Abstract
Sea cucumber ovum are high-value compounds that remain after the processing of sea cucumbers, and their optimal utilization has long posed a challenge. In this research, we systematically examined the therapeutic effects of sea cucumber ovum hydrolysate (SCH) on premature ovarian failure (POF) and its underlying mechanism. We utilized a model of ICR mice induced with 100 mg/kg cyclophosphamide (CP) to evaluate the therapeutic influence of SCH on ovarian performance. The ovarian and uterine indices were significantly decreased in the POF group compared to the control group; however, these trends were notably reversed following SCH intervention. The therapeutic effects of SCH were positively reflected by the alterations induced by CP in levels of estradiol (E2), follicle-stimulating hormone (FSH), testosterone (T), luteinizing hormone (LH), and anti-Müllerian hormone (AMH). Regarding oxidative stress, SCH was found to enhance superoxide dismutase (SOD) activity and decrease malondialdehyde (MDA) levels, while also alleviating apoptosis in ovarian granulosa cells. Metabolomics analysis revealed hypoxanthine, mannitol, neocnidilide, tryptophan, palmitoleic acid, and protoporphyrinogen IX as potential biomarkers. In conclusion, SCH effectively improves POF induced by CP, thereby reinforcing the potential application of SCH in the domain of functional foods.
1. Introduction
Premature ovarian failure (POF), now more commonly referred to as primary ovarian insufficiency (POI), is a condition characterized by low estrogen levels in women prior to the age of 40 [1]. Estrogen, a vital sex hormone in the female body, is primarily secreted by the ovaries and plays a crucial role in various physiological processes. It significantly influences women’s reproductive health, bone health, metabolic regulation, and cardiovascular health [2,3]. The primary etiological factors associated with this syndrome include hysterectomy, genetic disorders such as Turner syndrome, cancer treatments, and autoimmune disorders [4]. The prevalence of this heterogeneous disease has been reported to range from 1% to 5%. Unfortunately, the disease is irreversible, and currently available management strategies, including hormone replacement therapy (HRT), can only alleviate the symptoms and side effects associated with the disease and related disorders [5,6].
In contemporary research, natural products play a significant role in the development of pharmaceuticals and food products. Their multi-pathway, multi-target mechanisms, combined with high efficacy and low toxicity, have been demonstrated in numerous studies to confer a wide array of beneficial activities, including anti-inflammatory, antioxidant, anti-proliferative, proapoptotic, and germ-protective effects [7]. The impact of various natural products (polyphenols, saponins, alkaloids, and polysaccharides) on ovarian function and POF has revealed their considerable potential [8]. Among these, flavonoids, also referred to as phytoestrogens, are non-steroidal polyphenolic compounds derived from plants. Their molecular structure bears similarities to endogenous estrogens and estradiol, which predisposes these phytoestrogens to bind to estrogen receptors across different cell types, thereby eliciting estrogenic or anti-estrogenic effects [9]. Recently, the influence of food-derived bioactive peptides on ovarian function has garnered attention. Reports indicated that oral administration of oyster peptide could restore the d-galactose-induced irregular estrous cycle and correct disturbances in serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), while also reducing apoptosis in ovarian granulosa cells [10]. Furthermore, tilapia skin peptides have been shown to modulate the Bcl-2/Bax/caspase-3 apoptotic pathway and enhance the Nrf2/HO-1 signaling pathway, thereby improving POF by alleviating ovarian oxidative stress and reducing granulosa cell apoptosis [11].
Sea cucumbers, marine creatures widely distributed across the globe, have been utilized as tonics in Chinese culture [12,13]. They contain a variety of bioactive substances such as triterpene glycosides (saponins), chondroitin sulfate, glycosaminoglycans (GAGs), and peptides, which play crucial roles in numerous unique biological and pharmacological activities [14]. Recent studies have demonstrated that peptides derived from the sea cucumber (Acaudina leucoprocta) enhance sex hormone synthesis by upregulating the expression of StAR, Fshr and Cyp19a1 in the ovaries of mice with POF [15]. The potential of sea cucumbers to alleviate symptoms of POF is indicated by the presence of sex hormones (estradiol, progesterone, and testosterone) in their gonadal and neural tissues [16]. Sea cucumber ovum, a low-value by-product of industrial processing, represents a promising source of protein hydrolysates with multiple functional properties. Consequently, this study was designed to examine the mitigating effects of sea cucumber ovum hydrolysates (SCH) on POF. We examined and analyzed body weight, organ indices, estrous cycle, histopathology, hormone levels, antioxidant capacity and ovarian apoptosis. Additionally, metabolomics was employed to elucidate the potential mechanisms involved.
2. Materials and Methods
2.1. Reagents and Experimental Materials
The sea cucumber ovum used in this study was sourced from the Yantai Haizhongbao Seafood Trading Center (Yantai, China). Flavourzyme (15,000 U/g) was provided by Solarbio Biotechnology Co., Ltd. (Beijing, China). Cyclophosphamide (CP) was purchased from Shanghai Bichen Biochemical Technology Co., Ltd. (Shanghai, China), and soy isoflavones (SIF) was acquired from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Superoxide dismutase (SOD) and malondialdehyde (MDA) kits were supplied by the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for testosterone (T), estradiol (E2), FSH, LH, and anti-Müllerian hormone (AMH) were sourced from Jiangsu Aikang Science & Technology Co., Ltd. (Suzhou, China). All other chemical reagents utilized in this study were of analytical grade and acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Preparation of SCH
The cleaned sea cucumber ovum was hydrolyzed at 50 °C using flavourzyme for a duration of 5 h. Following hydrolysis, the mixture was heated to 100 °C for 15 min to inactivate the enzyme. Upon chilling the sample with ice-cold water, the supernatant was achieved by centrifugation at 10,000 rpm for 15 min at 4 °C. Finally, the harvested supernatant was subjected to freeze-drying to yield the end product, SCH.
2.3. Determination of Dynamic Light Scattering (DLS)
SCH was dissolved in ultrapure water to create a 0.5 M solution, following a modification of the method proposed by Li et al. [17]. The ζ-potential, particle size, and polydispersity index (PDI) were accurately determined using the DLS technique (Nano Brook, Brookhaven, Nashua, NH, USA).
2.4. Determination of Amino Acids Composition
Modifications were implemented to determine the amino acid composition of SCH, in accordance with the protocol established by Chen et al. [18]. SCH was subjected to acid hydrolysis using 6 M hydrochloric acid at 110 °C for 22 h. The amino acid composition of SCH was then determined employing an L-8900 high-speed amino acid analyzer (Hitachi, Tokyo, Japan).
2.5. Animals and Experimental Design
Forty-five female ICR mice (8–10 weeks old, body weight 30 ± 2 g) were purchased from Jinan Pengyue experimental animal breeding Co., Ltd. (Jinan, China) and were maintained in a sterile environment with a 1-week acclimatization period.
After the acclimatization period, a POF model was established following the modifications of Luo et al. [15]. The 45 female mice were randomly divided into five groups: the control group, the model group (POF group), the positive control group (POF + SIF group), the POF + SCH low-dose group, and the POF + SCH high-dose group, with 9 mice in each group. Mice in the POF group received intraperitoneal injections of 100 mg/kg of CP for three consecutive days, while the other four groups were administered an equal volume of saline. Subsequently, the mice in the POF + SIF group received a daily gavage of 400 mg/kg SIF for four weeks. Meanwhile, those in the POF + SCH low-dose and high-dose groups were administered 200 mg/kg and 600 mg/kg SCH daily over a period of four weeks. Mice were monitored daily for body weight, and vaginal smears were collected for 8 days during both weeks 2 and 4.
2.6. Observation of Estrous Cycle
At weeks 2 and 4, the estrous cycle was divided into four distinct phases through cytological assessment of vaginal smears: pre-estrus, estrus, mid-pregnancy, and diestrus. During this period, saline was applied to 2–3 cm of the mouse vagina using a cotton swab, and the secretions were collected by gently rotating the swab. The collected samples were then smeared and stained with 0.4% methylene blue for observation and determination of the estrus stage under a light microscope.
2.7. Organ Index and Area of the Ovary
Following the experimental period, mice were euthanized by cervical dislocation, and the bilateral ovaries and uterus of the mice were collected and weighed. The area of the ovaries was calculated using ImageJ software (version 1.53c). Subsequently, the samples were either cryopreserved at −80 °C or preserved in 4% paraformaldehyde for further experiments.
2.8. Determination of Biochemical Parameters
Serum and ovarian tissue samples were collected and analyzed for SOD, MDA, T, E2, FSH, LH and AMH in accordance with the instructions provided with the assay kits.
2.9. Histological Observations on the Ovaries and Uterus
The uterus and ovaries were immersed in 4% paraformaldehyde for a fixation period of 24 h, followed by dehydration and embedding in paraffin. Tissue sections were cut to a thickness of 5 µm and stained with hematoxylin and eosin (H&E) prior to observation under a light microscope.
2.10. TUNEL
Ovarian paraffin sections were deparaffinized in water, and then the tissue was covered with a proteinase K working solution for 20 min. After rinsing twice with PBS, the sections were inactivated with 0.3% methanol peroxidase for 15 min. Subsequently, the sections were gently shaken to remove excess liquid, and buffer was applied dropwise in a circular manner. Following the removal of the equilibrium solution, the prepared TUNEL reaction solution was added dropwise, and coverslips were placed on top to allow for a 1 h reaction. Subsequently, the sections were washed in PBS three times for 5 min each, re-stained with DAPI for 15 min, and finally sealed. The apoptotic cells in the ovary were observed under a fluorescence microscope (Leica, Wetzlar, Germany).
2.11. LC-MS Based Untargeted Metabolomics Analysis
A total of 100 μL of serum sample was combined with 400 μL of extraction solution (MeOH:ACN, 1:1 (v/v)), which contained a deuterium internal standard, and vortexed for 30 s. The sample was then subjected to sonication in an ice-water bath for 10 min, followed by incubation at −40 °C for 1 h. Subsequently, the sample was centrifuged (12,000 rpm, 4 °C, 15 min), after which the supernatant was collected into a fresh glass vial for analysis. Quality control (QC) samples were generated through the combination of identical volumes from the sample’s upper liquid layer. The online assay was conducted using chromatographic separation of the target compounds on a Waters ACQUITY UPLC BEH Amide column (2.1 mm × 50 mm, 1.7 μm), with the ultra-high-performance liquid chromatograph being a Vanquish (Thermo Fisher Scientific, Waltham, MA, USA) ultra-high-performance liquid chromatograph (UHPLC). Liquid chromatography Phase A was composed of a water-based solution comprising 25 mM ammonium acetate and 25 mM ammonia, while Phase B consisted of acetonitrile. Subsequent data processing was conducted via the platform available at https://www.genescloud.cn/login (accessed on 18 May 2025).
2.12. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 software, and results were expressed as mean ± SD. All tests were performed at least three times. Differences among various groups were assessed using the Tukey test in One-way ANOVA, with differences reported as p < 0.05.
3. Results
3.1. Structural Characterization of SCH
3.1.1. ζ-Potential, Particle Size Distribution, and PDI of SCH
The ζ-potential, also known as the interfacial kinetic potential, is closely related to the stability of peptide solution systems. The results presented in Table 1 indicated that the ζ-potential of SCH was −37.11 ± 1.52 mV. This value suggests that the peptide molecule has a high proportion of acidic amino acids, and the electronegativity greater than −30 mV implies that the peptide molecules are unlikely to agglomerate due to strong repulsive forces, thereby contributing to the stability of the system. Furthermore, the particle size of SCH was measured at 465.17 ± 1.09 nm, with a PDI of 0.29 ± 0.01, further demonstrating that the SCH system possessed excellent physical stability.

Table 1.
ζ-potential, particle size distribution, and PDI of SCH.
3.1.2. Amino Acid Composition of SCH
The results indicated that SCH contained 15 amino acids (Table 2). Among these, the negatively charged residues, specifically Glu and Asp, comprised 10.39% of the total, while the positively charged residues, including Arg, Lys, and His, accounted for 6.64%. This finding was consistent with the above reported negative ζ-potential. In addition, essential amino acids represented 16.91% of the total composition. Research indicates that arginine can enhance the ovarian antioxidant capacity during the luteal phase in ewes through the Nrf2/Keap1 signaling pathway [19]. Branched-chain amino acids (BCAAs), including Val, Leu, and Ile, serve as critical regulators of mammalian metabolic pathways and positively contribute to the treatment of ovarian diseases [20]. Consequently, SCH, which is rich in these amino acids, may have the potential to alleviate POF.

Table 2.
The amino acid composition of SCH.
3.2. Body Weight Changes and Organ Indices in Mice
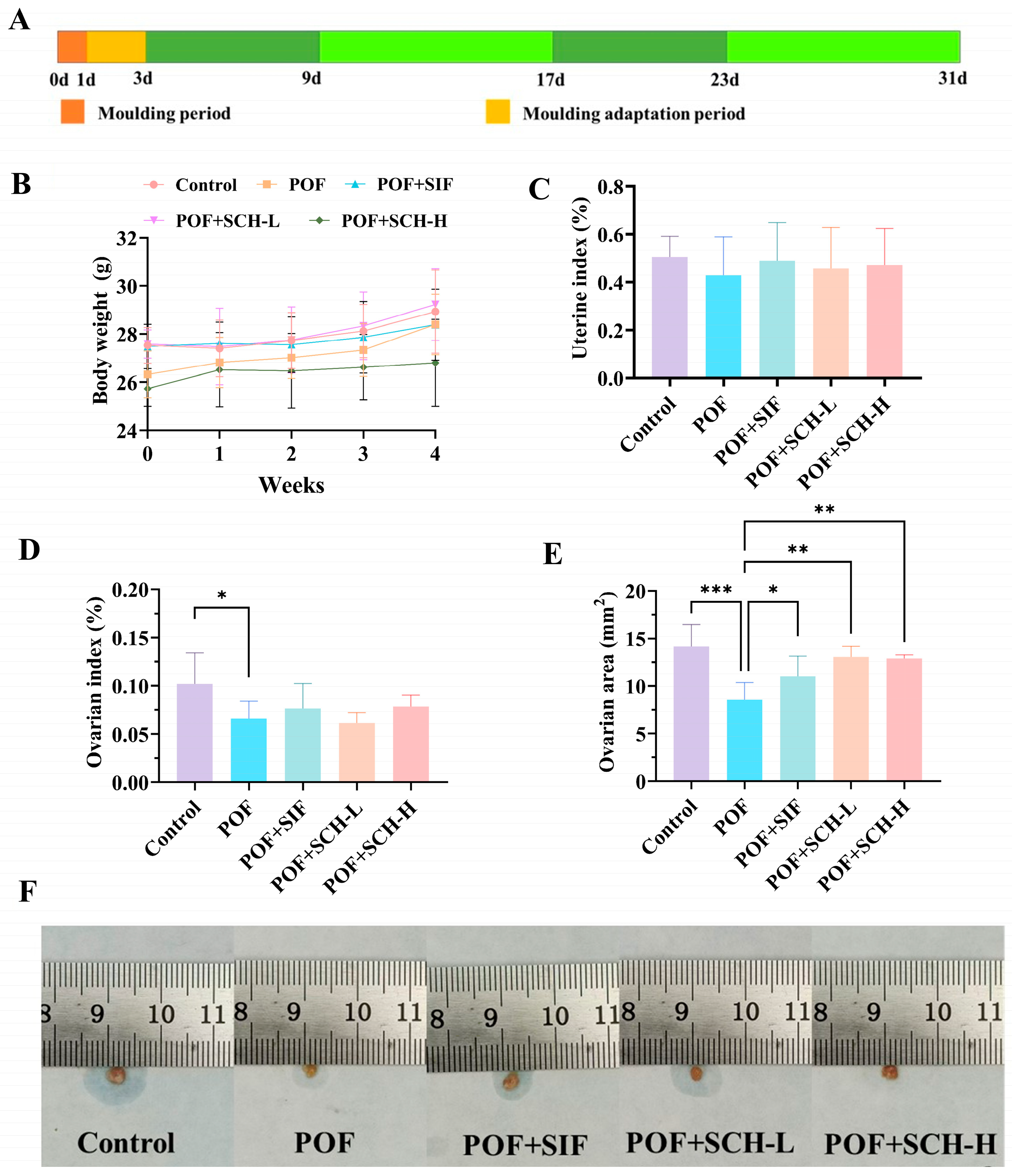
To evaluate the impact of SCH on CP-induced POF in mice, body weight changes and organ indices after 4 weeks of treatment were determined. The results of body weight, as depicted in Figure 1B, indicated no significant differences in SIF and SCH interventions when compared to the POF group. Similar findings were observed for uterine index (Figure 1C); although CP influenced the weight of the uterus in normal mice, this effect was not statistically significant. Furthermore, CP had a more pronounced impact on the ovaries, with a significantly lower ovarian index observed in the POF group compared to the control group (p < 0.05, Figure 1D). Notably, the results for ovarian area (Figure 1E) demonstrated that CP treatment significantly reduced the ovarian area (p < 0.001). Additionally, there was a significant increase in ovarian area among POF mice following interventions with SIF (p < 0.05), SCH-L (p < 0.01), and SCH-H (p < 0.01). These findings suggest a preliminary hypothesis that SCH may confer a protective effect on the ovaries of POF mice.
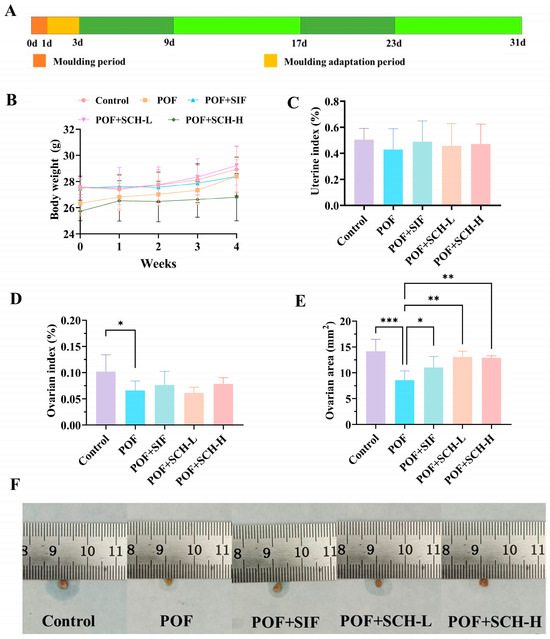
Figure 1.
Experimental design, body weight, and organ indices. (A) Experimental design; (B) Body weight; (C) Uterine index; (D) Ovarian index; (E) Ovarian area; (F) Physical picture of ovaries. Statistical significance is indicated as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.3. Effect of SCH on the Estrous Cycle in Mice with POF
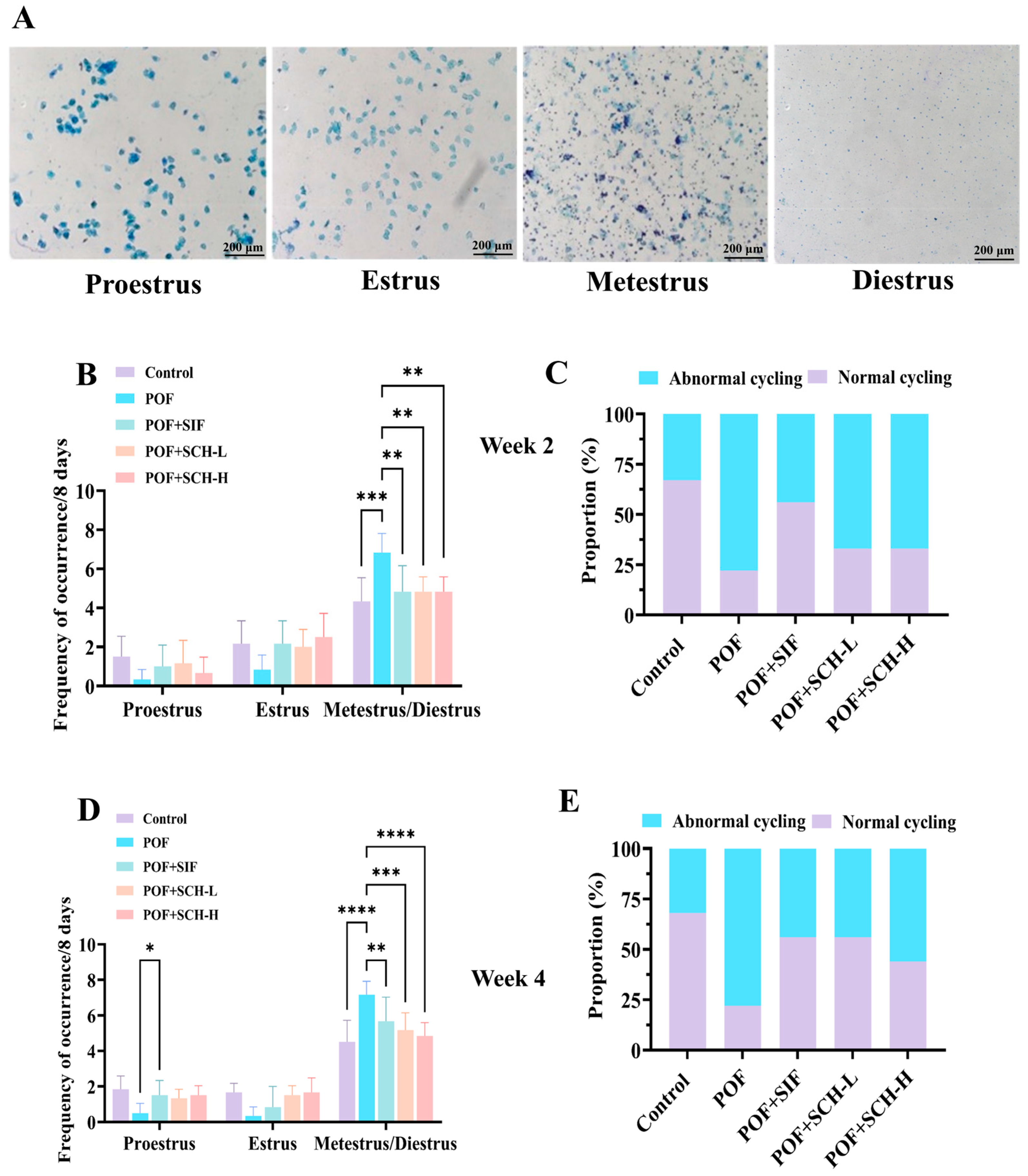
The impact of SCH on POF mice was assessed through vaginal smears to determine the estrus period in the subjects. The observation period was divided into two weeks: week 2 and week 4. During week 2, the estrous cycle status of the mice was first evaluated. In comparison to the control group, mice modeling POF exhibited a notable prolongation of metestrus and diestrus when exposed to CP (p < 0.001, Figure 2B). Conversely, a significant decrease in residence time was observed with the SIF, SCH-L, and SCH-H interventions (p < 0.01). Furthermore, the ability of the mice to complete an estrous cycle serves as an indicator of estrous cycle disorders. The proportion of mice that successfully completed an estrous cycle exhibited a marked reduction in the POF group relative to the control group. However, this proportion increased under the influence of SIF, SCH-L, and SCH-H, with SIF exhibiting the most pronounced effect (Figure 2C). In week 4, the effects of SIF (p < 0.01), SCH-L (p < 0.001), and SCH-H (p < 0.0001) on the residence time of POF mice during the metestrus and diestrus periods remained significant (Figure 2D). Notably, the proportion of mice capable of completing an estrous cycle increased in the SCH-L and SCH-H groups (Figure 2E).
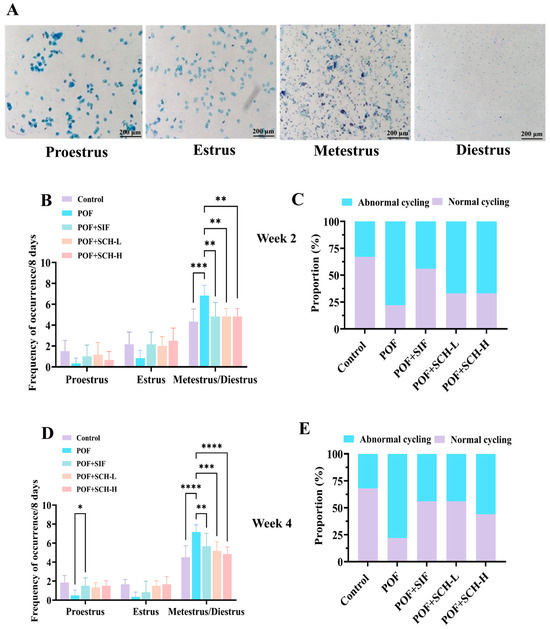
Figure 2.
The effect of SCH on the estrous cycle. (A) Stained smears of the distinct phases of the estrous cycle: proestrus, estrus, metestrus, and diestrus; (B) Estrous cycle in POF model mice at week 2; (C) Percentage of cycling mice exhibiting normal estrous cycle at week 2; (D) Estrous cycle in POF model mice at week 4; (E) Percentage of cycling mice with normal estrous cycle at week 4. Data were expressed as mean ± SD (n = 9), with significance levels indicated as * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.4. Effect of SCH on Serum Sex Hormones in POF Mice
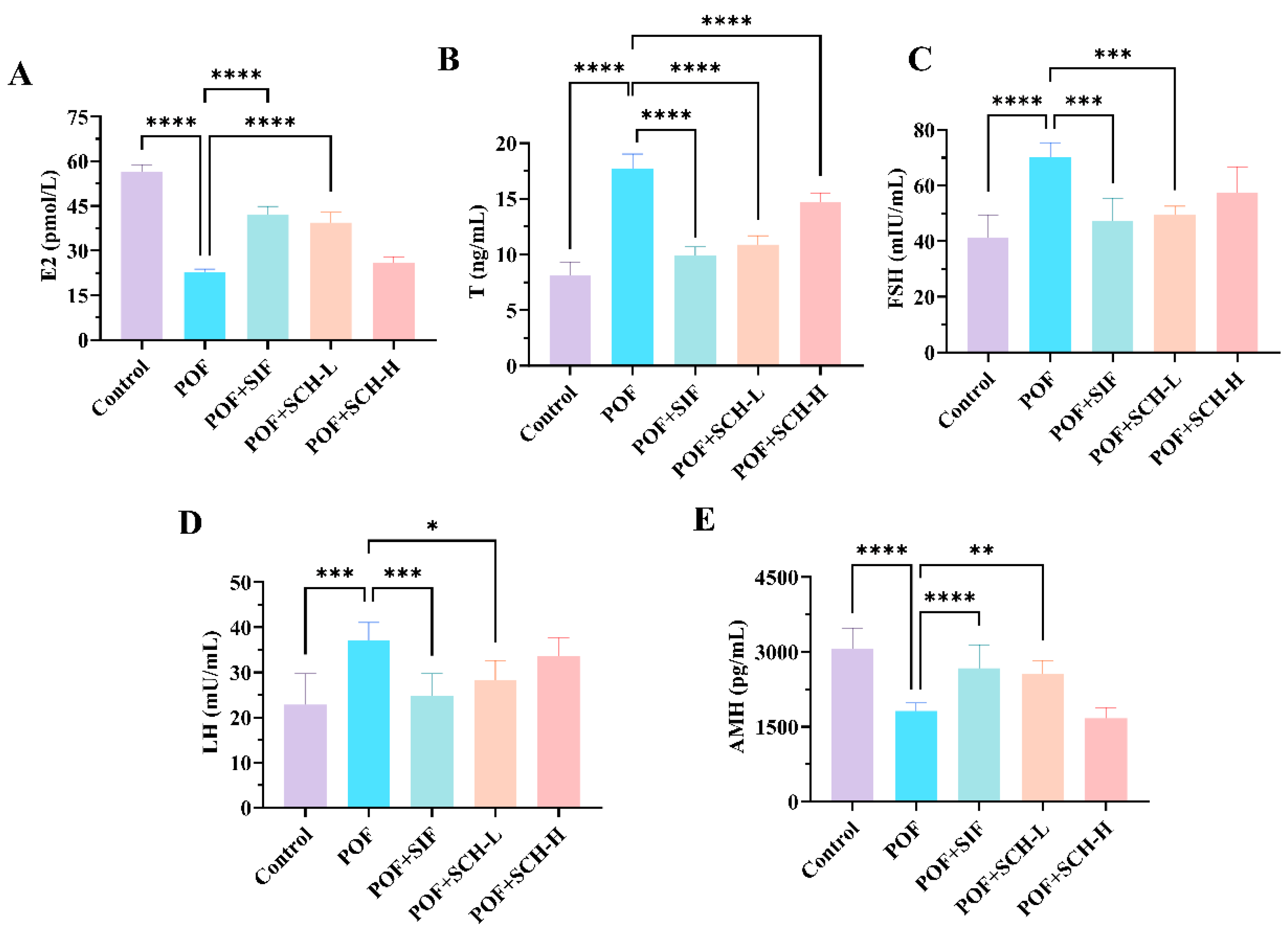
Having established that SCH exerted a restorative effect on estrous cycle disorders in POF mice, we further investigated its impact on serum hormone levels in these animals. Based on the data presented in Figure 3, the POF group exhibited a significant reduction in the hormone levels of E2 and AMH compared to the control group (p < 0.0001, Figure 3A,E), while simultaneously showing a marked increase in the levels of T (p < 0.0001), FSH (p < 0.0001), and LH (p < 0.001) (Figure 3B–D). Notably, both SIF and SCH-L interventions demonstrated a positive trend in reversing these changes. Specifically, SIF and SCH-L significantly elevated E2 levels (p < 0.0001) while concurrently reducing T (p < 0.0001) and FSH (p < 0.001). In terms of LH and AMH, the overall effects were less pronounced compared to SIF, although SCH-L still exhibited significant impacts on both hormones. It is important to note that SCH-H, while significantly decreasing the elevated levels of T hormone resulting from CP (p < 0.0001), did not produce a significant, albeit somewhat reversible, effect on the other four indices.
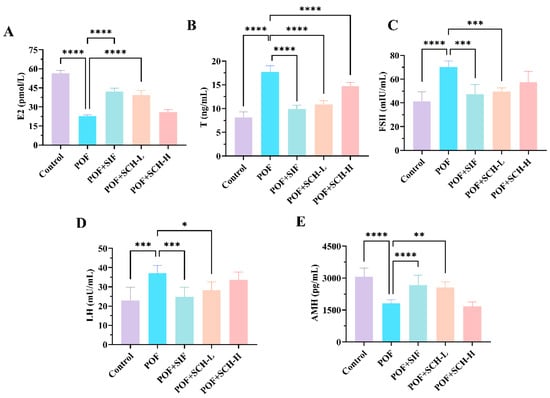
Figure 3.
The effect of SCH on serum sex hormones. (A) E2; (B) T; (C) FSH; (D) LH; (E) AMH. Statistical significance is indicated as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.5. Effect of SCH on Oxidative Stress
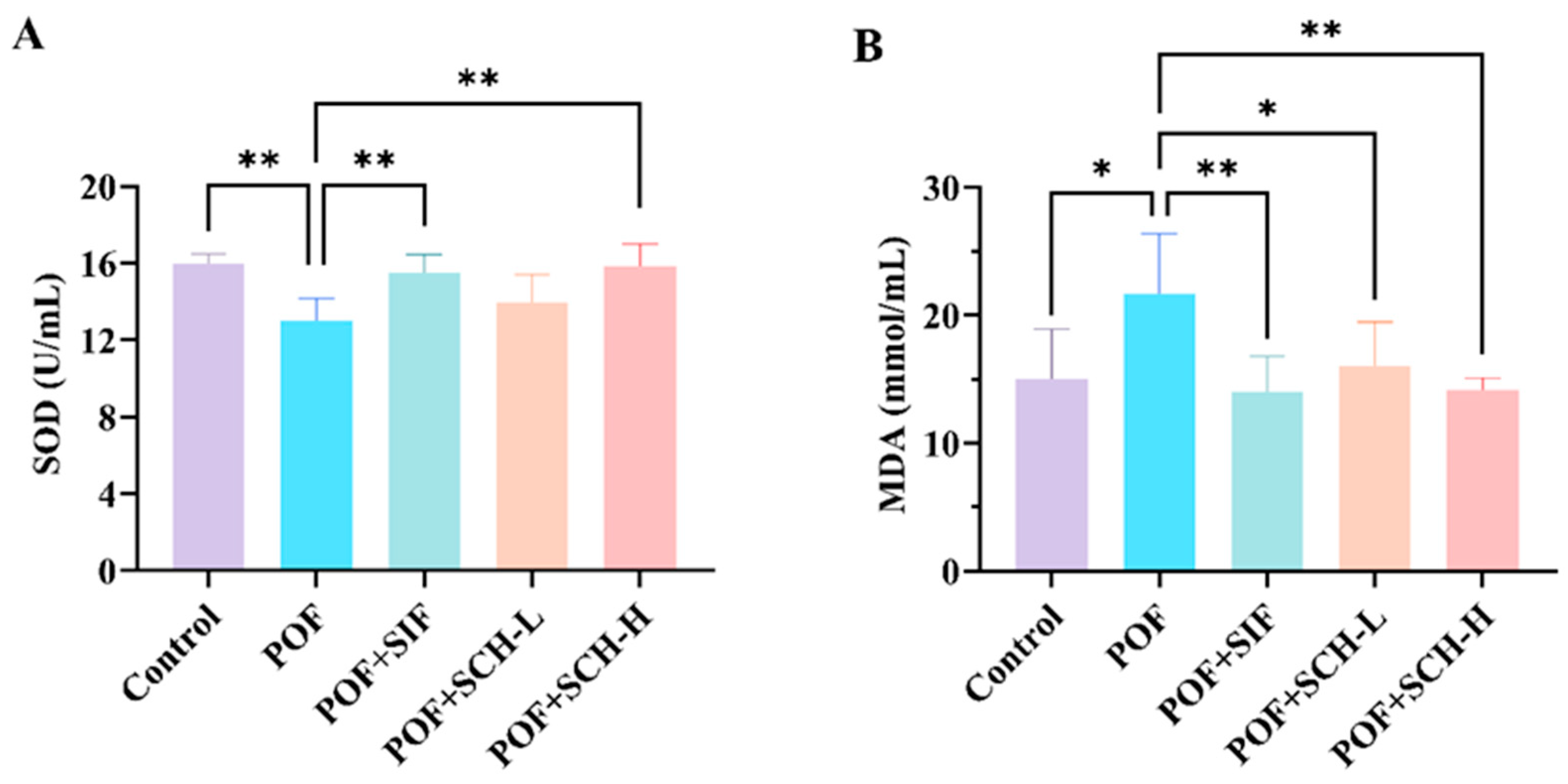
The activity of the SOD enzyme was significantly reduced in the POF group compared to the control group (p < 0.01), while MDA levels were considerably elevated (p < 0.05). Additionally, both SIF and SCH-H groups significantly restored SOD enzyme activity and reduced MDA levels in CP induced POF mice. However, the SCH-L group did not significantly elevate SOD enzyme activity (Figure 4A,B).
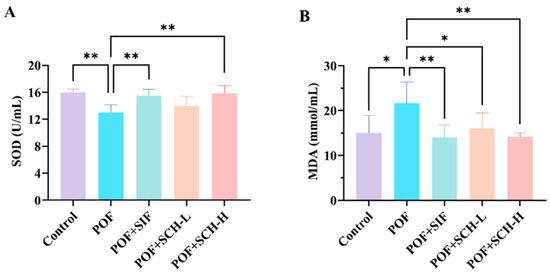
Figure 4.
Oxidative stress levels in mouse serum. (A) SOD; (B) MDA. Statistical significance is indicated as follows: * p < 0.05, ** p < 0.01.
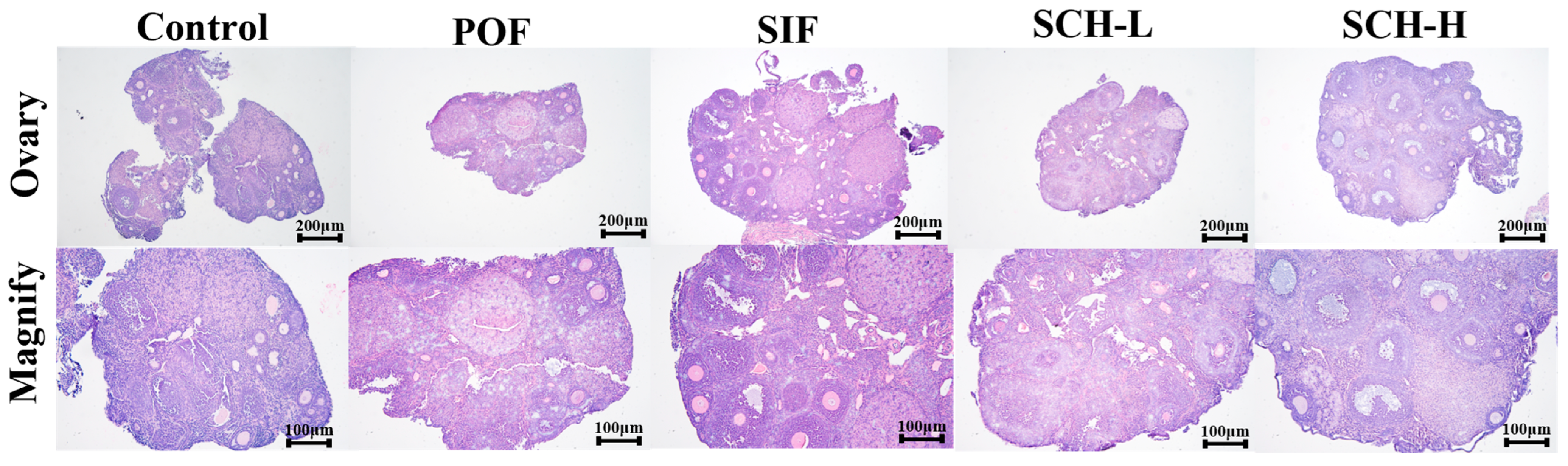
3.6. Histological Analysis
As illustrated in Figure 5, the induction of CTX led to the development of follicles at all stages towards atretic follicles in the normal mouse ovary when compared to the control group. However, this effect was ameliorated by the effective intervention of SIF and SCH, which resulted in an increased number of healthy follicles at various stages of maturation and a reduction in atretic follicles. Additionally, there was minimal congestion and inflammation in the ovarian stroma, and the overall structure of the ovary was well preserved.
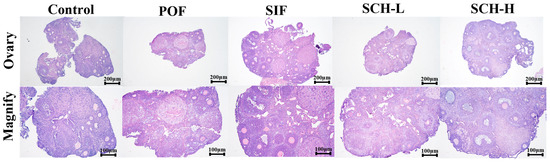
Figure 5.
Histological analysis of ovaries.
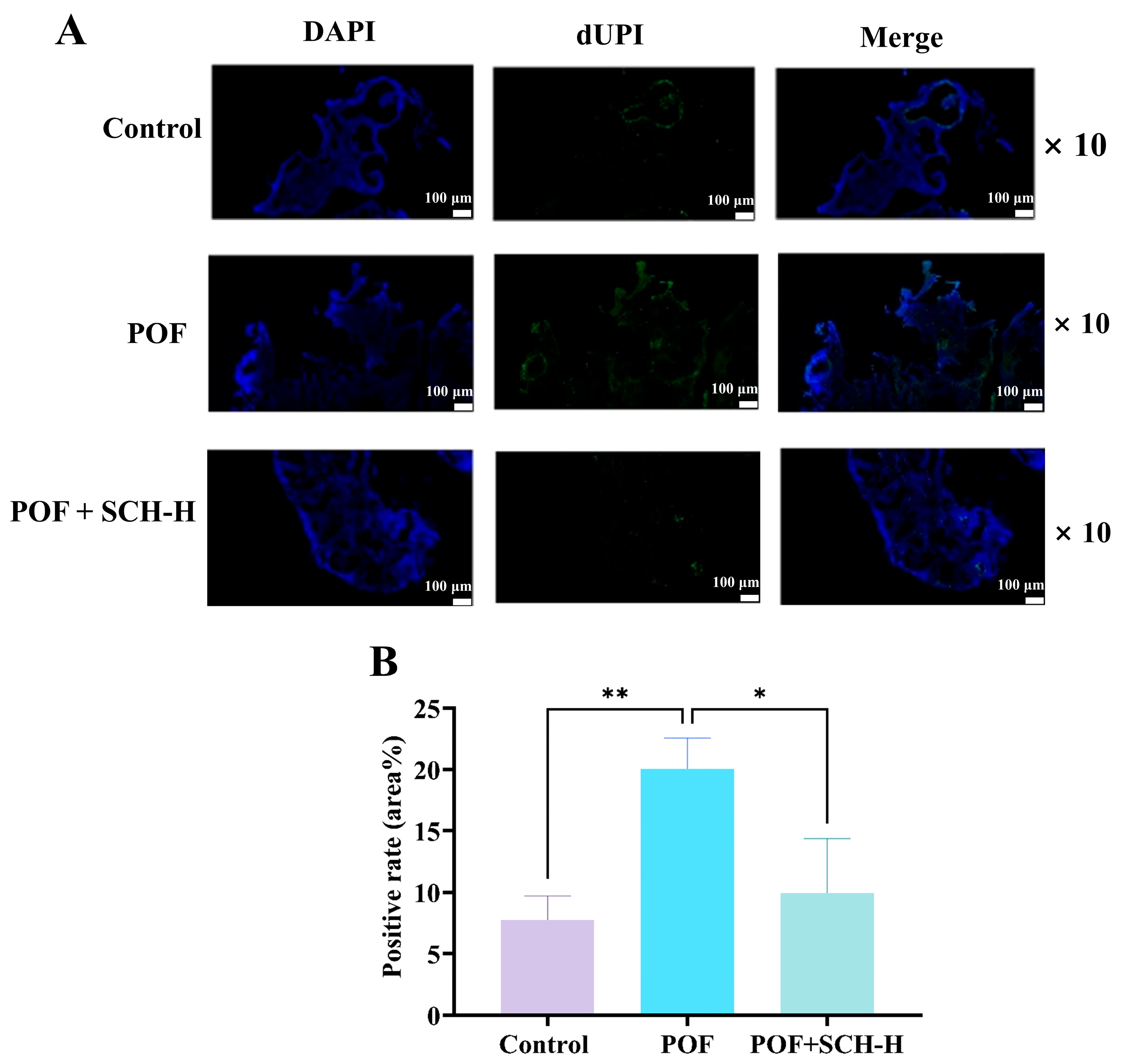
3.7. Impact of SCH on Ovarian Granulosa Cell Apoptosis in Mice with POF
The apoptosis of ovarian granulosa cells is a critical characteristic of POF. We investigated the impact of SCH on POF using TUNEL staining. The results indicated (Figure 6A,B) that POF significantly increased the apoptosis of ovarian granulosa cells compared to the control group (p < 0.01). However, apoptosis was notably reduced following SCH-H intervention (p < 0.05).
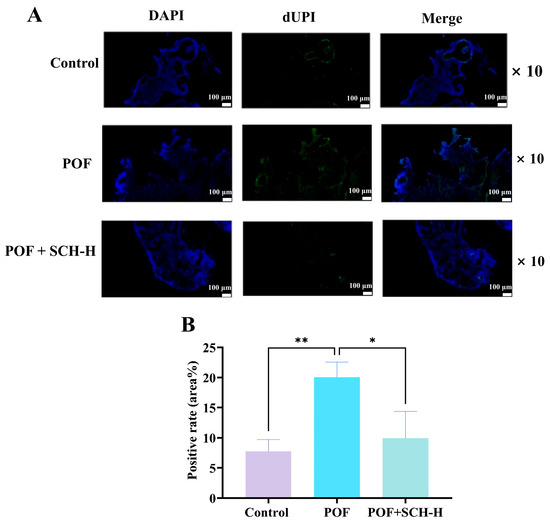
Figure 6.
Effect of SCH on apoptosis of ovarian granulosa cells. (A) TUNEL staining to detect ovarian apoptosis (scale bar: 100 μm); (B) Apoptotic optical density of ovarian granulosa cells (n = 3). Statistical significance is denoted as follows: * p < 0.05 and ** p < 0.01.
3.8. Effect of SCH on Serum Metabolic Profiles in POF Mice
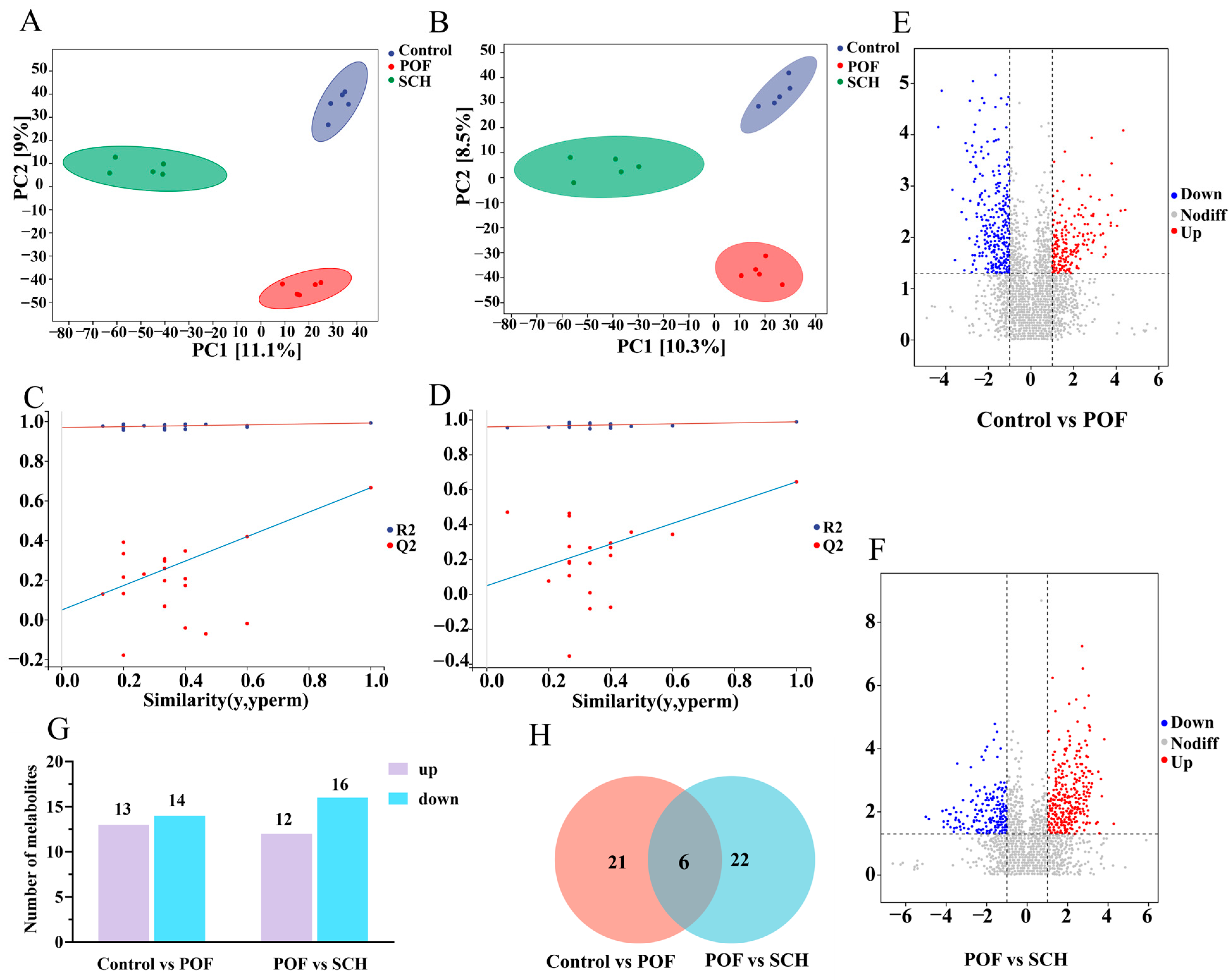
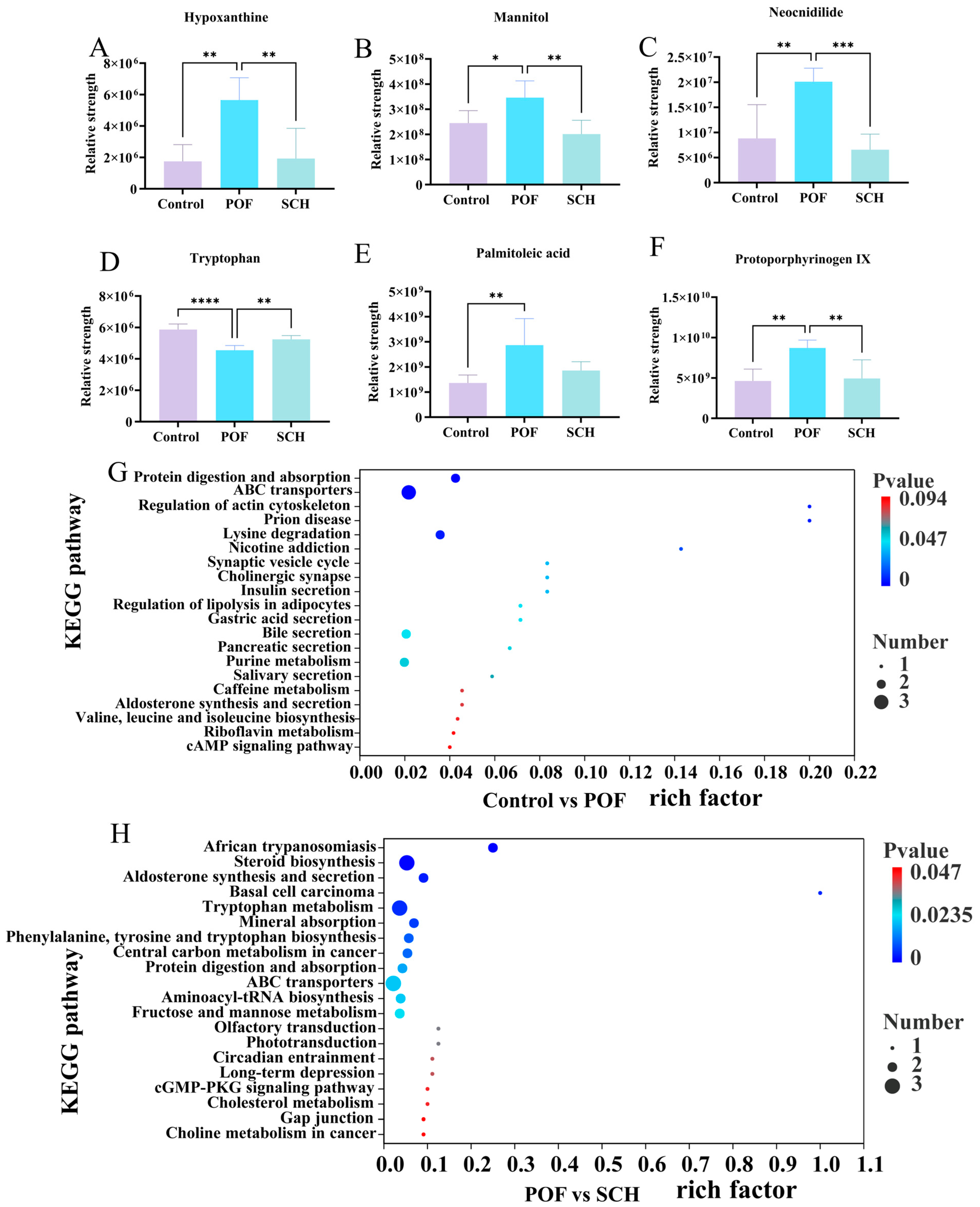
To elucidate the detailed mechanism of action of SCH concerning hormones, oxidative stress, and apoptosis, we analyzed serum metabolic profiles using UHPLC-Q-TOF-MS/MS to identify potential metabolites and metabolic pathways associated with POF. The results from the positive and negative partial least squares discriminant analysis model indicated (Figure 7A,B) that there was no overlapping region among the control, POF, and SCH groups, suggesting significant differences in metabolites across the three groups. Furthermore, the permutation test demonstrated (Figure 7C,D) that all Q2 points were lower than the original Q2 points on the far right, reinforcing the reliability and validity of our findings. Subsequently, univariate statistical analysis revealed differential metabolites between the control and POF groups, as well as between the POF and SCH groups (Figure 7E,F). Based on these findings, we screened differential substances for potential biomarkers using the criteria of p < 0.05 and VIP > 1. The results, illustrated in Figure 7G and Table 3, demonstrated that compared to the control group, 13 metabolites in the POF group were significantly up-regulated, while 14 metabolites were significantly down-regulated. Additionally, under the influence of SCH, 12 metabolites were significantly up-regulated and 16 metabolites were significantly down-regulated. Among these, eight metabolites associated with POF have been identified: hypoxanthine, mannitol, neocnidilide, tryptophan, 5-hydroxyindoleacetic acid, N6-acetyl-L-lysine, fisetin, and cyclic GMP. Additionally, mannitol, neocnidilide, tryptophan, 5-hydroxyindoleacetic acid, fisetin, and cyclic GMP may play a crucial role in preventing POF by inhibiting oxidative stress, repairing oocytes, preventing cell apoptosis, and regulating hormone levels. Notably, upon comparing the two data sets, we identified six significant metabolites common to all three groups (Figure 7H). Specifically, these metabolites included hypoxanthine, mannitol, neocnidilide, tryptophan, palmitoleic acid, and protoporphyrinogen IX (Figure 8A–F). Relative to the control group, the POF group demonstrated significant up-regulation of hypoxanthine, mannitol, neocnidilide, palmitoleic acid, and protoporphyrinogen IX, along with significant down-regulation of tryptophan. SCH treatment significantly reversed these trends.
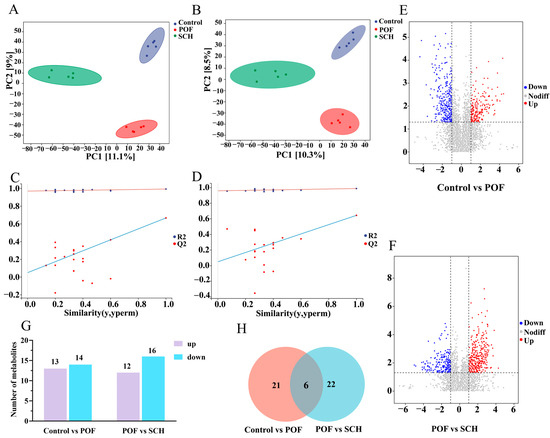
Figure 7.
Effect of SCH on serum metabolic profiles in POF mice. (A,B) Positive ion modeling partial least squares-dispersion analysis (PLS-DA) and displacement test; (C,D) Negative ion modeling PLS-DA and displacement test. The red and blue lines represent the regression lines of R2 and Q2, respectively; (E,F) Univariate statistical analysis (Control vs. POF and POF vs. SCH); (G) Differential metabolite screening bar chart; (H) Venn diagram (Control vs. POF and POF vs. SCH).

Table 3.
The differential metabolite screening.
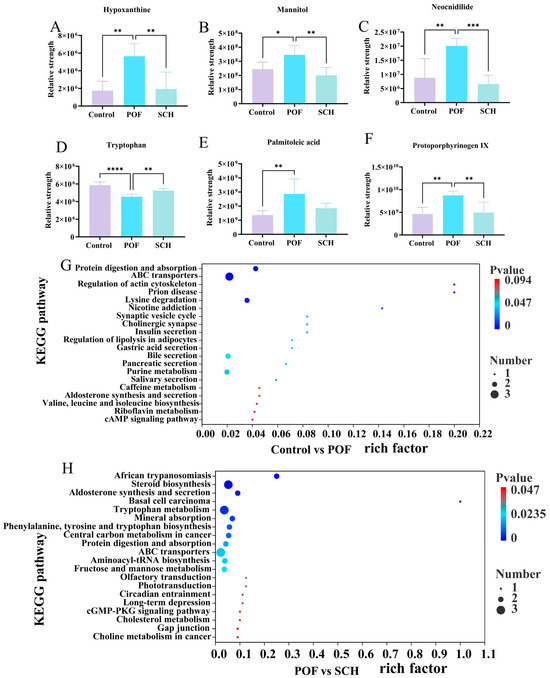
Figure 8.
Effect of SCH on serum metabolites and metabolic pathways in POF mice. (A–F) Common differential metabolite; (G,H) Enrichment pathway (Control vs. POF and POF vs. SCH). Statistical significance is indicated as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.001.
Finally, differential abundance plots were employed to analyze the metabolic pathways across the three groups (Figure 8G,H). The POF group exhibited significant changes in protein digestion and absorption, ABC transporters, regulation of actin cytoskeleton, prion disease, and lysine degradation. Additionally, in POF mice under SCH treatment, significant alterations were noted in African trypanosomiasis, steroid biosynthesis, aldosterone synthesis and secretion, basal cell carcinoma, and the tryptophan metabolism.
4. Discussion
POF is a known side effect of cancer chemotherapy and is characterized as a multifactorial condition in which ovarian function declines in women under 40 years of age. This condition is primarily marked by elevated levels of gonadotropins, along with a decrease in ovarian reserve and estrogen levels [21,22]. In this study, sea cucumber ovum-derived SCH was prepared through enzymatic hydrolysis, and its therapeutic effects were evaluated in a CP-induced POF mouse model at doses of 200 mg/kg and 600 mg/kg. The modeling success with a dose of 100 mg/kg CP was confirmed based on the irregularities observed in the estrous cycle, alongside reductions in E2 levels and increases in FSH levels, as reported in previous studies on mice with POF.
An ototoxicity study assessing the effects of CP metabolites on cultured mouse ovaries revealed that metabolites such as 4-hydroxycyclophosphamide (4-HC) and phosphonamidite mustard (PM) exerted significant damaging effects on primordial and primary follicles [23]. AMH, a glycoprotein secreted by granulosa cells of pre-sinus and small sinus follicles in the ovary, serves as a crucial marker of follicular health [24]. In the initial stages of CP-induced ovarian damage, the number of small follicles diminishes rapidly due to their heightened sensitivity, resulting in a more pronounced decline in serum AMH levels compared to other sex hormones. As ovarian function deteriorates, the hypothalamic-pituitary-ovarian (HPO) axis stimulates the secretion of FSH and LH [25,26]. Within follicular membrane cells, 17α-hydroxylase and 17, 20-lyase facilitate T synthesis in response to LH [27]. The effects of SIF and SCH-L on the serum hormone alterations induced by CP in this study align with anticipated outcomes, and similar findings have been observed with tilapia skin peptides [11]. The suboptimal effect noted with SCH-H may be attributed to the higher dosage administered.
Studies have demonstrated that oxidative stress plays a critical role in the development of POF. In the present study, CP administration resulted in a decrease in SOD enzyme activity as well as an increase in MDA levels in mice, thereby elevating oxidative stress within the ovarian tissues. This condition led to heightened oxidative damage in follicular membrane cells and granulosa cells. Recent findings indicated that curculigoside (CUR) effectively reduced oxidative stress in ovarian tissue and provided protection against CP-induced ovarian injury [28]. Notably, SOD and MDA levels in this study showed significant positive results following SCH intervention, representing that SCH also played a protective role in ovarian health.
CP induces an imbalance between oxidative and antioxidant activities, resulting in the accumulation of reactive oxygen species (ROS) that can lead to ovarian damage through the production of oxygen free radicals, ultimately causing DNA damage and granulosa cell apoptosis [29]. The aberrant apoptosis of granulosa cells contributes to follicular failure, ultimately resulting in ovarian senescence or follicular atresia [30]. In this study, the TUNEL method was employed to detect ovarian granulosa cell apoptosis, which was significantly influenced by SCH intervention. Additionally, tilapia skin peptides were found to reduce ovarian granulosa cell apoptosis, aiding in the restoration of POF, and protein immunoblotting experiments demonstrated a significant increase in the anti-apoptotic protein Bcl-2, alongside the inhibition of Bax and caspase-3 expression, thereby offering protective effects for the ovary [11]. In addition, sea cucumber peptides derived from Acaudina leucoprocta did not significantly alter the relative expression of Bcl-2, Bax, and caspase-3 in response to POF [15]. Although these results were not examined in the present study, it is reasonable to speculate on their potential implications.
Furthermore, this study employed untargeted metabolomics to elucidate the potential mechanisms through which SCH protected ovarian function. We identified common differential metabolites present in three groups: hypoxanthine, mannitol, neocnidilide, tryptophan, palmitoleic acid, and protoporphyrinogen IX. SCH treatment can synergistically reshape the ovarian microenvironment across multiple dimensions, including abnormal cell apoptosis, oxidative stress imbalance, and hormonal metabolism disorders, through precise regulation of several key metabolites, thereby exerting therapeutic effects on POF. Notably, the level of hypoxanthine in the POF group was significantly higher than that in the control group (p < 0.01), which can inhibit granulocyte proliferation and induce apoptosis by disrupting the activity of MPF (cyclin B-CDK1 complex) within the PKA-Wee1/Myt1 signaling axis [31]. This is also associated with oxidative stress-induced cell death, exacerbating endothelial dysfunction and damaging the normal physiological state of ovarian cells [32]. However, following SCH intervention (Figure 8A), the level of hypoxanthine significantly decreased (p < 0.01), suggesting that it may improve CP-induced POF by promoting the degradation metabolism of hypoxanthine, such as by activating the reverse regulatory pathway of xanthine oxidase. Mannitol’s osmotic diuretic effect can not only reduce tissue edema, but also improve microcirculation and neutralize ROS, thereby minimizing cell and mitochondrial damage to the greatest extent possible [33]. Mice induced by CP may exhibit abnormally elevated drug metabolism due to the diuretic effect of mannitol in the regulation of their immune mechanisms (Figure 8B). Neocnidilide protects cells by inhibiting inflammatory mediators and reducing oxidative stress damage [34]. Figure 8D illustrated a significant increase in tryptophan levels following SCH intervention (p < 0.01). Serotonin, the primary metabolite of tryptophan [35], serves multiple roles as a neurotransmitter within the central nervous system, a blood factor, and a neurohormone that regulates peripheral organ function [36]. Furthermore, it influences the release of gonadotropins and regulates ovarian hormone levels.
In summarizing the differential metabolites presented in Table 3, alongside the six common differential metabolites, we identified that 5-hydroxyindoleacetic acid, N6-acetyl-L-lysine, fisetin, and cyclic GMP have specific effects on POF. Research indicates that the levels of 5-hydroxyindoleacetic acid influence ovarian cell function by regulating the hypothalamic-pituitary-ovarian (HPO) axis [37]. N6-acetyl-L-lysine, an acetylated form of lysine, exhibits changes in levels that are associated with protein modification and cellular stress, which in turn affects the epigenetic regulation of ovarian cells, leading to impaired follicular development [38]. Furthermore, studies demonstrate that fisetin activates the Sirt1 pathway in mouse oocytes through its antioxidant and anti-aging properties, thereby reducing oxidative stress and mitochondrial dysfunction, and delaying post-ovulation oocyte aging [39]. In the comparison between the POF and SCH groups, fisetin exhibited reduced activity, indicating that SCH intervention may mitigate oxidative stress damage to mouse ovarian germ cells. Additionally, cyclic GMP signaling is crucial for regulating follicular development and ovulation; its dysregulation may exacerbate granulosa cell apoptosis, consequently promoting follicular atresia [40].
In summary, SCH modulates CP-induced metabolic alterations in mice. Notably, KEGG enrichment analysis indicates that the tryptophan and cyclic GMP pathways in SCH metabolism represent the core mechanisms underlying CP pathogenicity in normal mice. Furthermore, the steroid biosynthesis pathway serves as a fundamental route for ovarian hormone synthesis. By promoting the release of androstenedione, testosterone, and dehydroepiandrosterone sulfate (DHEAS), it alleviates the functional decline of ovarian granulosa cells and theca cells in individuals with POI [41]. In the metabolic pathways of CP, protein digestion and absorption, as well as the ABC transporter and lysine degradation, are significantly enriched. Studies have demonstrated that lysine succinylation is a conserved and novel post-translational modification (PTM) that can influence protease activity and gene expression [42]. Furthermore, succinylated lysine is highly enriched in mitochondrial (Mt) proteins and extra-Mt proteins that regulate cell proliferation, oxidative damage, and apoptosis [43,44,45]. Le et al. [46] confirmed that lysine succinylation levels in POI mouse ovaries are higher than those in normal ovaries, suggesting that elevated lysine succinylation levels may inhibit ovarian reproductive and endocrine functions.
5. Conclusions
In conclusion, the findings of this study demonstrated that the oral administration of SCH could modulate serum metabolites to improve POF in mice. Additionally, lower doses of SCH demonstrated more favorable effects regarding hormonal levels and oxidative stress outcomes; however, the specific functions of SCH require further clinical research. Consequently, more rigorous and extensive studies should be warranted before SCH could be considered for the development of functional foods in the future. The findings of this study are significant for the regulation of sex hormones and will contribute to the advancement of marine functional foods.
Author Contributions
Conceptualization, S.G. and J.G.; methodology, X.W., S.G. and J.G.; software, X.W., M.L. and Y.Z.; validation, M.L. and Y.Z.; formal analysis, S.H. and L.S. (Liqin Sun); investigation, S.H.; writing—original draft preparation, X.W., M.L., S.G. and L.S. (Leilei Sun); writing—review and editing, Y.Z., L.S. (Leilei Sun) and L.S. (Liqin Sun); visualization, S.H.; supervision, J.G.; project administration, L.S. (Leilei Sun); funding acquisition, Y.Z. and L.S. (Leilei Sun). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shandong Province (No. ZR2021QD030); the Yantai Science and Technology Innovation Development Program (No. 2023JCYJ088); National Natural Science Foundation of China (No. 42106111) and Fund of Yantai Key Laboratory of Quality and Safety Control and Deep Processing of Marine Food (No. QSCDP202304).
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee of Yantai University (protocol code 20231227s179, approved on 27 December 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could potentially bias the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| SCH | Sea cucumber ovum hydrolysate |
| POF | Premature ovarian failure |
| CP | Cyclophosphamide |
| E2 | Estradiol |
| FSH | Follicle-stimulating hormone |
| T | Testosterone |
| LH | Luteinizing hormone |
| AMH | Anti-Müllerian hormone |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| POI | Primary ovarian insufficiency |
| SIF | Soy isoflavones |
| ELISA | Enzyme-linked immunosorbent assay |
| HRT | Hormone replacement therapy |
| GAGs | Glycosaminoglycans |
| DLS | Determination of dynamic light scattering |
| PDI | Polydispersity index |
| H&E | Hematoxylin and eosin |
| QC | Quality control |
| UHPLC | Ultra-high-performance liquid chromatograph |
| ANOVA | One-way analysis of variance |
References
- Kuchakzadeh, F.; Ai, J.F.; Ebrahimi-Barough, S. Tissue engineering and stem cell-based therapeutic strategies for premature ovarian insufficiency. Regen. Ther. 2024, 25, 10–23. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen hormone biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar]
- Mauvais-Jarvis, F.; Lindsey, S.H. Current Topics in Developmental Biology. J. Clin. Investig. 2024, 134, e180073. [Google Scholar] [CrossRef]
- Kunicki, M.; Rzewuska, N.; Gross-Kępińska, K. Immunophenotypic profiles and inflammatory markers in premature ovarian insufficiency. J. Reprod. Immunol. 2024, 164, 104253. [Google Scholar] [CrossRef]
- Sanverdi, I.; Kilicci, C.; Cogendez, E.; Abide Yayla, C.; Ozkaya, E. Utility of complete blood count parameters to detect premature ovarian insufficiency in cases with oligomenorrhea/amenorrhea. J. Clin. Lab. Anal. 2018, 32, e22372. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; De Muinck Keizer-Schrama, S.; Hogervorst, E.; Janse, F.; et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef]
- Liu, X.L.; Ma, K.; Tao, W.H.; Xu, Z.K.; Liu, G.; Hu, C.Y.; Mao, W.W.; Gu, C.; Guo, Q. Natural products for treatment of premature ovarian failure: A narrative review. J. Tradit. Chin. Med. 2023, 43, 606–617. [Google Scholar]
- Hu, H.; Zhang, J.; Xin, X.; Jin, Y.; Zhu, Y.; Zhang, H.; Fan, R.; Ye, Y.; Li, D. Efficacy of natural products on premature ovarian failure: A systematic review and meta-analysis of preclinical studies. J. Ovarian. Res. 2024, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fang, F.; Wang, J.; Wong, C.W. Structural activity relationship of flavonoids with estrogen-related receptor gamma. FEBS. Lett. 2010, 584, 22–26. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Zhang, Z.; Han, X.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Huang, A.; Feng, Z.; et al. Oral oyster polypeptides protect ovary against D-galactose-induced premature ovarian failure in C57BL/6 mice. J. Sci. Food Agric. 2020, 100, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Yin, H.; Hu, C.; Zeng, J.; Shi, X.; Chen, S.; Zhang, K.; Zheng, W.; Wu, W.; Liu, S. Tilapia skin peptides restore cyclophosphamide-induced premature ovarian failure via inhibiting oxidative stress and apoptosis in mice. Food Funct. 2022, 13, 1668–1679. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Shen, F.; Du, J.; Feng, F. Sea cucumber (Acaudina leucoprocta) peptides exhibit anti-aging effect in Drosophila melanogaster via regulation of microbiota dysbiosis and metabolic disorder. Food Biosci. 2024, 60, 104476. [Google Scholar] [CrossRef]
- Wang, K.; Sun, N.; Li, D.; Cheng, S.; Song, L.; Lin, S. Enzyme-controlled hygroscopicity and proton dynamics in sea cucumber (Stichopus japonicus) ovum peptide powders. Food Res. Int. 2018, 112, 241–249. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Luo, X.; Liu, W.; Zhao, M.; Wang, J.; Gao, X.; Feng, F. The evaluation of sea cucumber (Acaudina leucoprocta) peptide on sex hormone regulation in normal and premature ovarian failure female mice. Food Funct. 2023, 14, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Thongbuakaew, T.; Suwansa-ard, S.; Chaiyamoon, A.; Cummins, S.F.; Sobhon, P. Sex steroids and steroidogenesis-related genes in the sea cucumber, Holothuria scabra and their potential role in gonad maturation. Sci. Rep. 2021, 11, 2194. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Shen, Y.; Li, M.; Sun, L. Integrated extraction, structural characterization, and activity assessment of squid pen protein hydrolysates and β-chitin with different protease hydrolysis. Int. J. Biol. Macromol. 2024, 262, 130069. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Li, L.; Wang, S. Cryoprotective activity and action mechanism of antifreeze peptides obtained from tilapia scales on Streptococcus thermophilus during cold stress. J. Agric. Food Chem. 2019, 67, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Z.Y.; Wu, Q.J.; Cheng, B.Y.; Zhai, Z.H.; Wang, Y.Q. Arginine Enhances Ovarian Antioxidant Capability via Nrf2/Keap1 Pathway during the Luteal Phase in Ewes. Animals 2022, 12, 2017. [Google Scholar] [CrossRef]
- Zeng, T.; Liu, Y.T.; Tang, X.; Fu, R.S.; Gao, Q.; Zhou, W.C.; Fang, J.W.; Zhang, J.; Zou, J.; Li, Y.K. BCAA metabolism: The Achilles’ heel of ovarian cancer, polycystic ovary syndrome, and premature ovarian failure. Front. Endocrinol. 2025, 16, 1579477. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Cha, L.; Li, L.; Zhu, D.; Fang, Z.; He, Z.; Huang, J.; Pan, Z. Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int. J. Mol. Sci. 2019, 20, 20143605. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Chen, X.; Huang, R.; Ma, L.; Weng, H.; Yu, Y.; Zong, X. GnRHa protects the ovarian reserve by reducing endoplasmic reticulum stress during cyclophosphamide-based chemotherapy. NPJ Breast. Cancer 2021, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, P.; Devine, P.J. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol. Sci. 2006, 90, 500–509. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, Y.; Li, S.; Liang, Y.; Qiu, Q.; Lin, H.; Zhang, Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J. Ovarian. Res. 2017, 10, 56. [Google Scholar] [CrossRef]
- Hall, J.E.; Gill, S. Neuroendocrine aspects of aging in women. Endocrinol. Metabol. Clin. North. Amer. 2001, 30, 631–646. [Google Scholar] [CrossRef]
- Downs, J.L.; Wise, P.M. The role of the brain in female reproductive aging. Mol. Cell. Endocrinol. 2009, 299, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Garmey, J.C.; Veldhuis, J.D. Interactive stimulation by luteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17α-hydroxylase/17,20-lyase (CYP17) genes in porcine theca cells. Endocrinology 2000, 141, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lyu, Y.J.; Gong, J.; Zou, Y.; Jiang, X.; Xiao, M.; Guo, J. Therapeutic effects of curculigoside on cyclophosphamide-induced premature ovarian failure in mice. Climacteric 2024, 27, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Turton, M.; Luong, B.T.; Tan, Y.; Luderer, U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol. Sci. 2007, 98, 216–230. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Liu, K.; Wang, Q.; Cao, J.; Xiao, P.; Yang, W.; Li, X.; Li, J.; Li, M.; et al. Alpha-ketoglutarate ameliorates induced premature ovarian insufficiency in rats by inhibiting apoptosis and upregulating glycolysis. Reprod. Biomed. Online 2023, 46, 673–685. [Google Scholar] [CrossRef]
- Li, C.; Meng, X.; Liu, S.; Li, W.; Zhang, X.; Zhou, J.; Yao, W.; Dong, C.; Liu, Z.; Zhou, J.; et al. Oocytes and hypoxanthine orchestrate the G2-M switch mechanism in ovarian granulosa cells. Development 2020, 147, 184838. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ryu, H.M.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kim, Y.L. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem. Biophys. Res. Commun. 2017, 482, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Gamazon, E.R.; Vandeven, T.; Buchheit, T.; Walsh, C.G.; Mishra, P.; Ramanujan, K.; Shaw, A. DNA methylation profiles are associated with complex regional pain syndrome after traumatic injury. Pain 2019, 160, 2328–2337. [Google Scholar] [CrossRef]
- Dubé, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life. Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Alyoshina, N.M.; Tkachenko, M.D.; Malchenko, L.A.; Shmukler, Y.B.; Nikishin, D.A. Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary. Int. J. Mol. Sci. 2022, 23, 14828. [Google Scholar] [CrossRef]
- Ke, C.F.; Hou, Y.; Zhang, H.Y.; Fan, L.J.; Ge, T.T.; Guo, B.; Zhang, F.; Yang, K.; Wang, J.T.; Lou, G. Large-scale profiling of metabolic dysregulation in ovarian cancer. Int. J. Cancer 2015, 136, 516–526. [Google Scholar] [CrossRef]
- Min, Z.Y.; Long, X.Y.; Zhao, H.C.; Zhen, X.M.; Li, R.; Li, M.; Fan, Y.; Yu, Y.; Zhao, Y.; Qiao, J. Protein Lysine Acetylation in Ovarian Granulosa Cells Affects Metabolic Homeostasis and Clinical Presentations of Women with Polycystic Ovary Syndrome. Front. Cell Dev. Biol. 2020, 8, 567028. [Google Scholar] [CrossRef]
- Xing, X.P.; Liang, Y.L.; Li, Y.A.; Zhao, Y.L.; Zhang, Y.X.; Li, Z.; Li, Z.C.; Wu, Z.F. Fisetin Delays Postovulatory Oocyte Aging by Regulating Oxidative Stress and Mitochondrial Function through Sirt1 Pathway. Molecules 2023, 28, 553. [Google Scholar] [CrossRef]
- Shuhaibar, L.C.; Egbert, J.R.; Norris, R.P.; Lampe, P.D.; Nikolaev, V.O.; Thunemann, M.; Wen, L.; Feil, R.; Jaffe, L.A. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. USA 2015, 112, 5527–5532. [Google Scholar] [CrossRef]
- Gleicher, N.; Kushnir, V.A.; Weghofer, A.; Barad, D.H. The importance of adrenal hypoandrogenism in infertile women with low functional ovarian reserve: A case study of associated adrenal insufficiency. Reprod. Biol. Endocrinol. 2016, 14, 23. [Google Scholar] [CrossRef][Green Version]
- Alleyn, M.; Breitzig, M.; Lockey, R.; Kolliputi, N. The dawn of succinylation: A posttranslational modification. J. Physiol. Cell Physiol. 2018, 314, C228–C232. [Google Scholar] [CrossRef]
- Park, J.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.J.; Dai, L.Z.; Xie, Z.Y.; Zhang, Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef]
- Li, F.; He, X.; Ye, D. NADP+-IDH mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol. Cell 2015, 60, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, K.; Hitosugi, S.; Wiese, E.K.; Boakye-Agyeman, F. Carnitine palmitoyltransferase 1A has a lysine succinyltransferase activity. Cell Rep. 2018, 22, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Li, J.; Zhang, D.; Yuan, Y.; Zhou, C.; He, J.X.; Huang, J.; Hu, L.L.; Luo, T.; Zheng, P.L. The emerging role of lysine succinylation in ovarian aging. Reprod. Biol. Endocrinol. 2023, 21, 38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).