The Influence of Storage Conditions on the Quality of Vacuum-Packed Water Caltrop Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Experimental Instruments and Equipment
2.3. Test Methods
2.3.1. Sample Treatment
2.3.2. Determination of Sensory Quality
2.3.3. Determination of Moisture Content
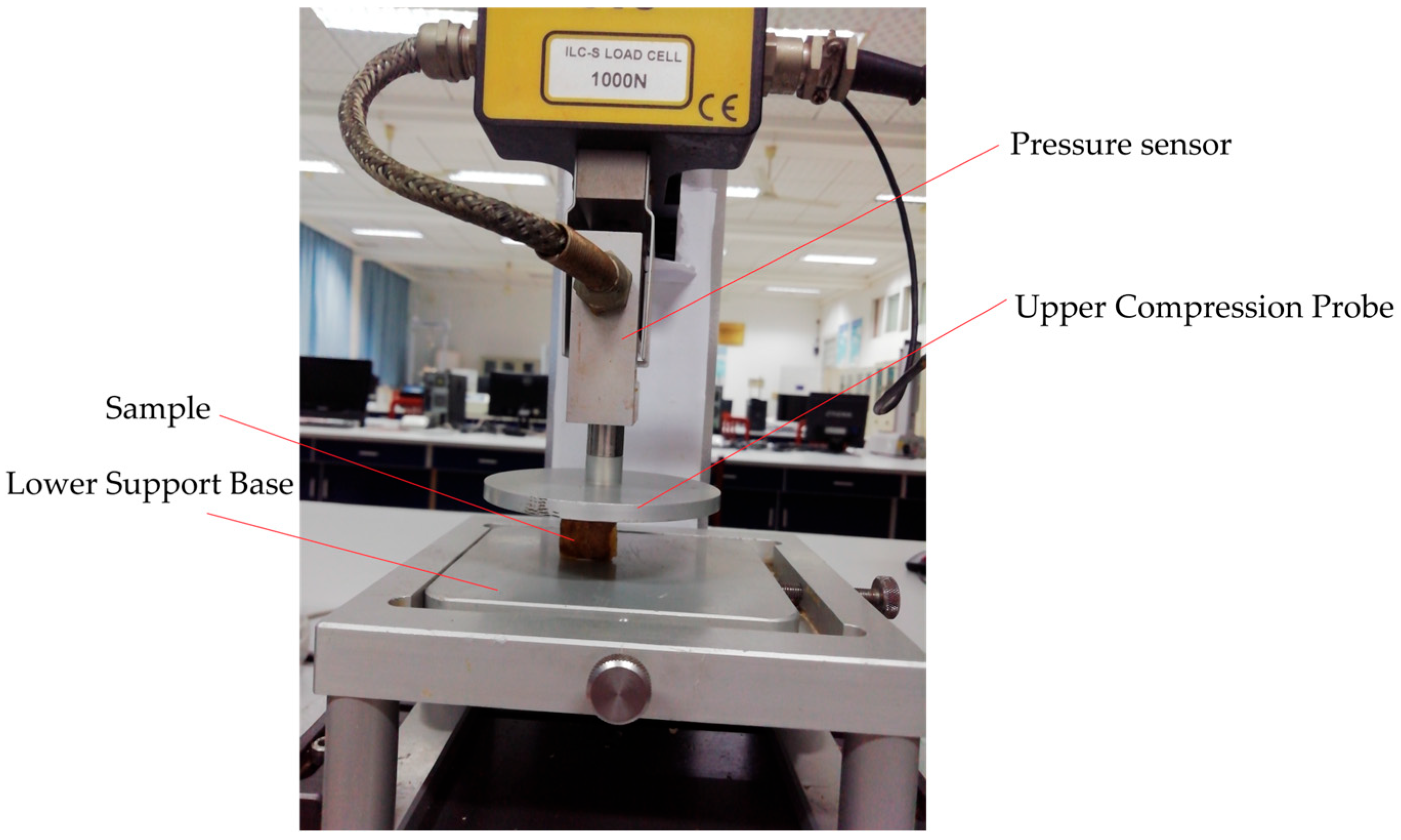
2.3.4. Measurement of Textural Characteristics
2.3.5. Microstructure Observation
2.3.6. Statistical Analysis
3. Results and Discussion
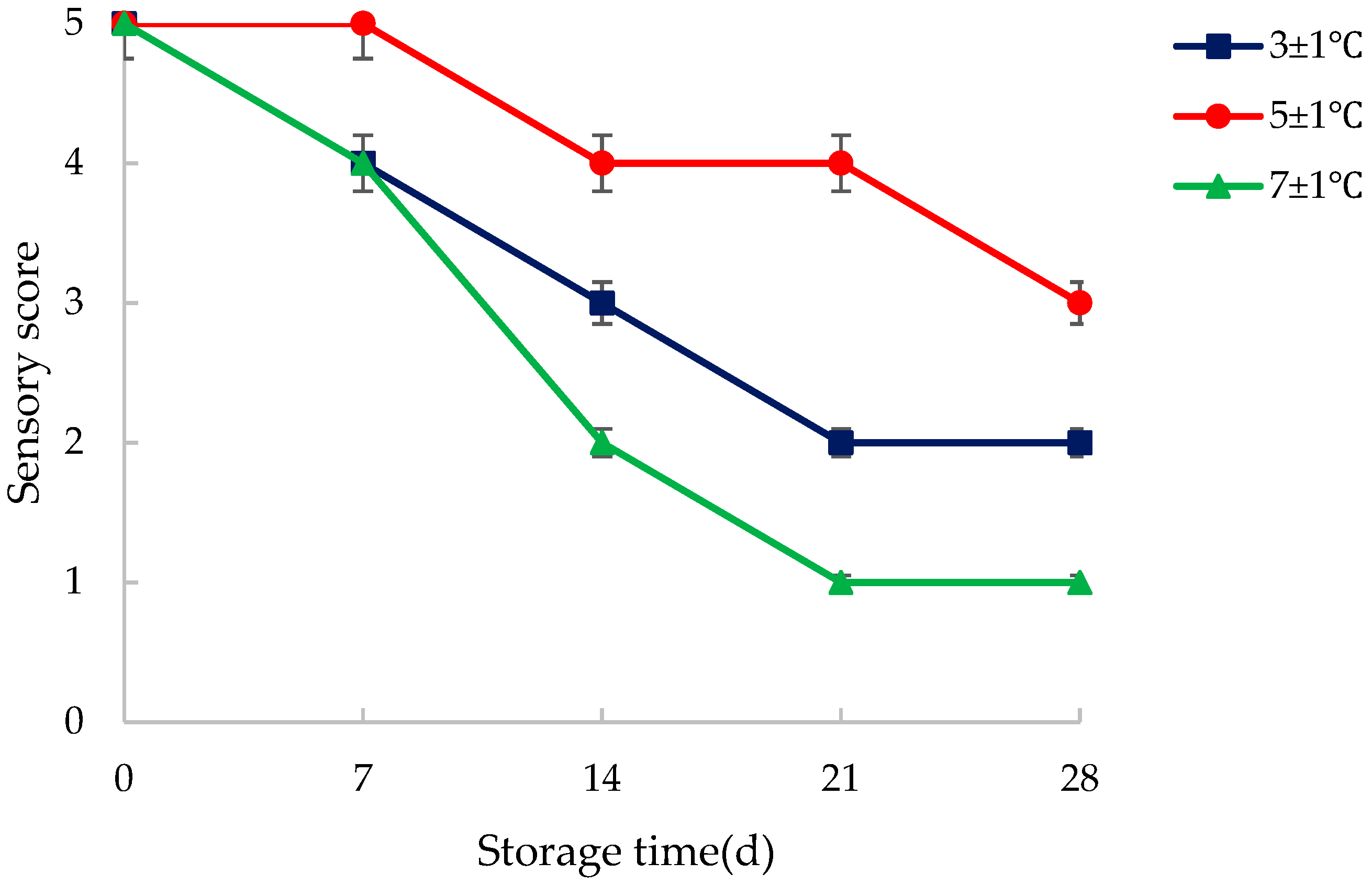
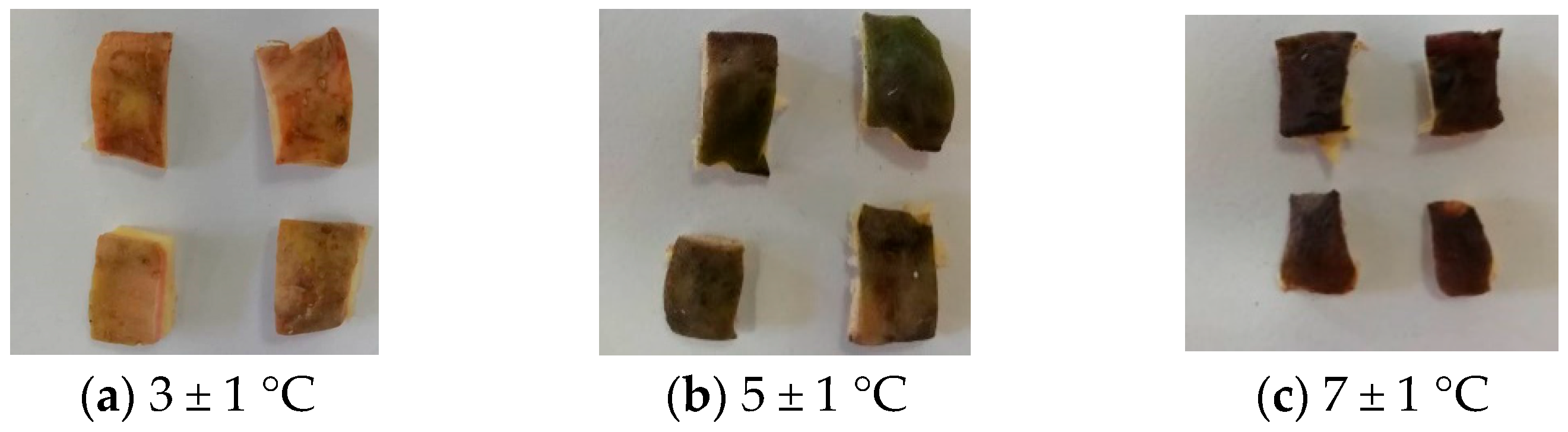
3.1. Sensory Quality
3.2. Moisture Content
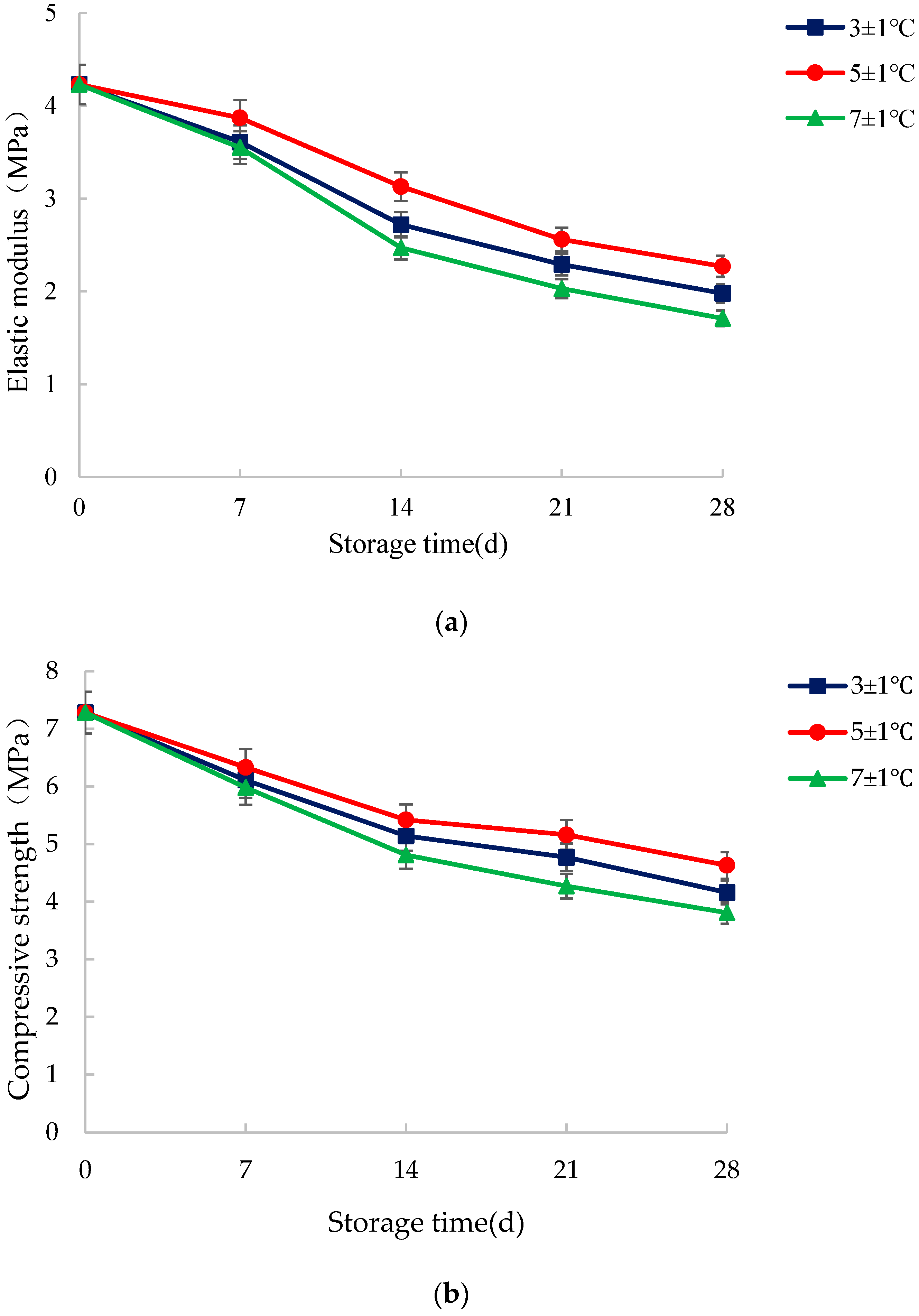
3.3. Texture Characteristics
3.4. Microstructure
3.5. Variance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WCS | water caltrop shell |
| SEM | scanning electron microscopy |
| SE | secondary electron |
Appendix A
| Evaluation Index | Evaluation Score | ||||
|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | |
| Color | Fresh green | Dark green | Dark yellow | Little black | Black |
| Odor | Fragrance | No mildew | Slight moldy | Mildew | Heavy mildew |
| Surface texture | Strong | Little hard | Little soft | Soft | Soft rotten |
| Factors and Indicators | Sensory Quality | Moisture Content (%) | Elastic Modulus (MPa) | Compressive Strength (MPa) | |
|---|---|---|---|---|---|
| 3 ± 1 °C | 0 d | 5 | 79.38 | 4.23 | 7.28 |
| 7 d | 4 | 78.92 | 3.61 | 6.11 | |
| 14 d | 3 | 77.63 | 2.72 | 5.14 | |
| 21 d | 2 | 77.22 | 2.29 | 4.77 | |
| 28 d | 2 | 76.87 | 1.98 | 4.16 | |
| 5 ± 1 °C | 0 d | 5 | 79.38 | 4.23 | 7.28 |
| 7 d | 5 | 78.81 | 3.87 | 6.33 | |
| 14 d | 4 | 77.59 | 3.13 | 5.42 | |
| 21 d | 4 | 77.18 | 2.56 | 5.16 | |
| 28 d | 3 | 76.44 | 2.27 | 4.63 | |
| 7 ± 1 °C | 0 d | 5 | 79.38 | 4.23 | 7.28 |
| 7 d | 4 | 78.49 | 3.55 | 5.98 | |
| 14 d | 2 | 77.27 | 2.47 | 4.81 | |
| 21 d | 1 | 77.16 | 2.03 | 4.27 | |
| 28 d | 1 | 76.63 | 1.71 | 3.81 | |
| Effect of storage temperature | F = 9.33 ** | F = 3.13 | F = 12.07 ** | F = 10.46 ** | |
| Effect of storage days | F = 14.29 ** | F = 201.70 ** | F = 148.14 ** | F = 131.93 ** | |
References
- Wang, H.; Chen, Y.; Yan, S.; Mei, D.; Zhao, D.; Zhang, J.; Wang, Q.; Li, J. Antibacterial activity and component analysis of water chestnut stem and water chestnut pericarp extracts. Sci. Technol. Food Ind. 2021, 42, 61–67. [Google Scholar]
- Wang, Y.; Lu, H.; Yi, Y.; Wang, L.; Wang, H.; Ai, Y.; Min, T. Nutritional quality and evaluation of Chinese water chestnuts from different origins. Horticulturae 2025, 11, 979. [Google Scholar] [CrossRef]
- Singh, I.; Tarate, S.; Thakur, A. Water chestnut (Trapa natans) a crop of high nutritional and economical importance-recent guidelines on farming practices. J. Sci. Res. Rep. 2024, 30, 310–321. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Yan, H.; Ni, X.; Sun, J. Effect of wax treatment on quality and physiology of Shaobo water chestnut during storage. Food Mach. 2014, 30, 142–146+162. [Google Scholar]
- Li, Y.; Zhang, J.; Song, W.; Wang, Y.; Yu, Z. Effect of temperature on qualities assessment of postharvest trapa taiwanensis. Food Sci. 2006, 1, 235–239. [Google Scholar]
- Farid, M.; Maryam, D. Effect of Vacuum and Modified Atmosphere Packaging on the Quality Attributes and Sensory Evaluation of Fresh Jujube Fruit. Int. J. Fruit Sci. 2020, 21, 82–94. [Google Scholar] [CrossRef]
- Ma, L.; Wang, B.; Liu, G.; Kang, N.; He, J.; Zhang, X.; Yang, G. Effect of pretreatment combined with modified atmosphere packaging on the storage quality of fresh Lycium barbarum. Food Ferment. Ind. 2021, 47, 195–200. [Google Scholar]
- Lwin, W.; Pongprasert, N.; Boonyaritthongchai, P.; Wongs, A.; Srilaong, V. Synergistic effect of vacuum packaging and cold shock reduce lignification of asparagus. J. Food Biochem. 2020, 44, e13479. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hu, W.; Saren, G.; Li, Y.; Lao, Y.; Zhao, M.; Liao, J. Precise fresh-keeping packaging for fresh-cut fruits and vegetables. Food Ferment. Ind. 2019, 45, 249–256. [Google Scholar]
- Fang, Z.; Duan, Z.; Dou, Z.; He, A.; Xie, H. Effects of different vacuum packaging treatment with low temperature on storage quality of fresh-cut pineapple. Sci. Technol. Food Ind. 2018, 39, 259–264. [Google Scholar]
- Sujeetha, A.; Meenatchi, R.; Patricia, P.; Negi, A. Effect of Vacuum Packaging on Quality of Pomegranate Arils during Storage. Curr. J. Appl. Sci. Technol. 2020, 39, 40–46. [Google Scholar] [CrossRef]
- Elda, E.; Dormita, D.; Roxanne, D.; Ryan, A. Vacuum packaging controlled crown rot of organically-grown Balangon (Musa acuminata AAA Group) banana. Horticulturae 2017, 3, 14. [Google Scholar]
- Rivia, D.; Benedito, C.; Montserrat, P.; Isabel, A.; Mara, L. A first approach of using ultrasound as an alternative for blanching in vacuum-packaged potato strips. Food Bioprocess Technol. 2016, 9, 1794–1801. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y. Application of oxygen absorbers and vacuum packaging on Chinese chestnut. Food Sci. Technol. 2015, 40, 306–309. [Google Scholar]
- Wang, G.; Xu, W. Research on fresh-keeping packaging and respiration rate of the peeled fresh lotus seed. J. Wuhan Polytech. Univ. 2013, 32, 16–18+36. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, C.; Wu, H.; Dong, C.; Pan, Y.; Wang, C. Effects of different precooling temperature on the storage quality of posthar-vest tomato. Food Res. Dev. 2018, 39, 188–193+200. [Google Scholar]
- Liu, J.; Wu, W.; Gao, H.; Liu, R.; Han, Q.; Chen, H. Effects of different storage temperatures on quality and microorganism of fresh-cut pitaya. J. Chin. Inst. Food Sci. Technol. 2017, 17, 168–175. [Google Scholar]
- Li, Y. Studies on Postharvest Quality, Biochem-Physiology and Enzymatic Browning of Trapa ssp. Master’s Thesis, Nanjing Agriculture University, Nanjing, China, 2006. [Google Scholar]
- Zubala, L.; Anjum, N.; Feroz, A.; Abid, H.; Syed, Z. Influence of xanthan, guar, CMC and gum acacia on functional properties of water chestnut (Trapa bispinosa) starch. Int. J. Biol. Macromol. 2017, 103, 220–225. [Google Scholar]
- Ma, M. Study on the Physicochemical Properties and Application of Water Caltrop. Master’s Thesis, Jiangnan University, Wuxi, China, 2013. [Google Scholar]
- Li, L.; Wen, Z.; Gui, Z.; Tan, N.; Chen, X.; Liu, Q. Preparation and quality of water chestnut composite vermicelli. Food Sci. Technol. 2016, 41, 74–78. [Google Scholar]
- Lv, H.; Jian, T.; Chen, J.; Ma, L.; Ren, B.; Li, W. Study on purification of polyphenols from shuck of Trapa quadrispinosa Roxb. and its α-glucosidase inhibitory activity. Lishizhen Med. Mater. Medica Res. 2017, 28, 2628–2630. [Google Scholar]
- Zhang, M.; Guo, Q.; Rui, L.; Zhao, J. A Method for Controlling and Monitoring the Deterioration of Starch Based Aquatic Vegetables. Chinese Patent CN202510564698.8, 18 July 2025. [Google Scholar]
- Jing, Y.; Meng, B.; Peng, Y.; Li, Q.; Lei, Y.; Li, Y. Metabolomics reveals the effect of vacuum packaging combined with moderate-temperature preservation on quality changes of tender ginger. Food Chem. X 2025, 25, 102168. [Google Scholar] [CrossRef]
- McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. The microbiology of beef steaks stored aerobically or anaerobically in vacuum pack films with different oxygen barrier properties. Food Packag. Shelf Life 2020, 26, 100597. [Google Scholar] [CrossRef]
- Zhang, P.; Lü, J.; Bi, J.; Zhou, L.; Yi, J.; Wu, X. Effect of osmotic dehydration on quality of peach chips prepared by explosion puffing drying. J. Chin. Inst. Food Sci. Technol. 2017, 17, 69–76. [Google Scholar]
- Yin, L.; Wang, C.; Wang, Y.; Xiang, C.; Chen, H. Sensory Quality, Texture and Chemical Composition Analysis of Pumpkin. Food Sci. 2013, 34, 26–30. [Google Scholar]
- Zheng, M.; Li, J.; Feng, J.; Lu, S. Effect of Drying Methods on Sensory Qualities of Loquat Flower Tea and Its Toxicological Evaluation. Food Sci. 2018, 39, 116–121. [Google Scholar]
- Liu, Y.; Chen, W. Ultrasonic-assisted extraction of chestnut shell pigment and its thermal degradation kinetics. China Food Addit. 2015, 8, 60–65. [Google Scholar]
- Ma, Q.; Guo, G.; Ma, J.; Lei, L.; Liu, K.; Long, H.; Li, J. Determination of mechanical characteristic parameters and extrusion crushing characteristics test for lotus seed kernel. Trans. Chin. Soc. Agric. Eng. 2018, 34, 263–271. [Google Scholar]
- Liao, J.; Hu, W.; Long, Y.; Zhao, M.; Yang, X.; Li, Y.; Ji, Y. Application of Electron Microscopy in the Study of Postharvest Preservation of Berries. Sci. Technol. Food Ind. 2021, 42, 356–361. [Google Scholar]
- Luo, M.; Zhang, J. Floral Morphogenesis in Semiaquilegia (Ranunculaceae) with Scanning Electron Microscopy. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1874–1880. [Google Scholar]
- Jing, X. Effects of Temperature and Harvesting Period on the Quality of Fresh Walnuts During Frozen Storage. Master’s Thesis, Gansu Agriculture University, Lanzhou, China, 2019. [Google Scholar]
- Jing, J.; Liu, X.; Deng, L.; Yao, S.; Zeng, K. Effects of harvesting maturity on the quality changes during storage of late maturing W. Murcott. Trans. Chin. Soc. Agric. Eng. 2021, 37, 303–309. [Google Scholar]
- Lv, C.; Zhang, A.; Cong, H.; Ma, R.; Guo, H.; Zhang, Y. Establishment and analysis of shelf life prediction models of hazelnut scraps with different packing and temperature storage conditions. J. Shenyang Agric. Univ. 2021, 52, 171–179. [Google Scholar]
- Hou, J. Research of Apple Texture Based on Microstructure and Modal Analysis. Ph.D. Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Yang, N.; Jin, Y.; Ma, Q.; Zhang, J.; Wu, F.; Xu, X. Study on rapid salting of lettuce by ion current treatment. Food Ferment. Ind. 2014, 40, 32–36. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Z.; Wang, W.; Liu, X.; Li, P.; Zhang, W. The Influence of Storage Conditions on the Quality of Vacuum-Packed Water Caltrop Shell. Foods 2025, 14, 3567. https://doi.org/10.3390/foods14203567
Wan Z, Wang W, Liu X, Li P, Zhang W. The Influence of Storage Conditions on the Quality of Vacuum-Packed Water Caltrop Shell. Foods. 2025; 14(20):3567. https://doi.org/10.3390/foods14203567
Chicago/Turabian StyleWan, Zhihua, Wangping Wang, Xiaopeng Liu, Pengju Li, and Wenhao Zhang. 2025. "The Influence of Storage Conditions on the Quality of Vacuum-Packed Water Caltrop Shell" Foods 14, no. 20: 3567. https://doi.org/10.3390/foods14203567
APA StyleWan, Z., Wang, W., Liu, X., Li, P., & Zhang, W. (2025). The Influence of Storage Conditions on the Quality of Vacuum-Packed Water Caltrop Shell. Foods, 14(20), 3567. https://doi.org/10.3390/foods14203567





