire Pigs Using Multi-Omics Techniques. Food Chem. 2024, 457, 139807. [Google Scholar] [CrossRef]
- Lu, S.; Xu, Y.; Song, X.; Li, J.; Jiang, J.; Qin, C.; Wu, K.; Cui, K.; Liu, Y.; Liu, Q.; et al. Multi-Omics Reveal the Effects and Regulatory Mechanism of Dietary Neutral Detergent Fiber Supplementation on Carcass Characteristics, Amino Acid Profiles, and Meat Quality of Finishing Pigs. Food Chem. 2024, 445, 138765. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhao, R.; Chen, J.; Yan, Z.; Sui, X.; Li, H.; Li, Q.; Du, X.; Liu, Y.; Yao, S.; et al. Integrative Transcriptomic, Proteomic and Metabolomic Analyses Yields Insights into Muscle Fiber Type in Cattle. Food Chem. 2025, 468, 142479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, M.; Wei, S.; Zhu, L.; Yan, R.; Jin, M.; Wang, Y. Variation of Meat Quality and Relationship to Gut Microbiota Among Different Pig Breeds. Microb. Biotechnol. 2025, 18, e70139. [Google Scholar] [CrossRef]
- GB/T 17236-2019; Operating Procedures of Livestock and Poultry Slaughtering—Pig. National Standardization Administration: Beijing, China, 2018.
- NY/T 821-2019; Technical Code of Practice for Pork Quality Assessment. National Standardization Administration: Beijing, China, 2019.
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary Lycopene Supplementation Improves Meat Quality, Antioxidant Capacity and Skeletal Muscle Fiber Type Transformation in Finishing Pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio Cloud 2024: Update Single-Cell and Multiomics Workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Potes, Y.; Bermejo-Millo, J.C.; Mendes, C.; Castelão-Baptista, J.P.; Díaz-Luis, A.; Pérez-Martínez, Z.; Solano, J.J.; Sardão, V.A.; Oliveira, P.J.; Caballero, B.; et al. p66Shc Signaling and Autophagy Impact on C2C12 Myoblast Differentiation during Senescence. Cell Death Dis. 2024, 15, 200. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, M.; Sun, W. Effect of pH on the Interaction of Porcine Myofibrillar Proteins with Pyrazine Compounds. Food Chem. 2019, 287, 93–99. [Google Scholar] [CrossRef]
- Yang, N.; Liang, X.; Cao, J.; Zhang, Q.; Tan, Y.; Xu, B.; Yang, Y.; Wang, Y.; Yang, Q.; Liu, H.; et al. Denaturation Manner of Sarcoplasmic Proteins in Pale, Soft and Exudative Meat Determines Their Positive Impacts on Myofibrillar Water-Holding Capacity. Meat Sci. 2022, 185, 108723. [Google Scholar] [CrossRef]
- Yu, T.; Tian, X.; Li, D.; He, Y.; Yang, P.; Cheng, Y.; Zhao, X.; Sun, J.; Yang, G. Transcriptome, Proteome and Metabolome Analysis Provide Insights on Fat Deposition and Meat Quality in Pig. Food Res. Int. 2023, 166, 112550. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kong, F.; Xu, H.; Zhu, A.; Yan, N.; Yan, C. Cryo-EM Structure of Human Glucose Transporter GLUT4. Nat. Commun. 2022, 13, 2671. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, M.T.; Myung, C.S. Garcinia Cambogia Improves High-Fat Diet-Induced Glucose Imbalance by Enhancing Calcium/CaMKII/AMPK/GLUT4-Mediated Glucose Uptake in Skeletal Muscle. Mol. Nutr. Food Res. 2022, 66, e2100669. [Google Scholar] [CrossRef]
- Das, D.; Afzal, N.U.; Wann, S.B.; Kalita, J.; Manna, P. A ~24 kDa Protein Isolated from Protein Isolates of Hawaijar, Popular Fermented Soy Food of North-East India Exhibited Promising Antidiabetic Potential via Stimulating PI3K/AKT/GLUT4 Signaling Pathway of Muscle Glucose Metabolism. Int. J. Biol. Macromol. 2023, 224, 1025–1039. [Google Scholar] [CrossRef]
- Deng, Z.; Song, C.; Chen, L.; Zhang, R.; Yang, L.; Zhang, P.; Xiu, Y.; Su, Y.; Luo, F.; Luo, J.; et al. Inhibition of CILP2 Improves Glucose Metabolism and Mitochondrial Dysfunction in Sarcopenia via the Wnt Signalling Pathway. J. Cachexia Sarcopeni. 2024, 15, 2544–2558. [Google Scholar] [CrossRef]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Low Intramuscular Fat (but High in PUFA) Content in Cooked Cured Pork Ham Decreased Maillard Reaction Volatiles and Pleasing Aroma Attributes. Food Chem. 2016, 196, 76–82. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Tu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; Tao, X. Mechanisms of Change in Gel Water-Holding Capacity of Myofibrillar Proteins Affected by Lipid Oxidation: The Role of Protein Unfolding and Cross-Linking. Food Chem. 2021, 344, 128587. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Li, X.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Zeng, M.; Zhao, Y. Effects of Microbial Diversity and Phospholipids on Flavor Profile of Caviar from Hybrid Sturgeon (Huso Dauricus × Acipenser Schrencki). Food Chem. 2022, 377, 131969. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal Muscle Mass, Meat Quality and Antioxidant Status in Growing Lambs Supplemented with Guanidinoacetic Acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Mizgier, M.L.; Casas, M.; Contreras-Ferrat, A.; Llanos, P.; Galgani, J.E. Potential Role of Skeletal Muscle Glucose Metabolism on the Regulation of Insulin Secretion. Obes. Rev. 2014, 15, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Langa, S.O.P.; Mukaratirwa, S.; Masola, B. Effects of Centella Asiatica on Skeletal Muscle Structure and Key Enzymes of Glucose and Glycogen Metabolism in Type 2 Diabetic Rats. Biomed. Pharmacother. 2019, 112, 108715. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Tang, X.W.; Qin, Q.X. miR-335-5p Induces Insulin Resistance and Pancreatic Islet β-Cell Secretion in Gestational Diabetes Mellitus Mice through VASH1-Mediated TGF-β Signaling Pathway. J. Cell. Physiol. 2019, 234, 6654–6666. [Google Scholar] [CrossRef] [PubMed]
- Nasu, T.; Maeshima, Y.; Kinomura, M.; Hirokoshi-Kawahara, K.; Tanabe, K.; Sugiyama, H.; Sonoda, H.; Sato, Y.; Makino, H. Vasohibin-1, a Negative Feedback Regulator of Angiogenesis, Ameliorates Renal Alterations in a Mouse Model of Diabetic Nephropathy. Diabetes 2009, 58, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Suzuki, Y.; Yamada, T.; Katagiri, H.; Sato, Y. Knockout of Vasohibin-1 Gene in Mice Results in Healthy Longevity with Reduced Expression of Insulin Receptor, Insulin Receptor Substrate 1, and Insulin Receptor Substrate 2 in Their White Adipose Tissue. J. Aging Res. 2017, 2017, 9851380. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Li, X.; Wu, Y.; Yin, F.; Liu, J. Trilobatin Ameliorates Insulin Resistance through IRS-AKT-GLUT4 Signaling Pathway in C2C12 Myotubes and Ob/Ob Mice. Chin. Med. 2020, 15, 110. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, F.; Lei, F.; Wen, X.; Song, J.; Sun, G.; Liu, Z. Ameliorative Effects of Malonyl Ginsenoside from Panax Ginseng on Glucose-Lipid Metabolism and Insulin Resistance via IRS1/PI3K/Akt and AMPK Signaling Pathways in Type 2 Diabetic Mice. Am. J. Chin. Med. 2022, 50, 863–882. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New Insights into Activation and Function of the AMPK. Nat. Rev. Mol. Cell. Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, X.; Wei, Z.; Yang, M.; Zhou, X.; Lei, J.; Bai, C.; Su, G.; Liu, X.; Yang, L.; et al. Myostatin Deficiency Enhances Antioxidant Capacity of Bovine Muscle via the SMAD-AMPK-G6PD Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3497644. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, L.; Li, J.; Xing, T.; Gao, F. Intracellular Calcium Overload and Activation of CaMKK/AMPK Signaling Are Related to the Acceleration of Muscle Glycolysis of Broiler Chickens Subjected to Acute Stress. J. Agric. Food Chem. 2023, 71, 4091–4100. [Google Scholar] [CrossRef]
- Bouche, A.; Borner, B.; Richard, C.; Grand, Y.; Hannouche, D.; Laumonier, T. In Vitro-Generated Human Muscle Reserve Cells Are Heterogeneous for Pax7 with Distinct Molecular States and Metabolic Profiles. Stem Cell Res. Ther. 2023, 14, 243. [Google Scholar] [CrossRef]
- Mei, Z.; Shen, Z.; Pu, J.; Liu, Q.; Liu, G.; He, X.; Wang, Y.; Yue, J.; Ge, S.; Li, T.; et al. NAT10 Mediated ac4C Acetylation Driven m6A Modification via Involvement of YTHDC1-LDHA/PFKM Regulates Glycolysis and Promotes Osteosarcoma. Cell Commun. Signal. 2024, 22, 51. [Google Scholar] [CrossRef]
| Items | Values |
|---|---|
| Formulation | |
| Maize | 47.6% |
| Soybean meal | 8.5% |
| Alfalfa, dehydrated | 1.5% |
| Wheat, soft | 8.0% |
| Barley | 6.0% |
| Wheat feed flour | 4.0% |
| Rice bran, oil > 5% | 16.0% |
| Rice, polished, broken | 5.0% |
| Limestone | 0.9% |
| Premix | 2.5% |
| Chemical composition | |
| Dry matter (DM) | 87.3% |
| Crude protein (CP) | 16.2% |
| Crude fiber (CF) | 4.3% |
| Ether extract (EE) | 5.7% |
| Ash | 5.0% |
| Neutral detergent fiber (NDF) | 13.0% |
| Acid detergent Fiber (ADF) | 4.7% |
| Total calcium (Ca) | 0.7% |
| Total phosphorus (P) | 0.8% |
| Digestible energy (DE) | 3358.2 Kcal/kg |
| Metabolic energy (ME) | 3226.1 Kcal/kg |
| Gross energy (GE) | 3892.0 Kcal/kg |
| Traits | JH (n = 6) | LY (n = 6) | p |
|---|---|---|---|
| pH45min | 6.66 ± 0.12 | 6.54 ± 0.62 | 0.06 |
| pH24h | 5.78 ± 0.25 | 5.71 ± 0.18 | 0.60 |
| DL24h | 3.85 ± 0.01 | 4.38 ± 0.01 | 0.34 |
| DL48h | 6.76 ± 0.01 | 6.90 ± 0.01 | 0.81 |
| Meat color | 3.00 ± 0.65 a | 2.04 ± 0.37 b | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Fu, Y.; Sun, J.; Zhao, W.; Gong, H.; Zhang, Z.; Wang, Z.; Wang, Q.; Pan, Y. Integrated Transcriptomic and Metabolomic Analysis Reveals VASH1 Influences Pork Quality by Regulating Skeletal Muscle Glycolysis. Foods 2025, 14, 3840. https://doi.org/10.3390/foods14223840
Wu F, Fu Y, Sun J, Zhao W, Gong H, Zhang Z, Wang Z, Wang Q, Pan Y. Integrated Transcriptomic and Metabolomic Analysis Reveals VASH1 Influences Pork Quality by Regulating Skeletal Muscle Glycolysis. Foods. 2025; 14(22):3840. https://doi.org/10.3390/foods14223840
Chicago/Turabian StyleWu, Fen, Yihan Fu, Jiabao Sun, Wei Zhao, Huanfa Gong, Zhe Zhang, Zhen Wang, Qishan Wang, and Yuchun Pan. 2025. "Integrated Transcriptomic and Metabolomic Analysis Reveals VASH1 Influences Pork Quality by Regulating Skeletal Muscle Glycolysis" Foods 14, no. 22: 3840. https://doi.org/10.3390/foods14223840
APA StyleWu, F., Fu, Y., Sun, J., Zhao, W., Gong, H., Zhang, Z., Wang, Z., Wang, Q., & Pan, Y. (2025). Integrated Transcriptomic and Metabolomic Analysis Reveals VASH1 Influences Pork Quality by Regulating Skeletal Muscle Glycolysis. Foods, 14(22), 3840. https://doi.org/10.3390/foods14223840






