Study on the Changes of Antioxidant System and Respiratory Metabolism in Rice Grains Under Nitrogen-Modified Atmosphere Storage from the Targeted Metabolomics Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Grain Materials and Storage Conditions
2.2. Determination of Fatty Acid Values (FAV)
2.3. Determination of Reactive Oxygen Species (ROS) Contents
2.4. Determination of Antioxidant Enzyme Activities
2.5. Determination of Pyridine Nucleotides Contents
2.6. Targeted Metabolomics Profiling of Central Carbon Metabolism
2.6.1. Metabolite Extraction and Standard Solution Preparation
2.6.2. HPIC-MRM-MS Analysis
2.7. Statistical Analysis
3. Results
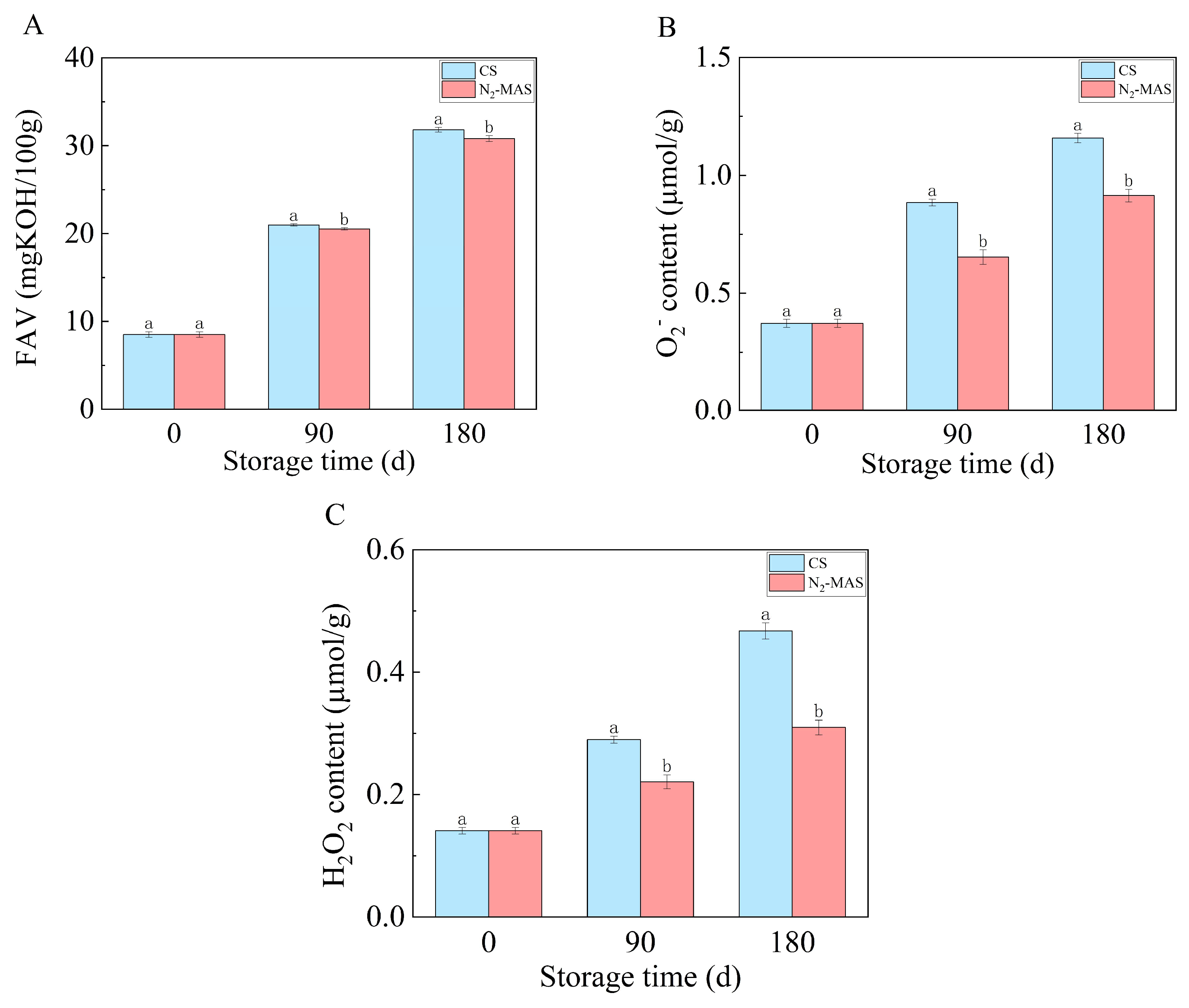
3.1. Effect of N2-MAS on Fatty Acid Values (FAV) and Reactive Oxygen Species (ROS) in Rice Grains
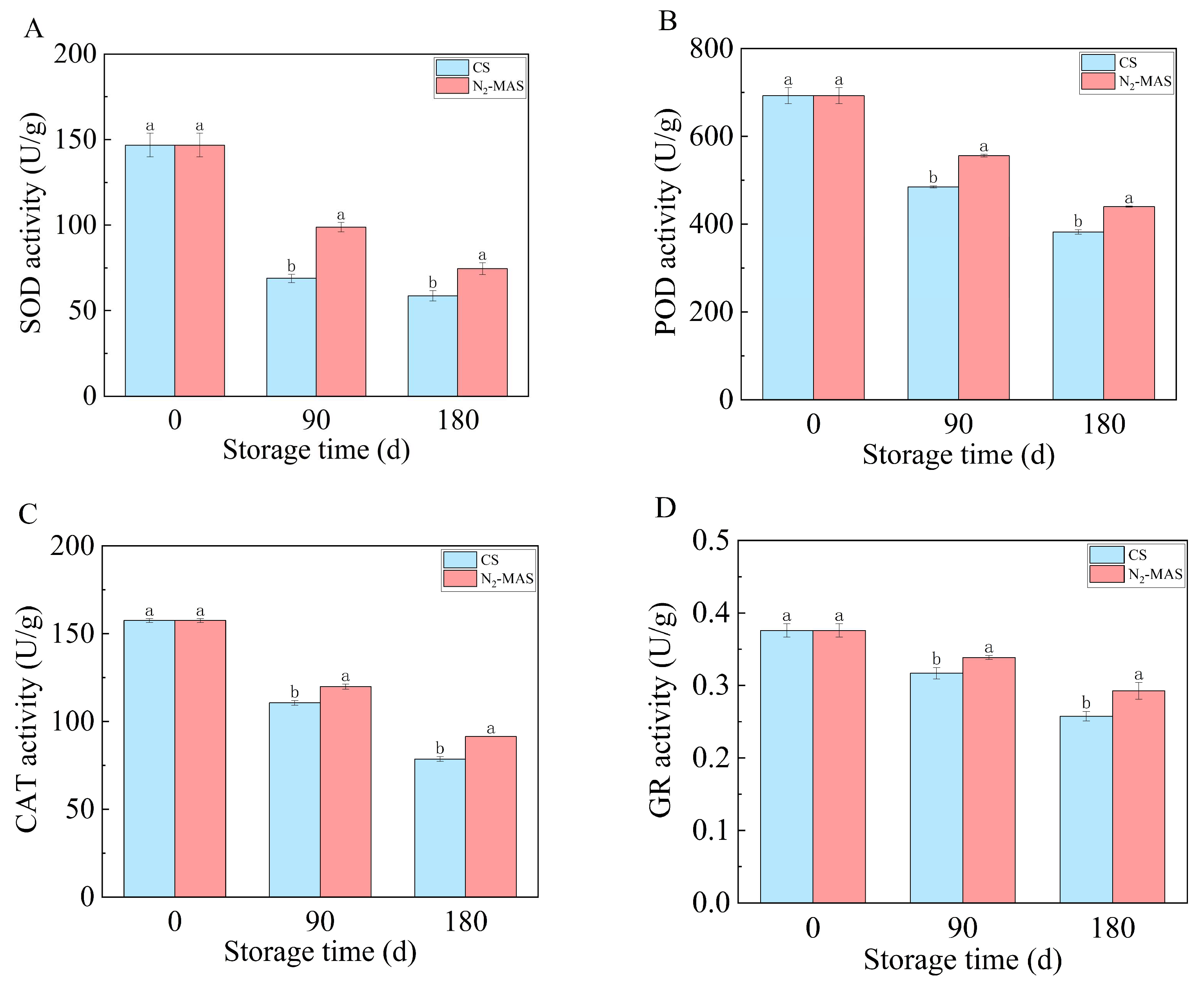
3.2. Effect of N2-MAS on the Activities of Antioxidant Enzymes
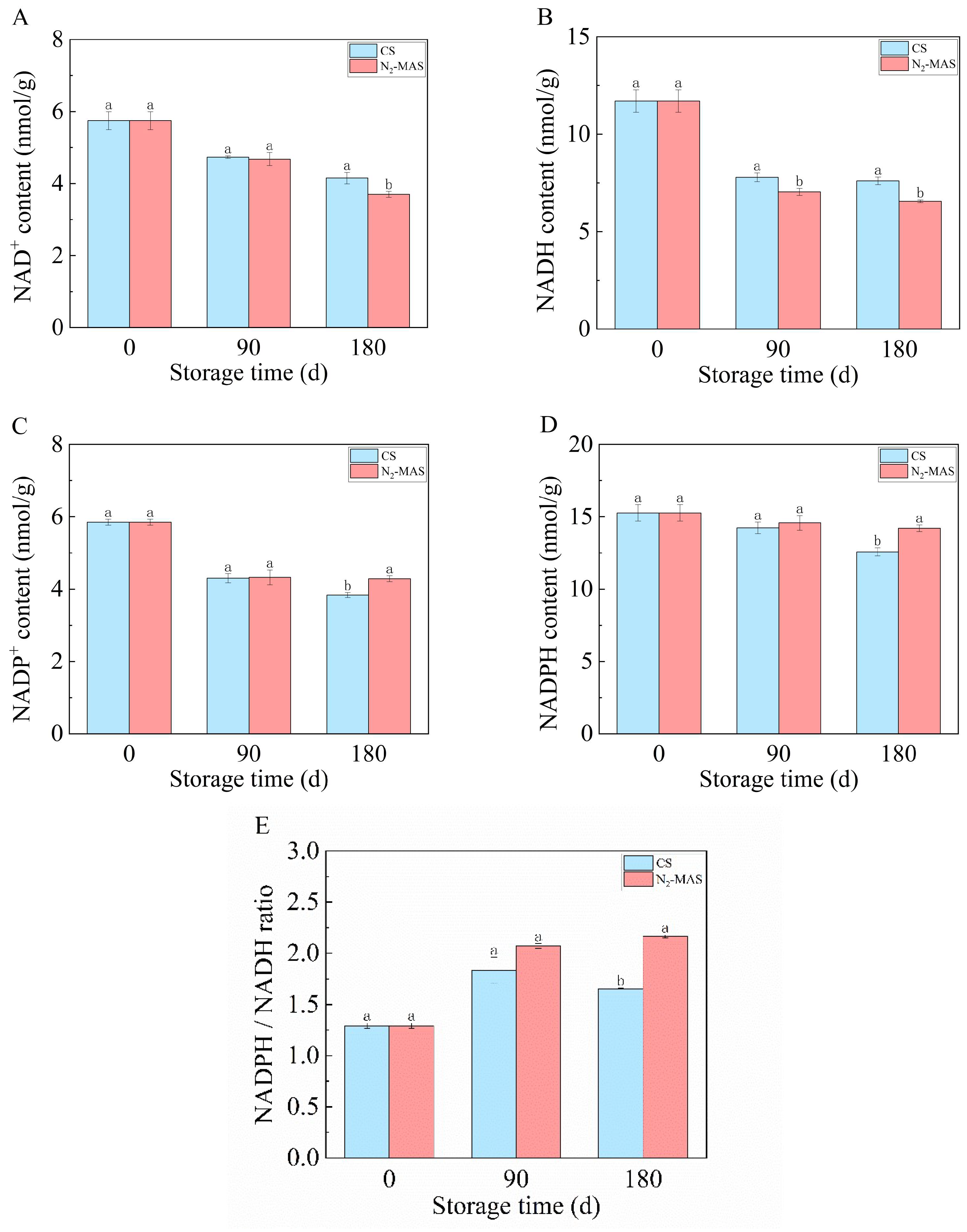
3.3. Effect of N2-MAS on the Contents of Pyridine Nucleotides in Rice Grains
3.4. Effect of N2-MAS on the Metabolites in Rice Grains
3.4.1. Comparative Metabolomic Profiling of Central Carbon Metabolites in Rice Grains Under N2-MAS and CS
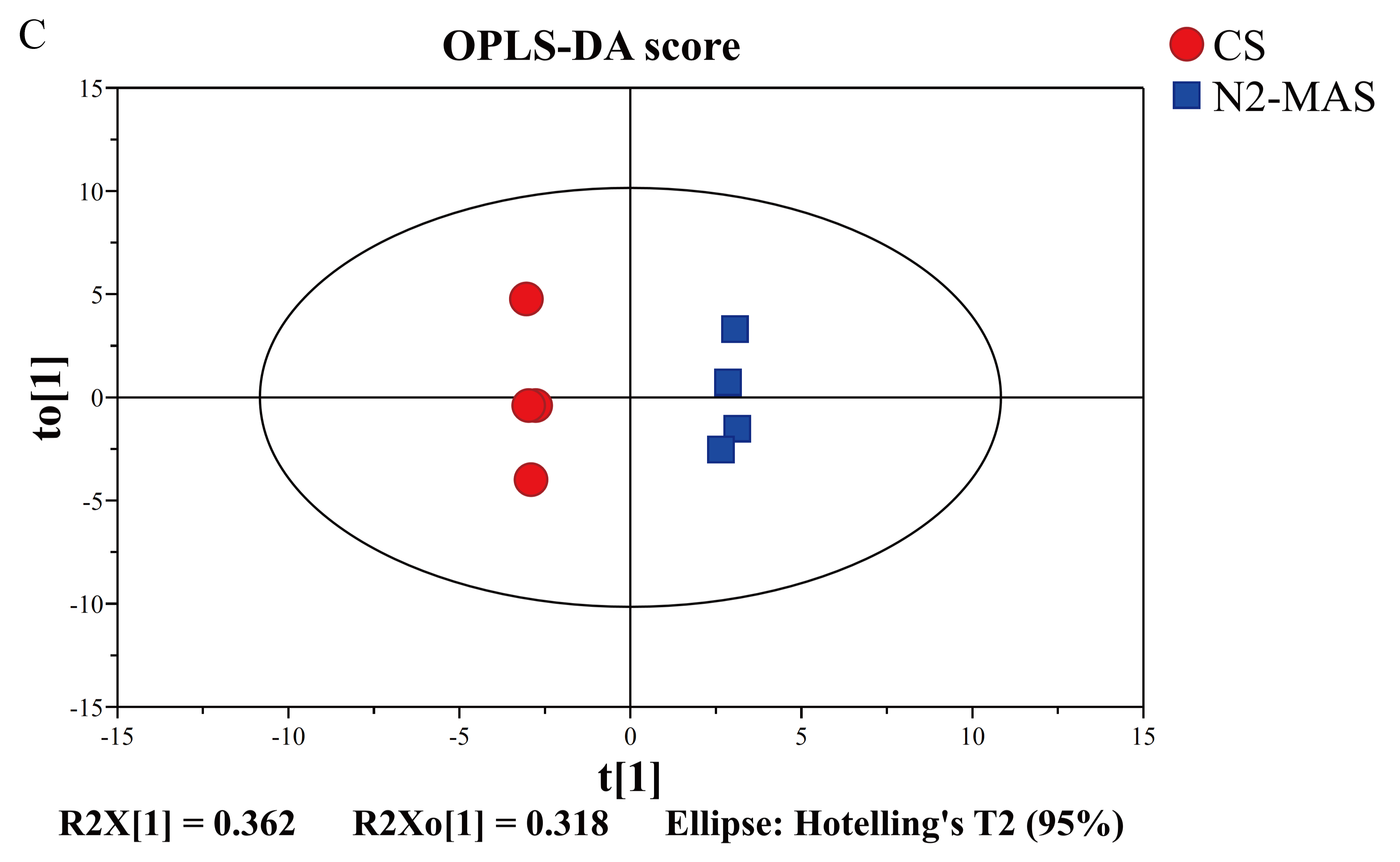
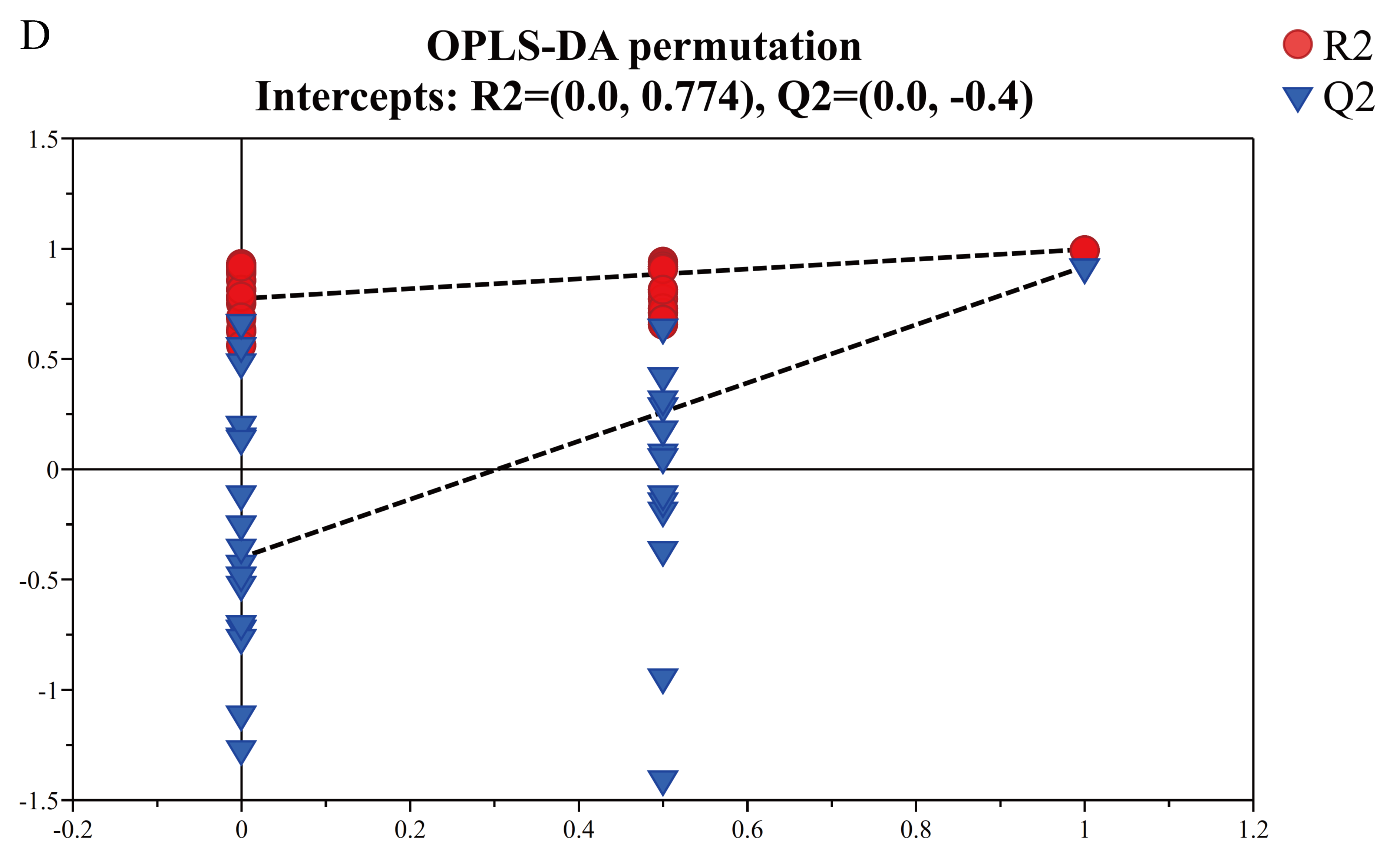
3.4.2. Multivariate Statistical Analysis
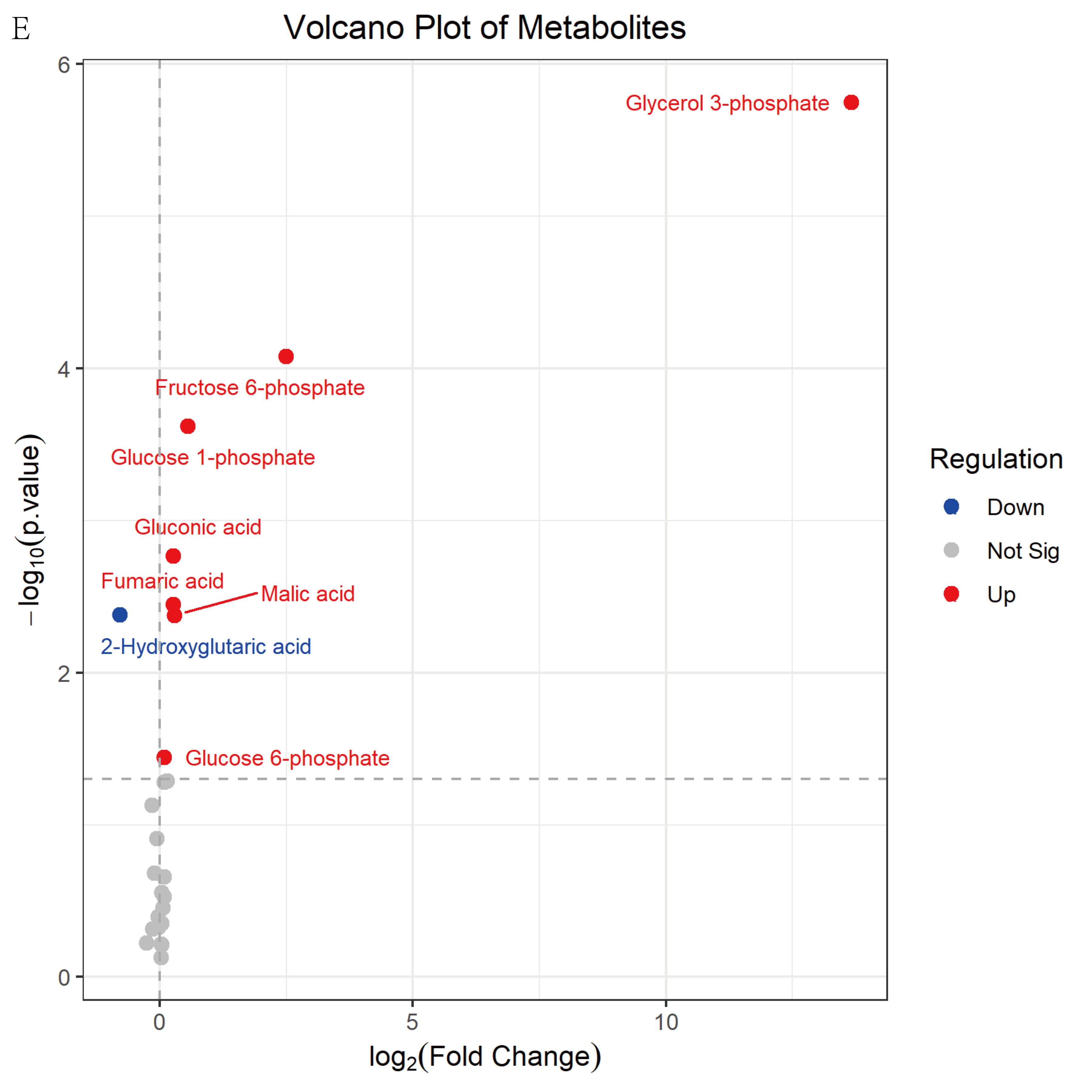
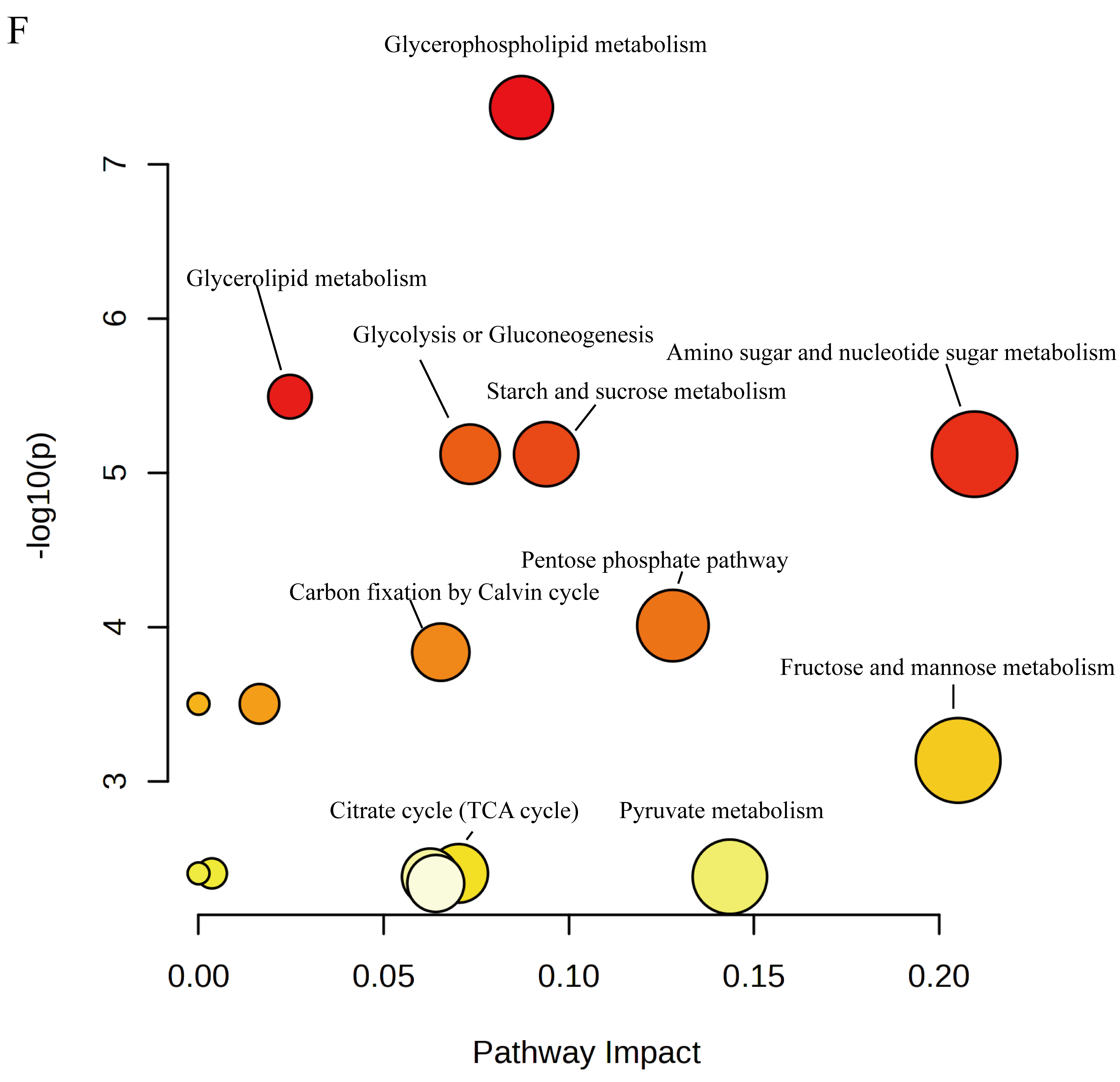
3.4.3. Identification of Key Metabolites in Rice Grains
4. Discussion
4.1. N2-MAS Induced Alterations in Oxidative Stability of Rice Grains
4.2. N2-MAS-Induced Alterations in Central Carbon Metabolism and Its Relationships to Antioxidant System of Rice Grains
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Guo, J.; Ye, C.; Chen, K.; Zhou, X.; Chen, D.; Xiao, X.; Liu, C. Low temperature storage alleviates aging of paddy by reducing lipid degradation and peroxidation. Food Chem. 2025, 465, 142140. [Google Scholar] [CrossRef]
- Qu, C.; Li, W.; Yang, Q.; Xia, Y.; Lu, P.; Hu, M. Metabolic mechanism of nitrogen modified atmosphere storage on delaying quality deterioration of rice grains. Food Chem. X 2022, 16, 100519. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.B.; Augustin, M.A.; Robertson, M.J.; Manners, J.M. The science of food security. NPJ Sci. Food 2018, 2, 14. [Google Scholar] [CrossRef]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain storage systems and effects of moisture, temperature and time on grain quality—A review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- De Sousa, I.G.; Oliveira, J.; Mexia, A.; Barros, G.; Almeida, C.; Brazinha, C.; Vega, A.; Brites, C. Advances in Environmentally Friendly Techniques and Circular Economy Approaches for Insect Infestation Management in Stored Rice Grains. Foods 2023, 12, 511. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Lu, Q. Exploration of Mechanisms for Internal Deterioration of Wheat Seeds in Postharvest Storage and Nitrogen Atmosphere Control for Properties Protection. Crop Sci. 2018, 58, 823–836. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Lin, M.; Ritenour, M.A.; Lin, Y. The role of ROS-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage. Food Chem. 2020, 305, 125439. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, M.; Zhen Wu, Z.; Qin, N.; Fu, Y.; Guo, S. Nutrient composition and functional constituents of daylily from different producing areas based on widely targeted metabolomics. Food Chem. X 2024, 21, 101239. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite Changes during Postharvest Storage: Effects on Fruit Quality Traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef]
- Olszewska-Czyz, I.; Kralik, K.; Tota, M.; Prpic, J. The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial. Antioxidants 2022, 11, 135. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Shi, J. Application of propyl gallate alleviates pericarp browning in harvested longan fruit by modulating metabolisms of respiration and energy. Food Chem. 2018, 240, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef]
- Katsuya-Gaviria, K.; Caro, E.; Carrillo-Barral, N.; Iglesias-Fernández, R. Reactive Oxygen Species (ROS) and Nucleic Acid Modifications during Seed Dormancy. Plants 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Sahu, B.; Sahu, A.K.; Thomas, V.; Naithani, S.C. Reactive oxygen species, lipid peroxidation, protein oxidation and antioxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. South. Afr. J. Bot. 2017, 112, 383–390. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Lin, Y.; Lin, Y.; Hung, Y.-C.; Chen, Y.; Wang, H.; Shi, J. Energy status regulates disease development and respiratory metabolism of Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-infected longan fruit. Food Chem. 2017, 231, 238–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, F.; He, F.; Wu, G.; Zhang, S.; Lin, H. Exogenous nitric oxide inhibits the respiratory metabolism of postharvest wax apple fruit and its role in the delayed cottony softening. Sci. Hortic. 2023, 317, 112043. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-García, I. Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem. 2016, 51, 1891–1903. [Google Scholar] [CrossRef]
- Li, W.; Lu, X.; Li, J. The effect of organic nutrient solution on flavor in ripe cherry tomato fruit—Transcriptome and metabolomic analyses. Environ. Exp. Bot. 2022, 194, 104721. [Google Scholar] [CrossRef]
- Tadege, M. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Chia, D.W.; Yoder, T.J.; Reiter, W.-D.; Gibson, S.I. Fumaric acid: An overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 2000, 211, 743–751. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, P.; Cui, Y.; Liu, D.; Li, J.; Zhao, Y.; Yang, S.; Zhang, B.; Zhou, R.; Sun, M.; et al. Enhanced production of seed oil with improved fatty acid composition by overexpressing NAD+-dependent glycerol-3-phosphate dehydrogenase in soybean. J. Integr. Plant Biol. 2021, 63, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.Y.; Ouyang, Q.; Tumanov, S.; Xu, J.; Rong, P.; Dong, F.; Lam, S.M.; Wang, X.; Lukmantara, I.; Du, X.; et al. AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway. Nat. Commun. 2021, 12, 6877. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, H.; Ren, Y.; Lu, L. GlcA-mediated glycerol-3-phosphate synthesis contributes to the oxidation resistance of Aspergillus fumigatus via decreasing the cellular ROS. Fungal Genet. Biol. 2021, 149, 103531. [Google Scholar] [CrossRef]
- Maurino, V.G.; Engqvist, M.K.M. 2-Hydroxy Acids in Plant Metabolism. Arab. Book. 2015, 13, e0182. [Google Scholar] [CrossRef]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A. Identification of the 2-Hydroxyglutarate and Isovaleryl-CoA Dehydrogenases as Alternative Electron Donors Linking Lysine Catabolism to the Electron Transport Chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef]
- Hüdig, M.; Maier, A.; Scherrers, I.; Seidel, L.; Jansen, E.E.W.; Mettler-Altmann, T.; Engqvist, M.K.M.; Maurino, V.G. Plants Possess a Cyclic Mitochondrial Metabolic Pathway similar to the Mammalian Metabolic Repair Mechanism Involving Malate Dehydrogenase and l-2-Hydroxyglutarate Dehydrogenase. Plant Cell Physiol. 2015, 56, 1820–1830. [Google Scholar] [CrossRef][Green Version]
- Fettke, J.; Malinova, I.; Albrecht, T.; Hejazi, M.; Steup, M. Glucose-1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis. Plant Physiol. 2011, 155, 1723–1734. [Google Scholar] [CrossRef]
- Kunz, H.H.; Häusler, R.E.; Fettke, J.; Herbst, K.; Niewiadomski, P.; Gierth, M.; Bell, K.; Steup, M.; Flügge, U.I.; Schneider, A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. 2010, 12 (Suppl. S1), 115–128. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Shan, S.; Cao, F.; Chen, G.; Zou, Y.; Huang, M.; Abou-Elwafa, S.F. Proteomic profiling reveals differentially expressed proteins associated with amylose accumulation during rice grain filling. BMC Genom. 2020, 21, 714. [Google Scholar] [CrossRef] [PubMed]
- Owusu, A.G.; Lv, Y.-P.; Liu, M.; Wu, Y.; Li, C.-L.; Guo, N.; Li, D.-H.; Gao, J.-S. Transcriptomic and metabolomic analyses reveal the potential mechanism of waterlogging resistance in cotton (Gossypium hirsutum L.). Front. Plant Sci. 2023, 14, 1088537. [Google Scholar] [CrossRef]
- Wang, C.; Lu, C.; Wang, J.; Liu, X.; Wei, Z.; Qin, Y.; Zhang, H.; Wang, X.; Wei, B.; Lv, W. Molecular mechanisms regulating glucose metabolism in quinoa (Chenopodium quinoa Willd.) seeds under drought stress. BMC Plant Biol. 2024, 24, 796. [Google Scholar] [CrossRef] [PubMed]



| Metabolites | Mean (nmol/g) | p-Value | VIP | Fold Change | |
|---|---|---|---|---|---|
| N2-MAS | CS | N2-MAS/CS | |||
| Malic acid | 19.2 | 15.8 | 0.00 | 1.33 | 1.22 |
| Glycerol 3-phosphate | 65.3 | - | 0.00 | 1.45 | +∞ |
| Glucose 1-phosphate | 0.185 | 0.126 | 0.00 | 1.38 | 1.47 |
| Gluconic acid | 180 | 150 | 0.00 | 1.36 | 1.20 |
| Fumaric acid | 7.86 | 6.55 | 0.00 | 1.33 | 1.20 |
| Fructose 6-phosphate | 28.0 × 10−3 | 5.00 × 10−3 | 0.00 | 1.36 | 5.61 |
| 2-Hydroxyglutaric acid | 3.98 | 6.91 | 0.00 | 1.29 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Ma, X.; Li, W.; Xue, F.; Qu, C. Study on the Changes of Antioxidant System and Respiratory Metabolism in Rice Grains Under Nitrogen-Modified Atmosphere Storage from the Targeted Metabolomics Perspective. Foods 2025, 14, 3643. https://doi.org/10.3390/foods14213643
Chen M, Ma X, Li W, Xue F, Qu C. Study on the Changes of Antioxidant System and Respiratory Metabolism in Rice Grains Under Nitrogen-Modified Atmosphere Storage from the Targeted Metabolomics Perspective. Foods. 2025; 14(21):3643. https://doi.org/10.3390/foods14213643
Chicago/Turabian StyleChen, Ming, Xia Ma, Wenhao Li, Feiyan Xue, and Chenling Qu. 2025. "Study on the Changes of Antioxidant System and Respiratory Metabolism in Rice Grains Under Nitrogen-Modified Atmosphere Storage from the Targeted Metabolomics Perspective" Foods 14, no. 21: 3643. https://doi.org/10.3390/foods14213643
APA StyleChen, M., Ma, X., Li, W., Xue, F., & Qu, C. (2025). Study on the Changes of Antioxidant System and Respiratory Metabolism in Rice Grains Under Nitrogen-Modified Atmosphere Storage from the Targeted Metabolomics Perspective. Foods, 14(21), 3643. https://doi.org/10.3390/foods14213643





