Improving the Texturization of Pea Protein Through the Addition of a Mung Bean Protein Extract Solution and Optimizing the Moisture Content, Screw Speed, and Extrusion Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mung Bean Protein Extract Solution
2.3. Extrusion Experiments and Sample Preparation
2.4. Textural Properties
2.5. Expansion Ratio and Bulk Density
2.6. Color Analysis
2.7. Water- and Oil-Holding Capacities (WHC and OHC)
2.8. Determination of Sulfhydryl (SH) Group and Disulfide Bond (DB) Contents
2.9. Surface Hydrophobicity (H0)
2.10. Scanning Electron Microscopy (SEM) Observations
2.11. Fourier Transform Infrared Spectroscopy (FTIR)
2.12. Intrinsic Tryptophan Fluorescence Spectroscopy
2.13. Response Surface Experimental Design
2.14. Statistical Analysis
3. Results
3.1. Textural Quality Characteristics Analysis
3.1.1. MP Addition Amount
3.1.2. Screw Speed
3.1.3. Extrusion Temperature
3.2. Color Analysis
3.2.1. MP Addition Amount
3.2.2. Screw Speed
3.2.3. Extrusion Temperature
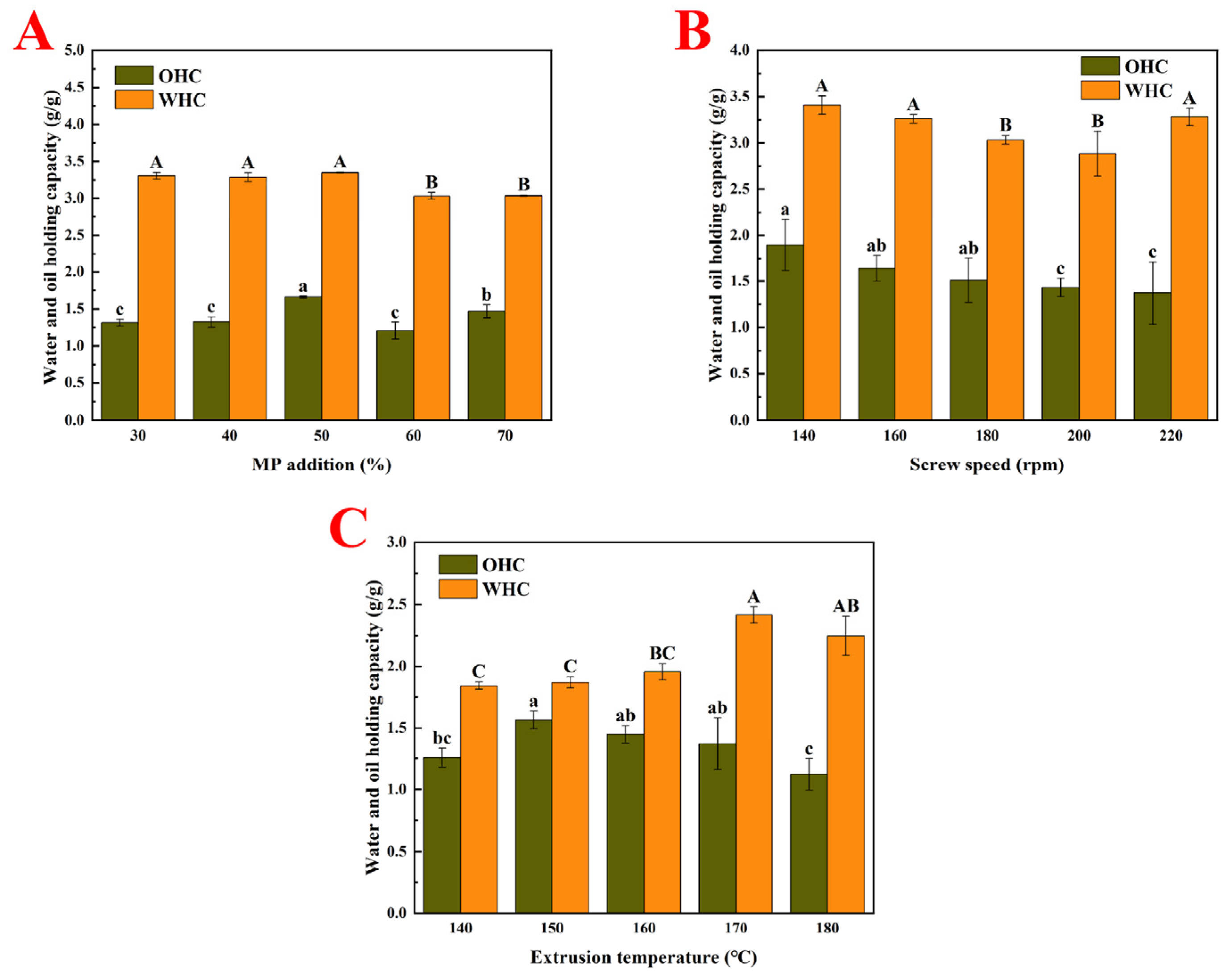
3.3. Oil- and Water-Holding Capacities (OHC and WHC)
3.3.1. MP Addition Amount
3.3.2. Screw Speed
3.3.3. Extrusion Temperature
3.4. Determination of Sulfhydryl (SH) Group and Disulfide Bond (DB) Contents
3.4.1. MP Addition Amount
3.4.2. Screw Speed
3.4.3. Extrusion Temperature
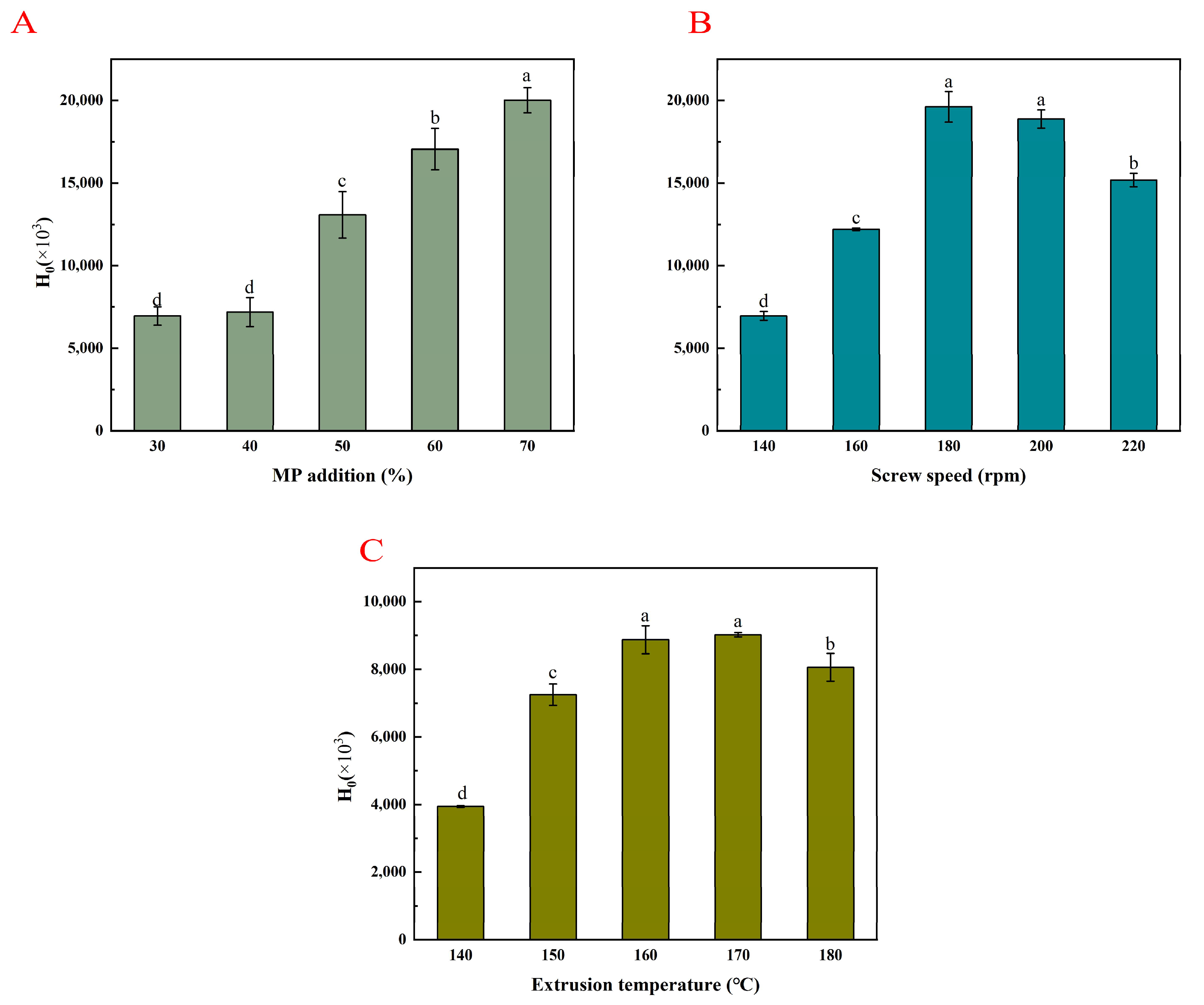
3.5. Surface Hydrophobicity (H0)
3.5.1. MP Addition Amount
3.5.2. Screw Speed
3.5.3. Extrusion Temperature
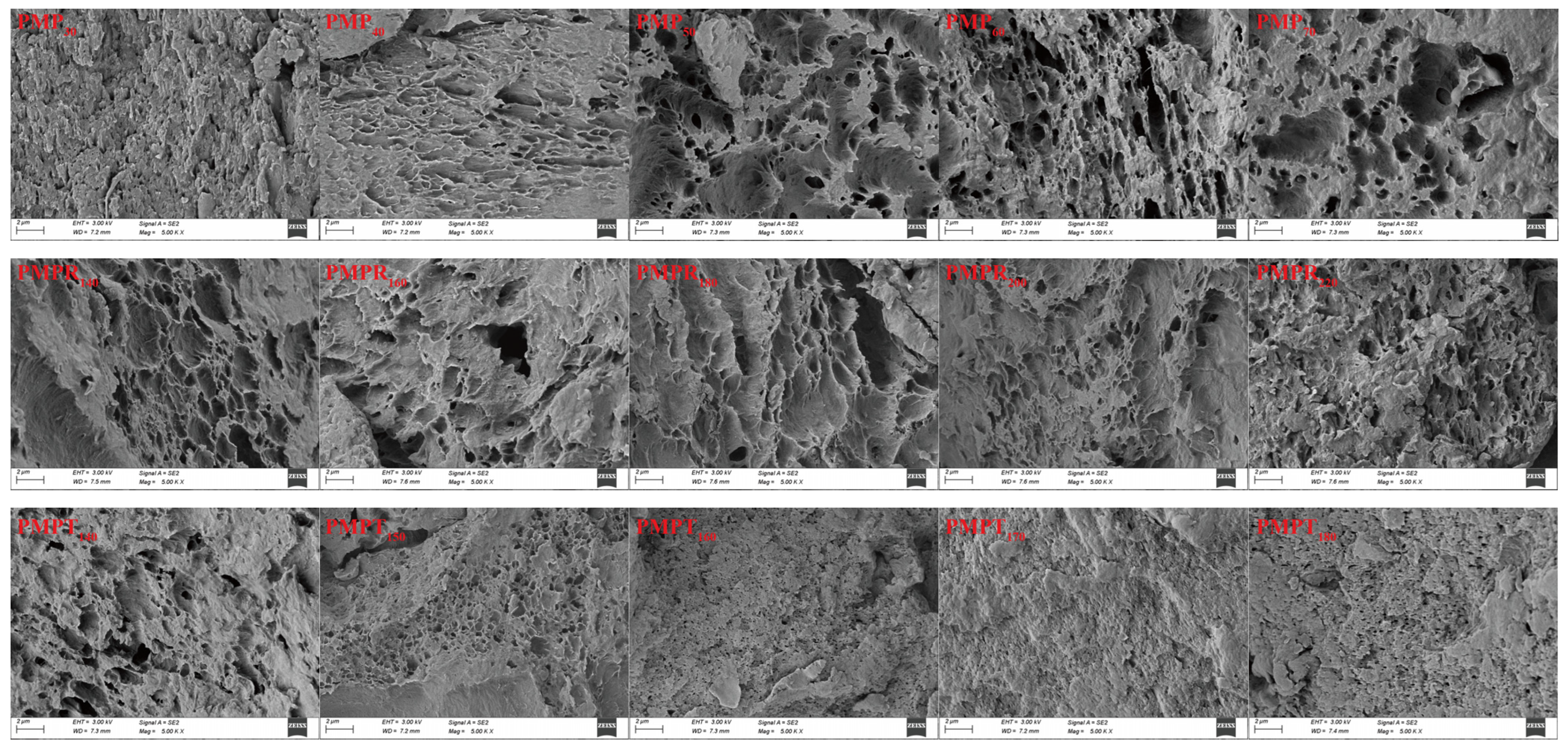
3.6. Scanning Electron Microscopy (SEM) Observations
3.6.1. MP Addition Amount
3.6.2. Screw Speed
3.6.3. Extrusion Temperature
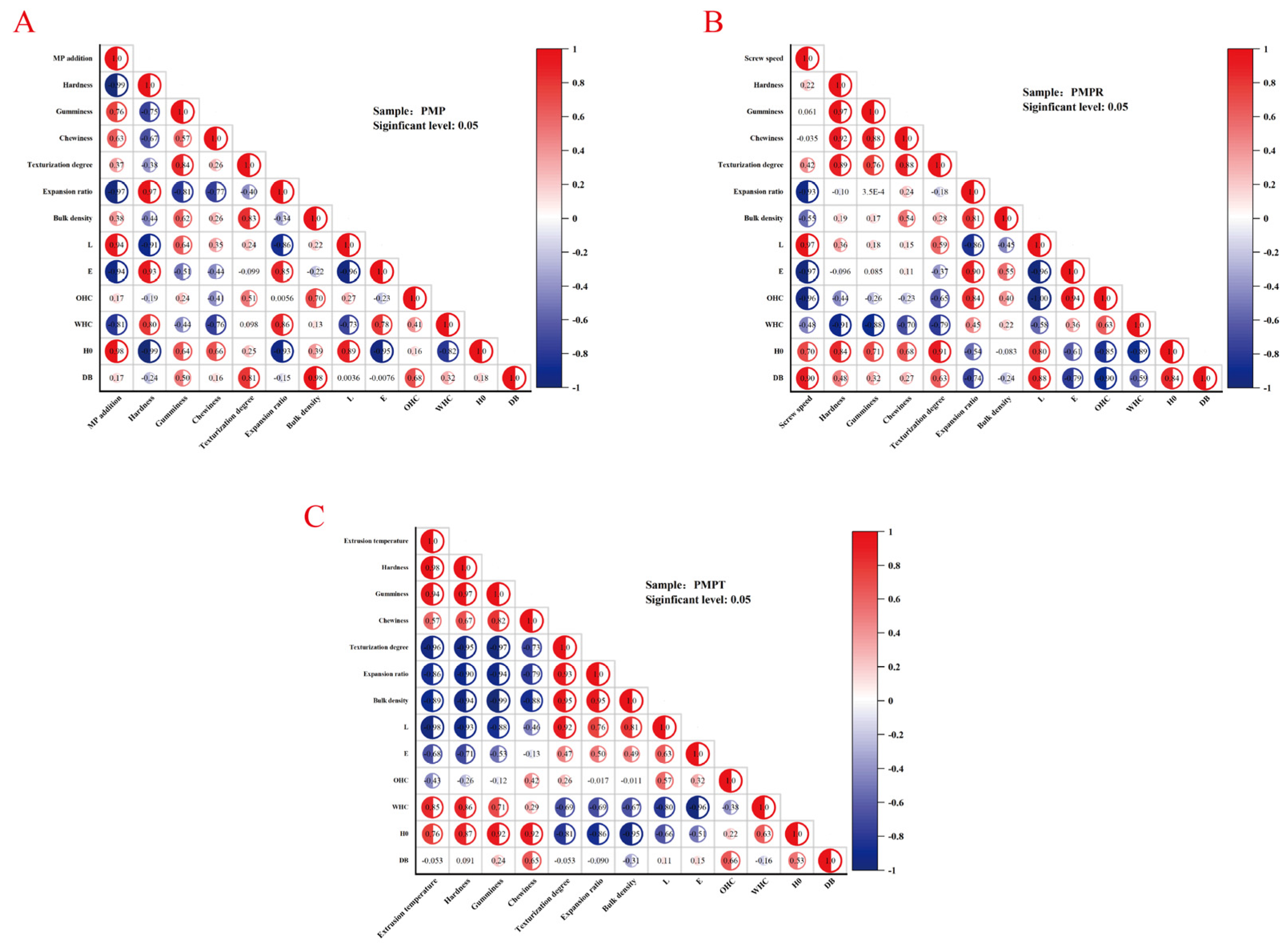
3.7. Correlation Analysis to Statistically Link the Textural Properties, Color Values, and Sulfhydryl/Disulfide Bond Data
3.7.1. MP Addition Amount
3.7.2. Screw Speed
3.7.3. Extrusion Temperature
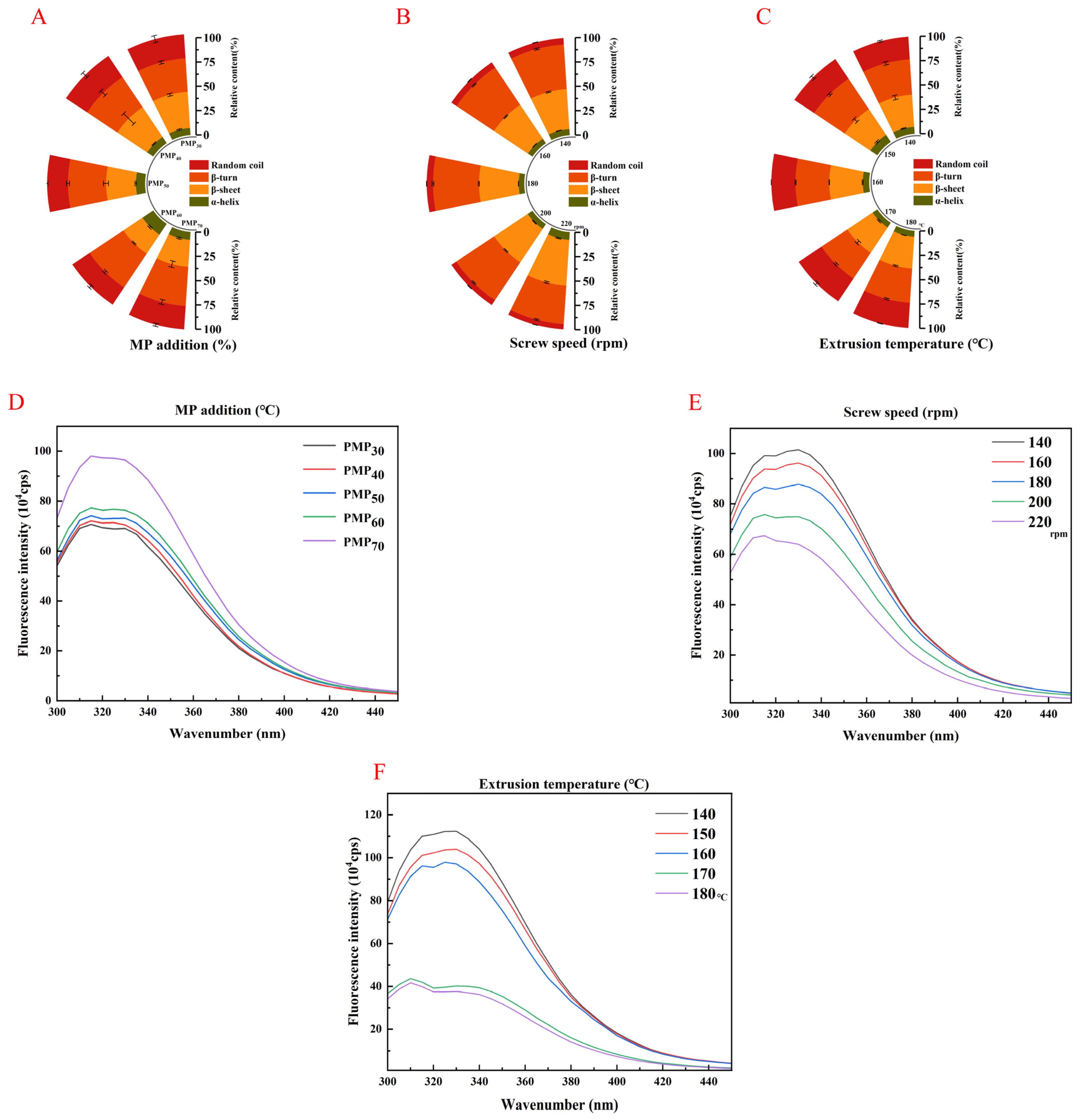
3.8. Fourier Transform Infrared Spectroscopy (FTIR)
3.9. Intrinsic Tryptophan Fluorescence
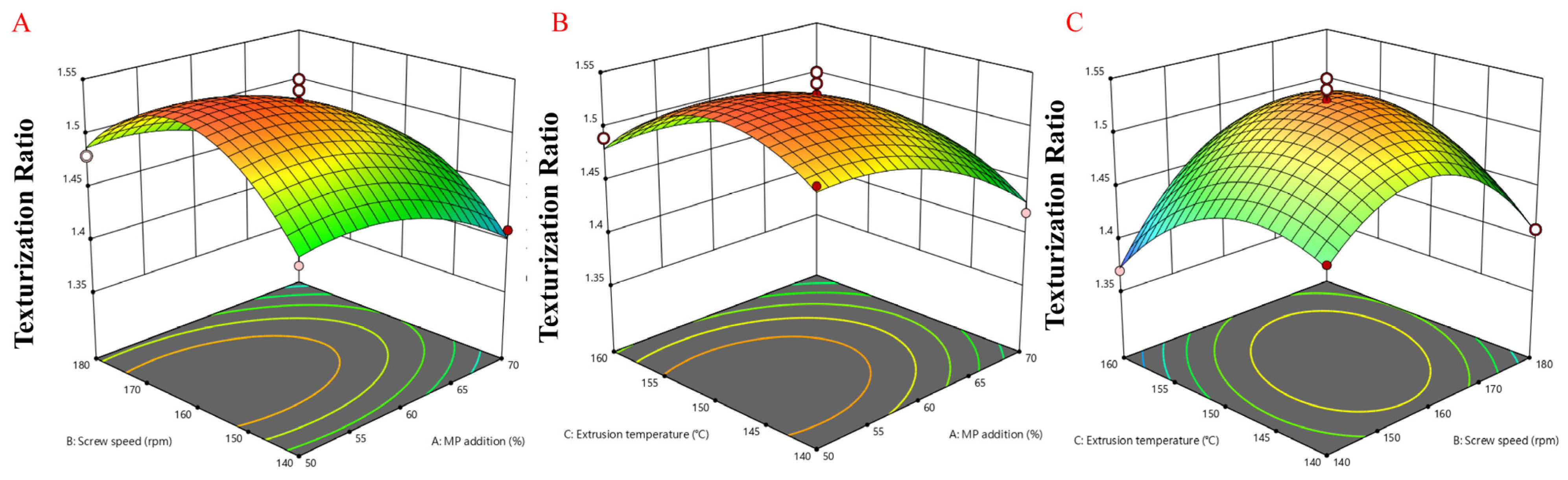
3.10. Three-Dimensional Response Surface Model for Effects of Different Extrusion Parameters on Response Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awol, S.J.; Kidane, S.W.; Bultosa, G. The Effect of Extrusion Condition and Blend Proportion on the Physicochemical and Sensory Attributes of Teff-Soybean Composite Flour Gluten Free Extrudates. Meas. Food 2024, 13, 100120. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- MacDonald, R.S.; Pryzbyszewski, J.; Hsieh, F.-H. Soy Protein Isolate Extruded with High Moisture Retains High Nutritional Quality. J. Agric. Food Chem. 2009, 57, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Singh, J.; Hodgkinson, S.; Farouk, M.; Kaur, L. Conformational Changes and Product Quality of High-Moisture Extrudates Produced from Soy, Rice, and Pea Proteins. Food Hydrocoll. 2024, 147, 109341. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Wang, S.; Qi, M.; Yue, M.; Zhang, S.; Song, J.; Wang, C.; Zhang, D.; Wang, X.; et al. High Moisture Extrusion of Pea Protein: Effect of l-Cysteine on Product Properties and the Process Forming a Fibrous Structure. Food Hydrocoll. 2022, 129, 107633. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Zhang, R.; Jiao, A.; Jin, Z. Effects of Different Polysaccharide Colloids on the Structure and Physicochemical Properties of Peanut Protein and Wheat Gluten Composite System under Extrusion. Int. J. Biol. Macromol. 2024, 272, 132773. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhu, S.; Wang, Q. Texturisation Behaviour of Peanut–Soy Bean/Wheat Protein Mixtures during High Moisture Extrusion Cooking. Int. J. Food Sci. Technol. 2018, 53, 2535–2541. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Hardiman, K.; Dam, A.B.; Dodge, N.; Aaslyng, M.D.; Petersen, I.L. Protein Blends and Extrusion Processing to Improve the Nutritional Quality of Plant Proteins. Food Funct. 2023, 14, 7361–7374. [Google Scholar] [CrossRef]
- Rolandelli, G.; Ozturk, O.K.; Giraldo, A.M.V.; Hamaker, B.R.; Campanella, O.H. Textural Improvement of Pea Protein-Based High-Moisture Extrudates with Corn Zein and Rice Starch. Int. J. Biol. Macromol. 2024, 281, 135960. [Google Scholar] [CrossRef]
- Gaber, S.M.; Knezevic, D.; Saldanha Do Carmo, C.; Zobel, H.; Knutsen, S.H.; Sahlstrøm, S.; Dessev, T. Meat Analogues from Pea Protein: Effect of Different Oat Protein Concentrates and Post Treatment on Selected Technological Properties of High-Moisture Extrudates. Appl. Sci. 2023, 13, 12354. [Google Scholar] [CrossRef]
- Chan, E.; Sinaki, N.Y.; Rodas-González, A.; Tulbek, M.; Koksel, F. Impact of Protein Blend Formulation and Extrusion Conditions on the Physical Properties of Texturised Pea Protein-extended Beef Burgers. Int. J. Food Sci. Technol. 2024, 59, 959–970. [Google Scholar] [CrossRef]
- González-Galeana, C.; Castañeda-Salazar, A.; Cortez-Trejo, M.D.C.; Gaytán-Martínez, M.; Campos-Vega, R.; Mendoza, S. Structural and Functional Properties of a High Moisture Extruded Mixture of Pea Proteins (Pisum sativum), Amaranth Flour (Amaranthus hypochondriacus), and Oat Flour (Avena sativa). Food Chem. 2025, 463, 141042. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Li, T.; Zhou, H.; Guo, F.; Wang, Q.; Zhang, J. Water Binding Ability Changes of Different Proteins during High-Moisture Extrusion. Food Hydrocoll. 2024, 152, 109935. [Google Scholar] [CrossRef]
- Sengar, A.S.; Beyrer, M.; McDonagh, C.; Tiwari, U.; Pathania, S. Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives. Foods 2023, 12, 3830. [Google Scholar] [CrossRef]
- Toldrà, M.; Parés, D.; Barnés-Calle, C.; Gou, P.; Fulladosa, E. Techno-Functional and Physico-Chemical Properties of High Moisture Extrudates from Faba Bean (Vicia faba) Protein Concentrate Using Different Extrusion Conditions. LWT 2025, 225, 117898. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, B.-J.; Hwang, N.; Ryu, G.-H. Optimization of High-Moisture Meat Analog Production with the Addition of Isolated Mung Bean Protein Using Response Surface Methodology. Foods 2025, 14, 1323. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.-S.; Chae, T.Y.; Yoon, C.S.; Han, J. Effect of Screw Speed and Die Temperature on Physicochemical, Textural, and Morphological Properties of Soy Protein Isolate-Based Textured Vegetable Protein Produced via a Low-Moisture Extrusion. Food Sci. Biotechnol. 2023, 32, 659–669. [Google Scholar] [CrossRef]
- Kirtil, E. Molecular Strategies to Overcome Sensory Challenges in Alternative Protein Foods. Food Bioprocess Technol. 2025, 18, 6964–6996. [Google Scholar] [CrossRef]
- Liu, L.; Jin, L.; Yang, S.; Li, H.; Chen, C.; Farouk, A.; Ban, Z.; Liang, H.; Huang, J. pH-Driven Formation of Soy Protein Isolate-Thymol Nanoparticles for Improved the Shelf Life of Fresh-Cut Lettuce. Food Control 2024, 160, 110306. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, Q.; Song, M.; Li, X.; Yang, A.; Tong, P.; Wu, Z.; Chen, H. The Structure and Potential Allergenicity of Peanut Allergen Monomers after Roasting. Food Funct. 2024, 15, 2577–2586. [Google Scholar] [CrossRef]
- Hwang, N.-K.; Gu, B.-J.; Zhang, Y.; Ryu, G.-H. Possibility of Isolated Mung Bean Protein as a Main Raw Material in the Production of an Extruded High-Moisture Meat Analog. Foods 2024, 13, 2167. [Google Scholar] [CrossRef]
- Hossain Brishti, F.; Chay, S.Y.; Muhammad, K.; Rashedi Ismail-Fitry, M.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and Rheological Changes of Texturized Mung Bean Protein Induced by Feed Moisture during Extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- Seetapan, N.; Raksa, P.; Limparyoon, N.; Srirajan, S.; Makmoon, T.; Israkarn, K.; Gamonpilas, C.; Methacanon, P.; Fuongfuchat, A. High Moisture Extrusion of Meat Analogues Using Mung Bean (Vigna radiata L.) Protein and Flour Blends: Investigations on Morphology, Texture and Rheology. Int. J. Food Sci. Technol. 2023, 58, 1922–1930. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Eze, C.R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Advances in Legume Protein Extraction Technologies: A Review. Innov. Food Sci. Emerg. Technol. 2022, 82, 103199. [Google Scholar] [CrossRef]
- Penchalaraju, M.; John Don Bosco, S. Legume Protein Concentrates from Green Gram, Cowpea, and Horse Gram. J. Food Process. Preserv. 2022, 46, e16477. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Liu, Q.; Xu, L.; Bao, H.; Ren, X.; Jin, Z.; Jiao, A. Effect of Wheat Gluten and Peanut Protein Ratio on the Moisture Distribution and Textural Quality of High-Moisture Extruded Meat Analogs from an Extruder Response Perspective. Foods 2023, 12, 1696. [Google Scholar] [CrossRef]
- Sahu, C.; Patel, S.; Tripathi, A.K. Effect of Extrusion Parameters on Physical and Functional Quality of Soy Protein Enriched Maize Based Extruded Snack. Appl. Food Res. 2022, 2, 100072. [Google Scholar] [CrossRef]
- Ganorkar, P.M.; Jain, R.K. Development of Flaxseed Fortified Rice—Corn Flour Blend Based Extruded Product by Response Surface Methodology. J. Food Sci. Technol. 2015, 52, 5075–5083. [Google Scholar] [CrossRef]
- Mazaheri Tehrani, M.; Ehtiati, A.; Sharifi Azghandi, S. Application of Genetic Algorithm to Optimize Extrusion Condition for Soy-Based Meat Analogue Texturization. J. Food Sci. Technol. 2017, 54, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, B.; Zhou, C.; Ren, W.; Wu, M. Effect of High-Moisture Extrusion Process on Quality Attribute and Fibrous Formation Mechanism of Pea Protein: Insight into Dynamic Changes of Textural Protein. Innov. Food Sci. Emerg. Technol. 2023, 89, 103486. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship Between Surface Hydrophobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. Int. J. Food Prop. 2015, 18, 1059–1074. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein-Protein Interactions during High-Moisture Extrusion for Fibrous Meat Analogues and Comparison of Protein Solubility Methods Using Different Solvent Systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Tabibloghmany, F.S.; Mazaheri Tehrani, M.; Koocheki, A. Optimization of the Extrusion Process through Response Surface Methodology for Improvement in Functional and Nutritional Properties of Soybean Hull. J. Food Sci. Technol. 2020, 57, 4054–4064. [Google Scholar] [CrossRef]
- Sahu, C.; Patel, S.; Khokhar, D.; Naik, R.K. Effect of Feed and Process Variables on Nutritional Quality of Maize-Millet Based Soy Fortified Extruded Product Using Response Surface Methodology. Appl. Food Res. 2022, 2, 100139. [Google Scholar] [CrossRef]
- Zang, Y.; Wang, S.; Gao, Y.; Sun, C.; Zhao, Y.; Cao, Y.; Lu, W.; Zhang, Y.; Fang, Y. High Moisture Extrusion of Pulse Proteins: Texture, Structure, and in Vitro Digestion Characteristics of Extrudates. Food Hydrocoll. 2025, 159, 110676. [Google Scholar] [CrossRef]
- Morejón Caraballo, S.; Fischer, S.V.; Masztalerz, K.; Lech, K.; Rohm, H.; Struck, S. Low Moisture Texturised Protein from Sunflower Press Cake. Int. J. Food Sci. Technol. 2024, 59, 8236–8247. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Shah, F.; Xu, Y.; Wang, Q. High-Moisture Extrusion of Peanut Protein-/Carrageenan/Sodium Alginate/Wheat Starch Mixtures: Effect of Different Exogenous Polysaccharides on the Process Forming a Fibrous Structure. Food Hydrocoll. 2020, 99, 105311. [Google Scholar] [CrossRef]
- Schmid, E.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High Moisture Extrusion Cooking of Meat Analogs: A Review of Mechanisms of Protein Texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, C.; Miao, Y.; Zhao, H.; Liu, Z.; Zhang, Y.; Zhao, L.; Peng, C.; Gong, J. Quality Characteristics and Fibrous Structure Formation Mechanism of Walnut Protein and Wheat Gluten Meat Analogues during High-Moisture Extrusion Cooking Process. Food Chem. 2025, 463, 141168. [Google Scholar] [CrossRef] [PubMed]
- Barnés-Calle, C.; Matas, G.; Claret, A.; Guerrero, L.; Fulladosa, E.; Gou, P. High Moisture Extrusion of Pea Protein Isolate to Mimic Chicken Texture: Instrumental and Sensory Insights. Food Hydrocoll. 2024, 154, 110129. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Y.; Tu, X.; Xia, S.; Xue, Y.; Xue, C. Effects of High Temperature, High Pressure, and High Shear during Extrusion on Maize Starch-Fish Protein Extrudates: Based on Physical Properties and Multiscale Structure. Food Chem. 2025, 468, 142364. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, S.; Pan, Y.; Li, H.; Liu, X.; Cao, J. Properties of Texturized Protein and Performance of Different Protein Sources in the Extrusion Process: A Review. Food Res. Int. 2023, 174, 113588. [Google Scholar] [CrossRef]
- Cagnin, C.; Morais, D.N.; Prudencio, S.H. Structural, Physicochemical and Technofunctional Properties of Corn Gluten Meal Modified by Extrusion. Food Res. Int. 2024, 196, 115067. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; Abd El-Aty, A.M. Impact of High Moisture Contents on the Structure and Functional Properties of Pea Protein Isolate during Extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Gasparre, N.; Van Den Berg, M.; Oosterlinck, F.; Sein, A. High-Moisture Shear Processes: Molecular Changes of Wheat Gluten and Potential Plant-Based Proteins for Its Replacement. Molecules 2022, 27, 5855. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, J.; Yang, X.; Li, L. Structure of Pea Protein-Based Complexes on High-Moisture Extrusion: Raw Materials and Extrusion Zones. LWT 2024, 194, 115823. [Google Scholar] [CrossRef]
- Ma, W.; Qi, B.; Sami, R.; Jiang, L.; Li, Y.; Wang, H. Conformational and Functional Properties of Soybean Proteins Produced by Extrusion-Hydrolysis Approach. Int. J. Anal. Chem. 2018, 2018, 9182508. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Sun, B.; Zhu, Y.; Gao, Y.; Liu, L.; Huang, Y.; Zhu, X. Low-Moisture Extruded Mung Bean Protein Isolate and Wheat Gluten: Structure, Techno-Functional Characteristics and Establishment of Rehydration Kinetics of the Products. LWT 2024, 210, 116844. [Google Scholar] [CrossRef]
- Li, J.; Li, L. Effect of Extrusion Temperature on the Structure and Emulsifying Properties of Soy Protein Isolate-Oat β-Glucan Conjugates Formed during High Moisture Extrusion. Food Chem. 2023, 429, 136787. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.-H.; An, H.-Z.; Ma, Y.-X.; Guo, Y.-T.; Du, Y.; Zhu, X.-Q.; Luo, Q. Effects of Lysine on the Physiochemical Properties of Plant-Protein High-Moisture Extrudates. LWT 2023, 173, 114410. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Zhao, C.; Lin, H.; Wang, Z.; Wu, F. Structural and Functional Properties of Rice Bran Protein Oxidized by Peroxyl Radicals. Int. J. Food Prop. 2017, 20, 1456–1467. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Yuan, Y.; Shi, J.; Duan, Y.; Wang, L.; Wang, P.; Xiao, Z. Changes in Physicochemical and Structural Properties of Pea Protein during the High Moisture Extrusion Process: Effects of Carboxymethylcellulose Sodium and Different Extrusion Zones. Int. J. Biol. Macromol. 2023, 251, 126350. [Google Scholar] [CrossRef]
- Xia, S.; Shen, S.; Ma, C.; Li, K.; Xue, C.; Jiang, X.; Xue, Y. High-Moisture Extrusion of Yeast-Pea Protein: Effects of Different Formulations on the Fibrous Structure Formation. Food Res. Int. 2023, 163, 112132. [Google Scholar] [CrossRef]
- Xiao, T.; Su, X.; Jiang, R.; Zhou, H.; Xie, T. Low Moisture Extrusion of Soybean Protein Isolate: Effect of β-Glucan on the Physicochemical Properties of the Product. LWT 2023, 179, 114660. [Google Scholar] [CrossRef]
| Sample | Hardness/g | Gumminess/g∙sec | Chewiness | Texturization Degree | Expansion Ratio | Bulk Density/g∙cm3 |
|---|---|---|---|---|---|---|
| PMP30 | 1805.33 ± 9.50 a | 1376.33 ± 18.93 b | 984.67 ± 11.85 c | 1.24 ± 0.07 d | 1.057 ± 0.014 a | 2.00 ± 0.23 c |
| PMP40 | 1796.33 ± 24.58 bc | 1467.33 ± 18.50 a | 995.67 ± 9.29 bc | 1.53 ± 0.01 b | 1.047 ± 0.010 ab | 2.17 ± 0.04 bc |
| PMP50 | 1780.00 ± 19.92 abc | 1478.33 ± 17.10 a | 1004.33 ± 19.50 bc | 1.65 ± 0.02 a | 1.044 ± 0.018 ab | 3.00 ± 0.27 a |
| PMP60 | 1768.33 ± 9.45 bc | 1484.67 ± 8.08 a | 1076.33 ± 15.31 a | 1.50 ± 0.05 bc | 1.032 ± 0.009 b | 2.47 ± 0.09 b |
| PMP70 | 1759.67 ± 8.39 c | 1476.33 ± 14.98 a | 1016.33 ± 14.19 b | 1.43 ± 0.04 c | 1.031 ± 0.008 b | 2.31 ± 0.11 bc |
| PMPR140 | 1058.00 ± 11.79 f | 993.33 ± 10.02 c | 885.67 ± 14.15 c | 1.22 ± 0.15 b | 1.047 ± 0.006 a | 2.79 ± 0.15 ab |
| PMPR160 | 1270.00 ± 15.62 c | 1069.67 ± 14.50 b | 935.33 ± 17.95 b | 1.45 ± 0.11 ab | 1.045 ± 0.012 a | 2.82 ± 0.14 ab |
| PMPR180 | 1780.00 ± 19.92 a | 1478.33 ± 17.10 a | 1004.33 ± 19.50 a | 1.65 ± 0.02 a | 1.044 ± 0.018 a | 3.00 ± 0.06 a |
| PMPR200 | 1674.67 ± 14.57 b | 1460.00 ± 13.75 a | 944.67 ± 20.23 b | 1.51 ± 0.04 a | 1.033 ± 0.010 a | 2.51 ± 0.27 b |
| PMPR220 | 1092.67 ± 16.29 d | 852.67 ± 8.39 d | 875.33 ± 18.50 c | 1.40 ± 0.19 ab | 1.032 ± 0.011 a | 2.61 ± 0.11 b |
| PMPT140 | 1780.00 ± 19.92 a | 1478.33 ± 17.10 c | 1004.33 ± 19.50 c | 1.65 ± 0.02 a | 1.044 ± 0.018 a | 3.00 ± 0.27 a |
| PMPT150 | 1850.33 ± 22.50 c | 1524.67 ± 22.28 b | 1099.67 ± 24.09 ab | 1.56 ± 0.02 b | 1.038 ± 0.007 a | 2.65 ± 0.14 b |
| PMPT160 | 1899.67 ± 17.50 b | 1548.33 ± 9.07 ab | 1124.33 ± 22.50 a | 1.53 ± 0.03 b | 1.039 ± 0.012 a | 2.53 ± 0.06 b |
| PMPT170 | 1953.33 ± 19.86 a | 1552.33 ± 11.85 ab | 1088.67 ± 8.14 b | 1.52 ± 0.04 b | 1.037 ± 0.004 a | 2.51 ± 0.06 b |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| PMP30 | 78.64 ± 0.21 a | 4.84 ± 0.18 a | 23.70 ± 0.23 b | 30.47 ± 0.26 a |
| PMP40 | 79.42 ± 0.52 a | 4.68 ± 0.15 a | 24.48 ± 0.44 a | 30.54 ± 0.54 a |
| PMP50 | 79.78 ± 0.50 b | 4.32 ± 0.31 b | 23.90 ± 0.30 b | 29.81 ± 0.31 b |
| PMP60 | 80.02 ± 0.22 c | 3.70 ± 0.19 c | 23.76 ± 0.22 b | 29.44 ± 0.20 b |
| PMP70 | 81.76 ± 0.60 d | 2.90 ± 0.31 d | 23.70 ± 0.32 b | 28.24 ± 0.46 c |
| PMPR140 | 77.34 ± 0.68 c | 1.48 ± 0.48 c | 19.76 ± 0.36 b | 28.11 ± 0.77 a |
| PMPR160 | 78.44 ± 0.42 b | 1.76 ± 0.34 bc | 20.00 ± 0.37 ab | 27.50 ± 0.41 ab |
| PMPR180 | 78.84 ± 0.58 ab | 1.84 ± 0.18 abc | 20.38 ± 0.34 a | 27.48 ± 0.50 ab |
| PMPR200 | 79.26 ± 0.80 a | 2.22 ± 0.26 a | 20.22 ± 0.49 ab | 27.13 ± 0.37 bc |
| PMPR220 | 79.62 ± 0.28 a | 1.94 ± 0.22 ab | 19.94 ± 0.38 ab | 26.64 ± 0.47 c |
| PMPT140 | 79.90 ± 0.37 a | 2.66 ± 0.64 b | 23.18 ± 0.33 a | 28.93 ± 0.37 a |
| PMPT150 | 79.72 ± 0.22 a | 4.12 ± 0.87 a | 23.02 ± 0.39 a | 29.15 ± 0.39 a |
| PMPT160 | 78.68 ± 0.59 b | 1.88 ± 0.43 c | 22.14 ± 0.65 b | 28.86 ± 0.72 a |
| PMPT170 | 78.24 ± 0.61 c | 3.90 ± 0.16 a | 18.62 ± 0.50 c | 27.03 ± 0.71 c |
| PMPT180 | 77.20 ± 0.54 d | 4.32 ± 0.33 a | 19.02 ± 0.24 c | 28.14 ± 0.40 b |
| Sample | FSH (μmol/g) | TSH (μmol/g) | DB (μmol/g) |
|---|---|---|---|
| PMP30 | 11.44 ± 0.21 b | 45.02 ± 0.62 d | 16.77 ± 0.20 d |
| PMP40 | 11.48 ± 0.07 b | 46.73 ± 0.75 c | 17.62 ± 0.34 c |
| PMP50 | 11.56 ± 0.25 b | 55.96 ± 0.37 a | 22.20 ± 0.06 a |
| PMP60 | 11.73 ± 0.19 b | 49.42 ± 0.79 b | 18.85 ± 0.30 b |
| PMP70 | 12.87 ± 0.61 a | 47.54 ± 0.64 c | 17.34 ± 0.02 cd |
| PMPR140 | 22.11 ± 0.50 d | 45.83 ± 0.49 a | 11.86 ± 0.04 a |
| PMPR160 | 23.91 ± 0.47 c | 48.20 ± 0.37 c | 12.15 ± 0.38 a |
| PMPR180 | 27.73 ± 0.74 a | 58.25 ± 0.75 a | 15.26 ± 0.34 bc |
| PMPR200 | 26.50 ± 0.55 b | 56.13 ± 0.74 b | 14.82 ± 0.55 c |
| PMPR220 | 24.15 ± 0.62 c | 55.47 ± 0.74 b | 15.66 ± 0.14 a |
| PMPT140 | 20.40 ± 0.40 b | 38.06 ± 0.66 d | 8.83 ± 0.23 bc |
| PMPT150 | 21.22 ± 0.29 b | 40.77 ± 0.69 c | 9.77 ± 0.47 b |
| PMPT160 | 22.29 ± 0.63 a | 45.55 ± 0.71 a | 11.62 ± 0.66 a |
| PMPT170 | 22.53 ± 0.33 a | 42.12 ± 0.28 b | 9.79 ± 0.30 b |
| PMPT180 | 23.02 ± 0.74 a | 40.26 ± 0.77 c | 8.62 ± 0.71 c |
| No. | MP Addition Amount (%) | Screw Speed (rpm) | Extrusion Temperature (°C) | Texturization Degree |
|---|---|---|---|---|
| 1 | 50 | 140 | 150 | 1.45 |
| 2 | 70 | 140 | 150 | 1.41 |
| 3 | 50 | 180 | 150 | 1.48 |
| 4 | 70 | 180 | 150 | 1.42 |
| 5 | 50 | 160 | 140 | 1.51 |
| 6 | 70 | 160 | 140 | 1.42 |
| 7 | 50 | 160 | 160 | 1.49 |
| 8 | 70 | 160 | 160 | 1.42 |
| 9 | 60 | 140 | 140 | 1.45 |
| 10 | 60 | 180 | 140 | 1.41 |
| 11 | 60 | 140 | 160 | 1.37 |
| 12 | 60 | 180 | 160 | 1.45 |
| 13 | 60 | 160 | 150 | 1.53 |
| 14 | 60 | 160 | 150 | 1.52 |
| 15 | 60 | 160 | 150 | 1.55 |
| 16 | 60 | 160 | 150 | 1.51 |
| 17 | 60 | 160 | 150 | 1.54 |
| Source of variance | Texturization degree | Dependent variable (Y) | Equation | |
| F | p | |||
| Quadratic model | 23.48 | 0.0002 | Texturization degree | Y = 1.53 − 0.0325A + 0.01B − 0.0075C − 0.005AB + 0.005AC + 0.03BC − 0.025A2 − 0.065B2 − 0.045C2 |
| MP addition (A) | 39.43 | 0.0004 | ||
| Screw speed (B) | 3.73 | 0.0946 | ||
| Extrusion temperature (C) | 2.1 | 0.1906 | ||
| AB | 0.4667 | 0.5165 | ||
| AC | 0.4667 | 0.5165 | ||
| BC | 16.8 | 0.0046 | ||
| A2 | 12.28 | 0.0099 | ||
| B2 | 83.02 | <0.0001 | ||
| C2 | 39.79 | 0.0004 | ||
| R2 | 0.97 | |||
| Radj2 | 0.93 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Ma, S.; Shen, R.; Dong, J.; Li, Y. Improving the Texturization of Pea Protein Through the Addition of a Mung Bean Protein Extract Solution and Optimizing the Moisture Content, Screw Speed, and Extrusion Temperature. Foods 2025, 14, 3750. https://doi.org/10.3390/foods14213750
Cheng Z, Ma S, Shen R, Dong J, Li Y. Improving the Texturization of Pea Protein Through the Addition of a Mung Bean Protein Extract Solution and Optimizing the Moisture Content, Screw Speed, and Extrusion Temperature. Foods. 2025; 14(21):3750. https://doi.org/10.3390/foods14213750
Chicago/Turabian StyleCheng, Zhe, Shunzhang Ma, Ruiling Shen, Jilin Dong, and Yunlong Li. 2025. "Improving the Texturization of Pea Protein Through the Addition of a Mung Bean Protein Extract Solution and Optimizing the Moisture Content, Screw Speed, and Extrusion Temperature" Foods 14, no. 21: 3750. https://doi.org/10.3390/foods14213750
APA StyleCheng, Z., Ma, S., Shen, R., Dong, J., & Li, Y. (2025). Improving the Texturization of Pea Protein Through the Addition of a Mung Bean Protein Extract Solution and Optimizing the Moisture Content, Screw Speed, and Extrusion Temperature. Foods, 14(21), 3750. https://doi.org/10.3390/foods14213750






