Phlorotannin–Alginate Extract from Nizimuddinia zanardinii for Melanosis Inhibition and Quality Preservation of Pacific White Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Seaweed Collection
2.3. Preliminary Solvent Screening and Extraction Procedure
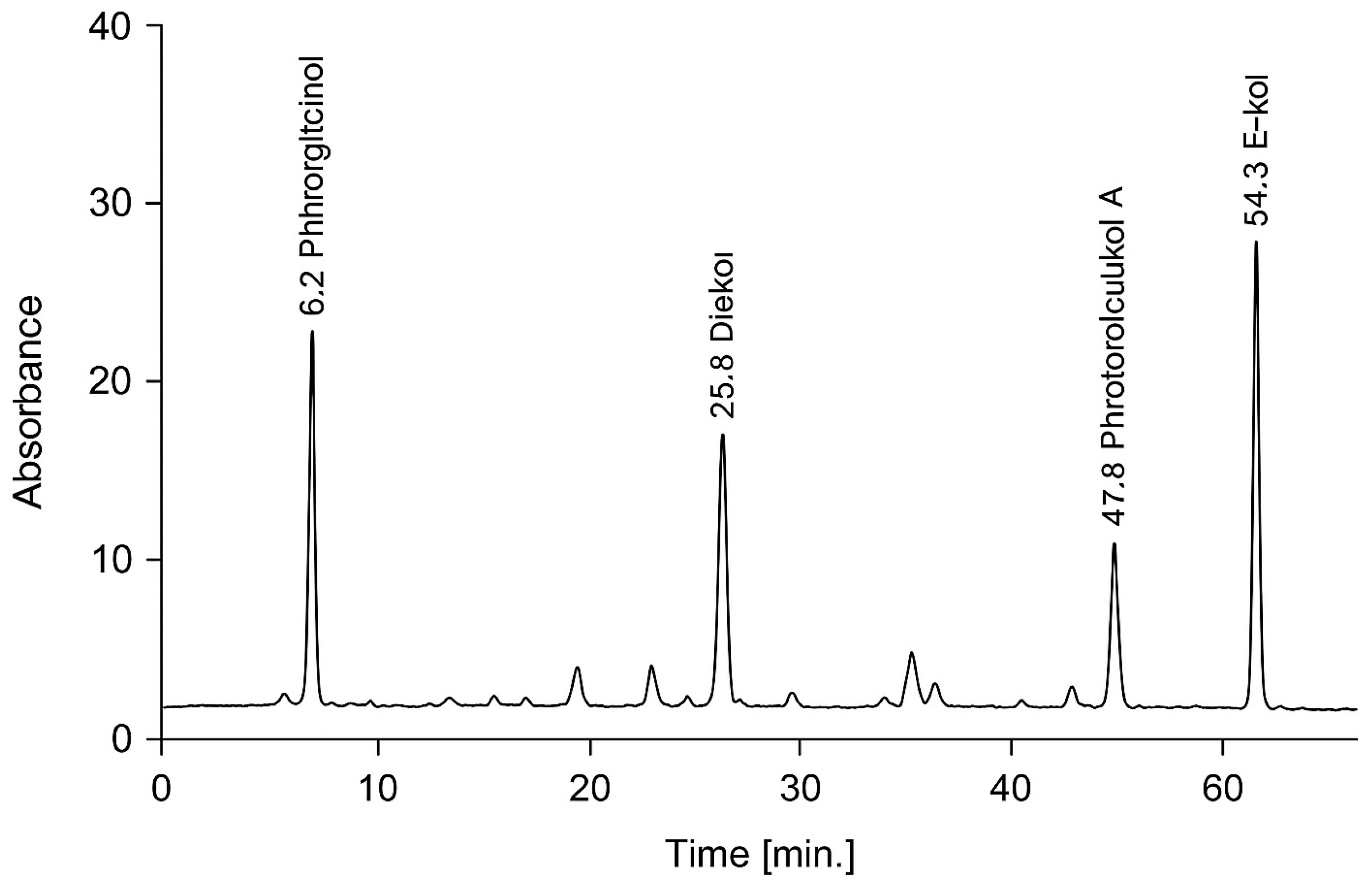
2.4. Extraction and Assay of Phlorotannins (PT)
2.5. Extraction of Alginate
2.6. Cupric Ion Chelating Activity
2.7. Effect of Phlorotannins and Alginate Extract on the Inhibition of Pacific White Shrimp PPO
2.7.1. Shrimp Sample Preparation
2.7.2. Extraction of PPO
2.7.3. PPO Inhibitory Activity of Phlorotannins and Alginate
2.8. Effect of Phlorotannins and Alginate Extract on the Quality of Pacific White Shrimp During Iced Storage
2.8.1. Treatment of Shrimp with Phlorotannins (PT) and Alginate (AG)
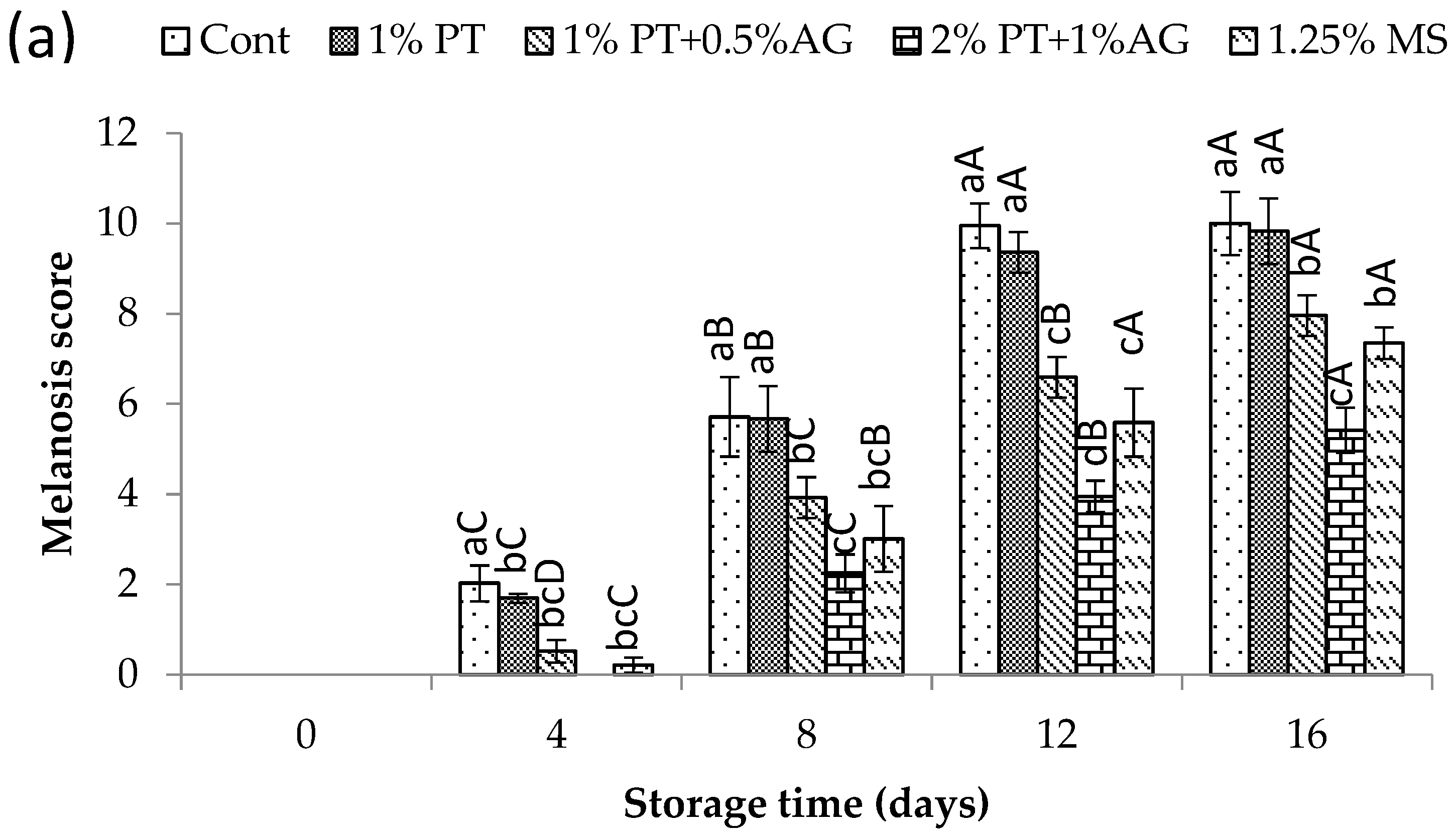
2.8.2. Melanosis Evaluation
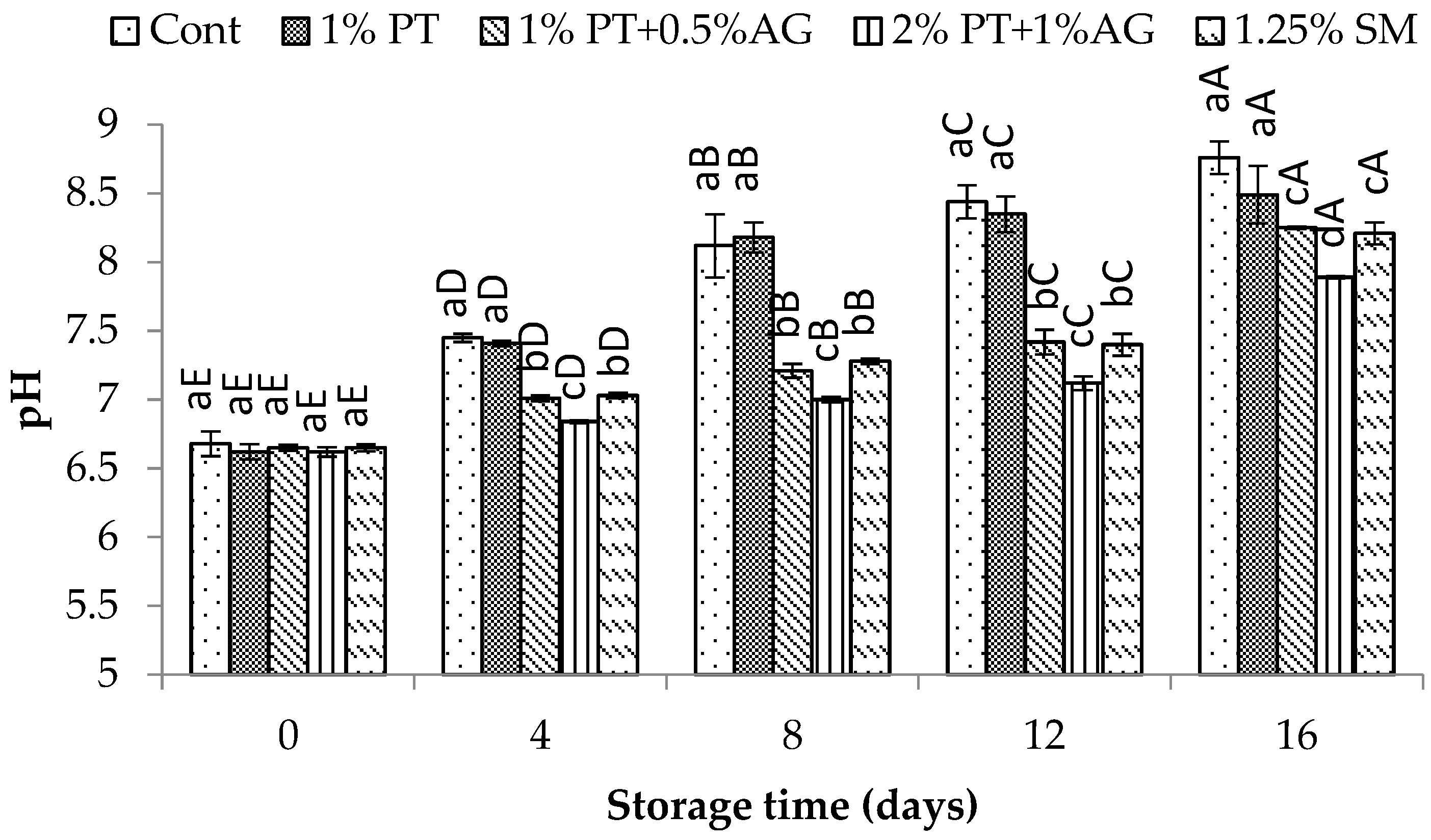
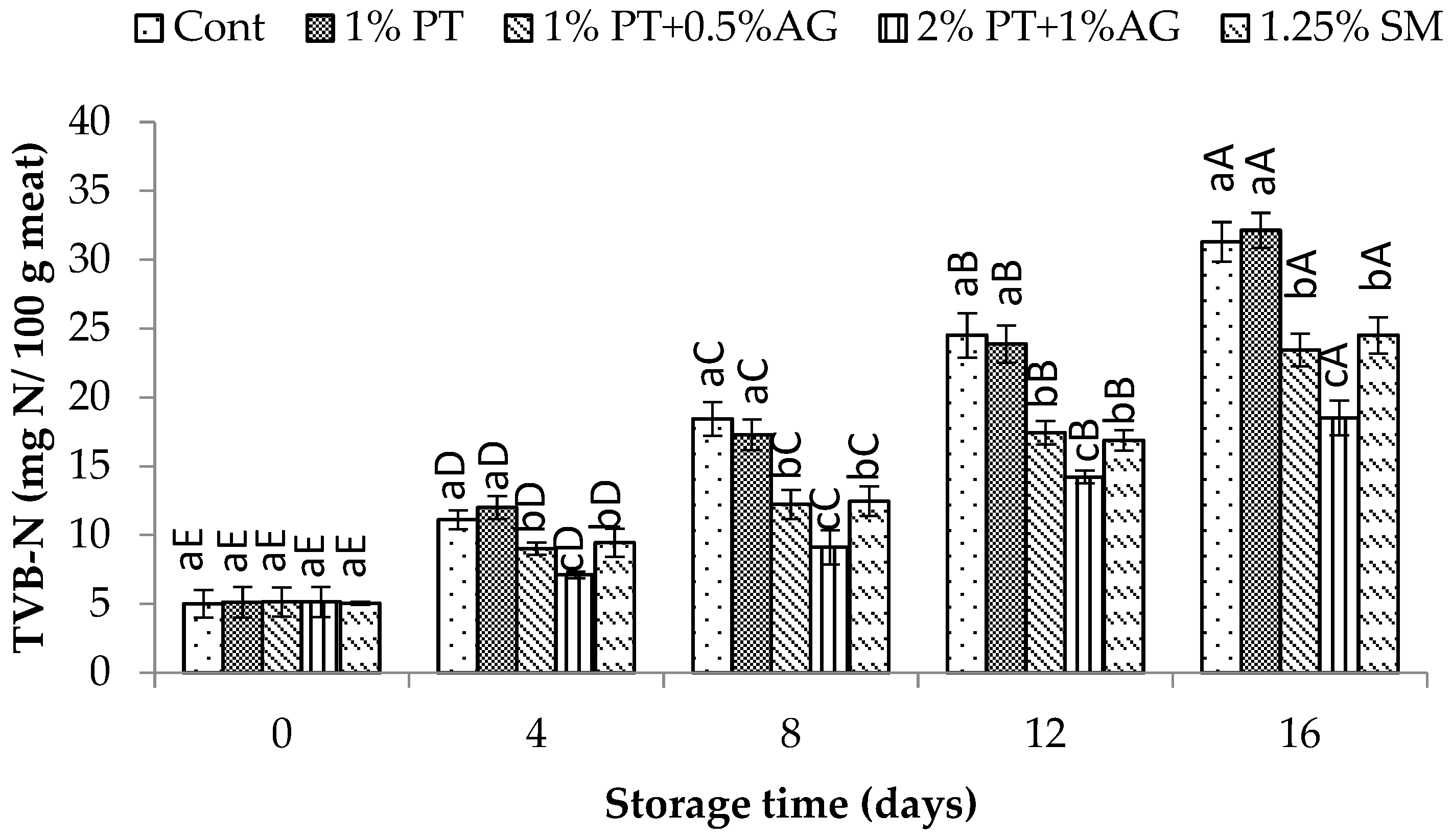
2.8.3. pH and Total Volatile Basic Nitrogen (TVB-N) Measurement
2.8.4. Peroxide Value (PV) Determination
2.8.5. Thiobarbituric Acid (TBA) Determination
2.8.6. Microbial Analyses
2.8.7. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Solvent Screening
3.2. Phlorotannins Content and Bioactivities of N. zanardinii Extracts
3.3. Yield and Bioactivities of Alginate from N. zanardinii
3.4. PPO Inhibitory Activity of Phlorotannins and Alginate
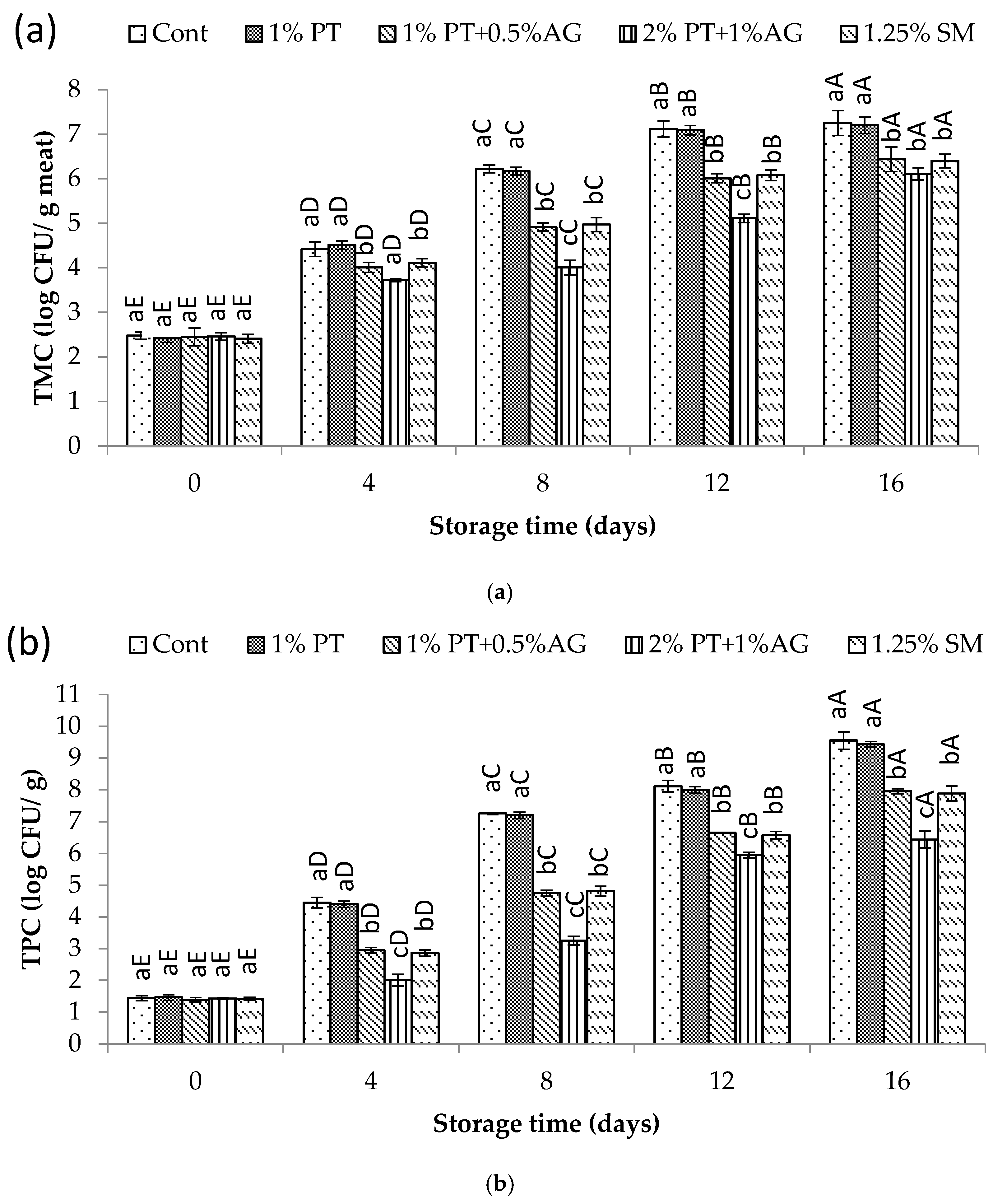
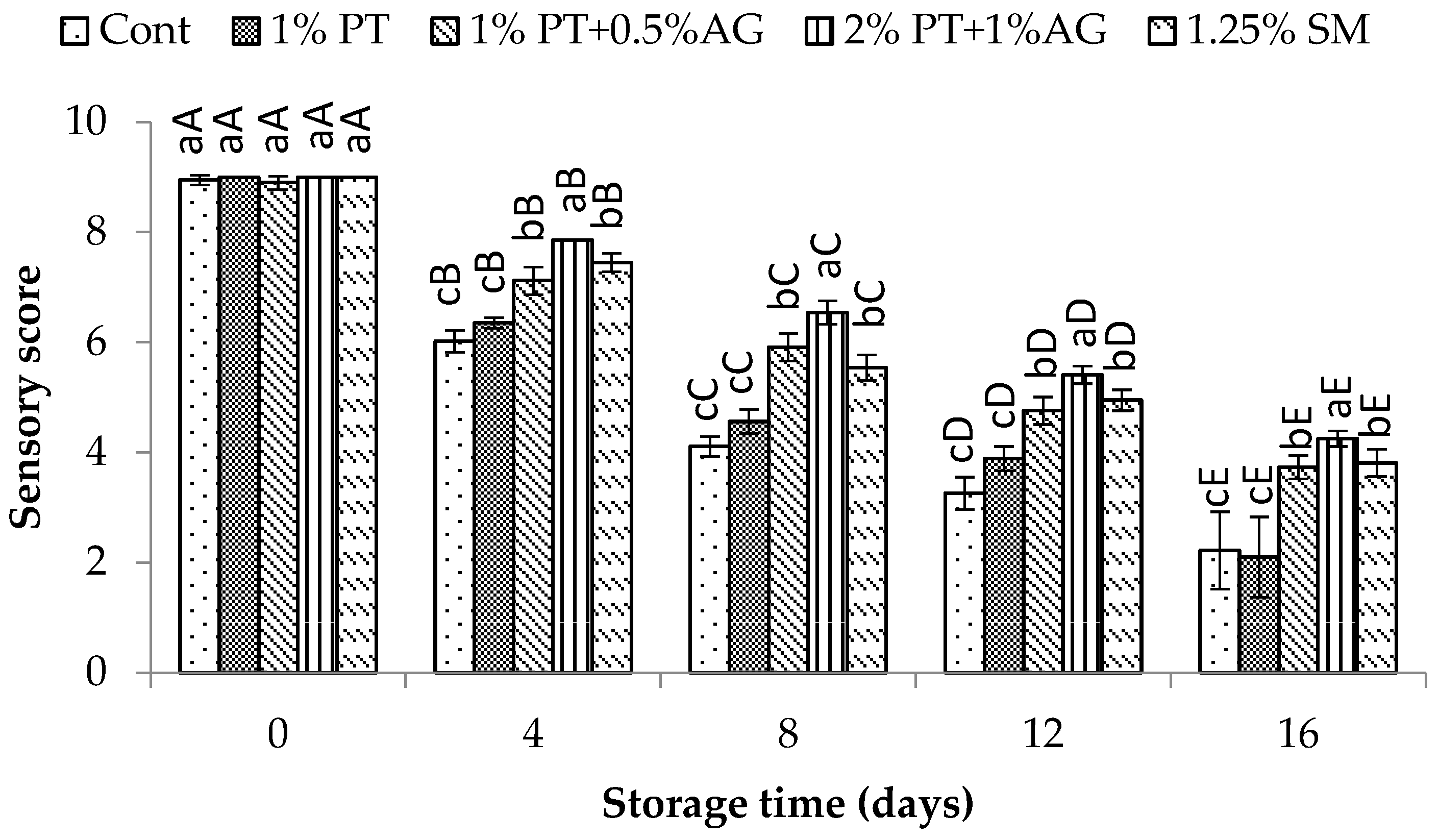
3.5. Effect of Phlorotannins and Alginate Extract on the Quality of Pacific White Shrimp During Iced Storage
3.5.1. Melanosis Changes
3.5.2. Physicochemical Changes
3.5.3. Microbial Changes
3.5.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifian, S.; Shabanpour, B.; Taheri, A.; Kordjazi, M. Effect of phlorotannins on melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2019, 298, 124980. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Yang, R.; Zhao, W. Combined effect of slightly acidic electrolyzed water and ascorbic acid to improve quality of whole chilled freshwater prawn (Macrobrachium rosenbergii). Food Control. 2020, 108, 106820. [Google Scholar] [CrossRef]
- Shiekh, K.H.; Benjakul, S.; Sae-leaw, T. Effect of Chamuang (Garcinia cowa Roxb.) leaf extract on inhibition of melanosis and quality changes of Pacific white shrimp during refrigerated storage. Food Chem. 2019, 270, 554–561. [Google Scholar] [CrossRef]
- Khan, F.; Jeong, G.-J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef]
- Jayakody, M.M.; Vanniarachchy, M.P.G.; Wijesekara, I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: A review. Food Meas. 2022, 16, 1195–1227. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, R.; Li, W.; Song, J.; Cheng, F. Improved postharvest preservation effects of Pholiota nameko mushroom by sodium alginate–based edible composite coating. Food Bioprocess Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Shams, M.; Afsharzadeh, S.; Balali, G. Taxonomic Study of Six Sargassum Species (Sargassaceae, Fucales) with Compressed Primary Branches in the Persian Gulf and Oman Sea Including S. binderi Sonder New Record Species for Algal Flora, Iran. J. Sci. Islamic Repub. Iran 2015, 26, 7–16. [Google Scholar]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process Biochem. 2017, 58, 289–297. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Kılınç, B.; Çaklı, Ş.; Kışla, D. Quality changes of sardine (Sardina pilchardus W., 1792) during frozen storage. Ege J. Fish. Aquat. Sci. 2003, 20, 139–146. [Google Scholar]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of the Official Analysis Chemists, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze-thaw cycles. Food Chem. 2001, 77, 207–217. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Fang, W.; Li, Y. Inhibitory effects of chitosan coating combined with organic acids on Listeria monocytogenes in refrigerated ready-to-eat shrimps. J. Food Prot. 2013, 76, 1377–1383. [Google Scholar] [CrossRef]
- Puspita, M.; Deniel, M.; Widowati, I. Total phenolic content and biological activities of enzymatic extraction from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activity of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Almeida, B.; Barroso, S.; Ferreira, A.S.D.; Adão, P.; Mendes, S.; Gil, M.M. Seasonal Evaluation of Phlorotannin-Enriched Extracts from Brown Macroalgae Fucus spiralis. Molecules 2021, 26, 4287. [Google Scholar] [CrossRef]
- Kirke, D.A.; Rai, D.K.; Smyth, T.J.; Stengel, D.B. An assessment of temporal variation in the low molecular weight phlorotannin profiles in four intertidal brown macroalgae. Algal Res. 2019, 41, 101550. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Le Lann, K.; Jégou, C.; Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: Comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol. Res. 2008, 56, 238–245. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Chaieb, K.; Bouraoui, A. Evaluation of antimicrobial activity of organic fractions of six marine algae from Tunisian Mediterranean coasts. Int. J. Pharm. Pharm. Sci. 2012, 4, 534–537. [Google Scholar]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D. A comprehensive analysis of alginate content and biochemical composition of leftover pulp from brown seaweed Sargassum wightii. Algal Res. 2017, 23, 233–239. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Kurihara, H.; Kujira, K. Phlorotannins derived from the brown alga Colpomenia bullosa as tyrosinase inhibitors. Nat. Prod. Commun. 2021, 16, 1–5. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Li, J. Shelf-life extension of Pacific white shrimp using algae extracts during refrigerated storage. J. Sci. Food Agric. 2017, 97, 291–298. [Google Scholar] [CrossRef]
- Xu, L.; Gao, L.; Meng, J.; Chang, M. Browning inhibition and postharvest quality of button mushrooms (Agaricus bisporus) treated with alginate and ascorbic acid edible coating. Int. Food Res. J. 2022, 29, 106–115. [Google Scholar] [CrossRef]
- Nazoori, F.; Poraziz, S.; Mirdehghan, S.H.; Esmailizadeh, M.; Zamani Bahramabadi, E. Improving shelf life of strawberry through application of sodium alginate and ascorbic acid coatings. Int. J. Hortic. Sci. Technol. 2020, 7, 279–293. [Google Scholar] [CrossRef]
- Afrin, F.; Ahsan, T.; Mondal, M.N.; Rasul, M.G.; Afrin, M.; Silva, A.A.; Yuan, C.; Shah, A.K.M.A. Evaluation of antioxidant and antibacterial activities of some selected seaweeds from Saint Martin’s Island of Bangladesh. Food Chem. Advanc. 2023, 3, 100393. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Sae-leaw, T.; Zhang, B.; Singh, P.; Kim, J.T.; Benjakul, S. Impact of Ethanolic Thai Indigenous Leaf Extracts on Melanosis Prevention and Shelf-Life Extension of Refrigerated Pacific White Shrimp. Foods 2023, 12, 3649. [Google Scholar] [CrossRef]



| Sargassum cristaefolium | Nizimudiana zanardinii | |||||
|---|---|---|---|---|---|---|
| Extraction Solvent | Extract Yield 1 | PT Content 2 | DPPH Assay 3 | Extract Yield | PT Content | DPPH Assay |
| MeOH100% | 4.23 ± 0.11 b* | 6.39 ± 0.07 c | 52.63 ± 0.99 b | 6.43 ± 0.09 a | 9.39 ± 0.27 b | 73.27 ± 0.94 b |
| MeOH70% | 5.50 ± 0.29 a | 5.33 ± 0.09 d | 48.08 ± 0.96 c | 5.05 ± 0.09 b | 6.40 ± 0.14 d | 52.48 ± 0.81 d |
| MeOH30% | 3.73 ± 0.11 c | 2.23 ± 0.11 e | 25.35 ± 0.70 d | 4.41 ± 0.21 c | 4.56 ± 0.11 e | 45.56 ± 0.93 e |
| H2O100% | 3.74 ± 0.08 c | 1.41 ± 0.15 f | 15.55 ± 0.61 f | 4.51 ± 0.22 c | 2.59 ± 0.17 g | 22.56 ± 0.95 g |
| EtOAc100% | 2.19 ± 0.11 f | 8.56 ± 0.08 a | 60.63 ± 0.54 a | 3.57 ± 0.14 d | 12.46 ± 0.14 a | 84.38 ± 1.14 a |
| EtOAc70% | 2.78 ± 0.05 e | 6.43 ± 0.10 b | 53.90 ± 0.65 b | 2.35 ± 0.09 e | 8.36 ± 0.17 c | 66.92 ± 0.63 c |
| EtOAc30% | 3.26 ± 0.06 d | 2.37 ± 0.10 e | 18.65 ± 0.72 e | 2.88 ± 0.07 e | 3.62 ± 0.09 f | 27.74 ± 0.95 f |
| Fractions | Extract Yield 1 | PT Content 2 | DPPH Assay 3 | Copper Chelating 3 |
|---|---|---|---|---|
| First extract | 6.43 ± 0.09 a* | 9.39 ± 0.27 b | 73.27 ± 0.94 b | 43.45 ± 1.17 b |
| TCM fraction | 0.28 ± 0.03 e | 1.35 ± 0.96 d | 5.98 ± 0.43 e | 2.34 ± 1.05 e |
| DCM faction | 0.85 ± 0.04 d | 5.05 ± 1.03 c | 52.36 ± 0.81 c | 34.42 ± 1.55 c |
| EtOAc fraction | 2.54 ± 0.12 b | 19.14 ± 0.65 a | 98.95 ± 0.74 a | 73.44 ± 1.64 a |
| Aqueous fraction | 2.16 ± 0.15 c | 1.51 ± 0.53 d | 13.66 ± 1.06 d | 11.43 ± 0.88 d |
| 0.1% PT | 0.5% PT | 1% PT | 1% SM | 1% AG | 1% PT + 0.5% AG | 2% PT + 1% AG | 1% AA | |
|---|---|---|---|---|---|---|---|---|
| PPO inhibition | 13.30 ± 0.85 g | 32.67 ± 1.22 e | 68.37 ± 0.65 d | 67.65 ± 1.42 d | 27.39 ± 1.05 f | 76.65 ± 1.27 b | 84.51 ± 2.15 a | 72.43 ± 1.42 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifian, S.; Bita, S. Phlorotannin–Alginate Extract from Nizimuddinia zanardinii for Melanosis Inhibition and Quality Preservation of Pacific White Shrimp. Foods 2025, 14, 3736. https://doi.org/10.3390/foods14213736
Sharifian S, Bita S. Phlorotannin–Alginate Extract from Nizimuddinia zanardinii for Melanosis Inhibition and Quality Preservation of Pacific White Shrimp. Foods. 2025; 14(21):3736. https://doi.org/10.3390/foods14213736
Chicago/Turabian StyleSharifian, Salim, and Seraj Bita. 2025. "Phlorotannin–Alginate Extract from Nizimuddinia zanardinii for Melanosis Inhibition and Quality Preservation of Pacific White Shrimp" Foods 14, no. 21: 3736. https://doi.org/10.3390/foods14213736
APA StyleSharifian, S., & Bita, S. (2025). Phlorotannin–Alginate Extract from Nizimuddinia zanardinii for Melanosis Inhibition and Quality Preservation of Pacific White Shrimp. Foods, 14(21), 3736. https://doi.org/10.3390/foods14213736






