Associations Between Mineral Composition and Aflatoxin B1 in Maize (Zea mays L.) Seeds: Toward Contamination Indicators and Food Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
2.3. ICP-OES and ICP-QMS
2.4. Data Analysis
3. Results
3.1. Contents of Macro- and Microelements
3.2. Principal Component Analysis and Correlation Analysis
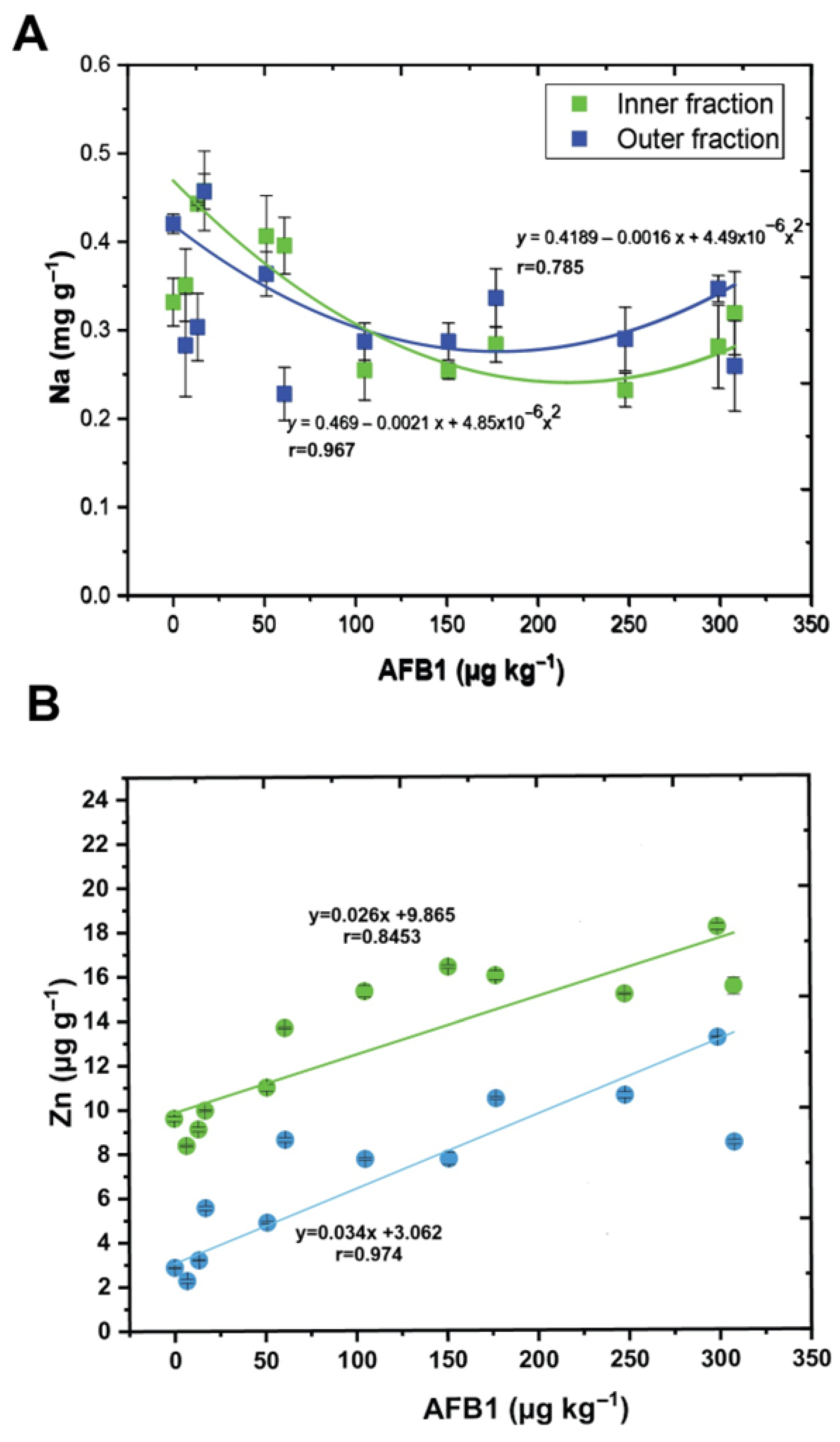
3.3. Relationship Between Macro- and Microelements and AFB1 Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; 224p. [Google Scholar]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Prod. Process. Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Fountain, J.C.; Khera, P.; Yang, L.; Nayak, S.N.; Scully, B.T.; Lee, R.D.; Chen, Z.Y.; Kemerait, R.C.; Varshney, R.K.; Guo, B. Resistance to Aspergillus flavus in maize and peanut: Molecular biology, breeding, environmental stress, and future perspectives. Crop J. 2015, 3, 229–237. [Google Scholar] [CrossRef]
- Messina, L.; Licata, P.; Bruno, F.; Litrenta, F.; Costa, G.L.; Ferrantelli, V.; Nava, V. Occurrence and health risk assessment of mineral composition and aflatoxin M1 in cow milk samples from different areas of Sicily, Italy. J. Trace Elem. Med. Biol. 2024, 85, 127478. [Google Scholar] [CrossRef]
- Bondoc, I. Foundations of Veterinary Sanitary and Food Safety Legislation (Bazele Legislației Sanitar-Veterinare și Pentru Siguranța Alimentelor); Ion Ionescu de la Brad Iași Publishing: Iași, Romania, 2015; Volume 1, ISBN 978-973-147-162-4. [Google Scholar]
- Bondoc, I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part One: The Role of European Institutions in Laying Down and Passing Laws Specific to the Veterinary Sanitary and Food Safety Area. Univ. Jurid. Supl. 2016, 12–15. [Google Scholar]
- Bondoc, I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Two: Regulations. Univ. Jurid. Supl. 2016, 16–19. [Google Scholar]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, A.L.; Simonović Radosavljević, J.; Prokopijević, M.; Spasojević, D.; Kovačević, J.; Prodanović, O.; Todorović, B.; Matović, B.; Stanković, M.; Maksimović, V.; et al. Cell Wall Response to UV Radiation in Needles of Picea omorika. Plant Physiol. Biochem. 2021, 161, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-H. Lignin Biosynthesis and Its Diversified Roles in Disease Resistance. Genes 2024, 15, 295. [Google Scholar] [CrossRef]
- Yadav, S.; Chattopadhyay, D. Lignin: The Building Block of Defense Responses to Stress in Plants. J. Plant Growth Regul. 2023, 42, 6652–6666. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Bartolić, D.; Mojović, M.; Prokopijević, M.; Djikanović, D.; Kalauzi, A.; Mutavdžić, D.; Baošić, R.; Radotić, K. Lignin and organic free radicals in maize (Zea mays L.) seeds in response to aflatoxin B1 contamination: An optical and EPR spectroscopic study. J. Sci. Food Agric. 2022, 102, 2500–2505. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Bartolić, D.; Maksimović, V.; Maksimović, J.D.; Stanković, M.; Krstović, S.; Baošić, R.; Radotić, K. Variations in Polyamine Conjugates in Maize (Zea mays L.) Seeds Contaminated with Aflatoxin B1: A Dose–Response Relationship. J. Sci. Food Agric. 2020, 100, 2905–2910. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, W.; Wang, F.; Zou, X.; Li, X.; Liu, C.M.; Zhang, W.; Yan, S. Integrated Metabolomics and Lipidomics Analyses Suggest the Temperature-Dependent Lipid Desaturation Promotes Aflatoxin Biosynthesis in Aspergillus flavus. Front. Microbiol. 2023, 14, 1137643. [Google Scholar] [CrossRef] [PubMed]
- Koley, S.; Jyoti, P.; Lingwan, M.; Wei, M.; Xu, C.; Chu, K.L.; Williams, R.B.; Koo, A.J.; Thelen, J.J.; Xu, D.; et al. Persistent Fatty Acid Catabolism during Plant Oil Synthesis. Cell Rep. 2025, 44, 115492. [Google Scholar] [CrossRef]
- Tiwari, R.P.; Mittal, V.; Bhalla, T.C.; Saini, S.S.; Singh, G.; Vadehra, D.V. Effect of Metal Ions on Aflatoxin Production by Aspergillus parasiticus. Folia Microbiol. 1986, 31, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Day, D.M.; Linz, J.E. Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus. Toxins 2016, 8, 171. [Google Scholar] [CrossRef]
- Cuero, R.; Ouellet, T. Metal ions modulate gene expression and accumulation of the mycotoxin aflatoxin and zearalenone. J. Appl. Microbiol. 2005, 98, 598–605. [Google Scholar] [CrossRef]
- Imran, M.; Garbe-Schönberg, D.; Neumann, G.; Boelt, B.; Mühling, K.H. Zinc distribution and localization in primed maize seeds and its translocation during early seedling development. Environ. Exp. Bot. 2017, 143, 91–98. [Google Scholar] [CrossRef]
- Jeevanraj, R.; Sivakumar, R.; Boominathan, P.; Kumar, P.S.; Selvi, C.S. The essential role of macronutrients and micronutrients in improving crop resilience to biotic stress. Russ. J. Plant Physiol. 2025, 72, 126. [Google Scholar] [CrossRef]
- Bityutskii, N.; Magnitski, S.; Lapshina, I.; Lukina, E.; Soloviova, A.; Patsevitch, V. Distribution of Micronutrients in Maize Grain and Their Mobilisation during Germination. In Plant Nutrition; Horst, W.J., Diatkova, O., Schenk, M.K., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2001; Volume 92, pp. 218–219. [Google Scholar] [CrossRef]
- Lung’aho, M.G.; Mwaniki, A.M.; Szalma, S.J.; Hart, J.J.; Rutzke, M.A.; Kochian, L.V.; Glahn, R.P.; Hoekenga, O.A. Genetic and Physiological Analysis of Iron Biofortification in Maize Kernels. PLoS ONE 2011, 6, e20429. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Z.X.; Kopittke, P.M.; Harper, S.M.; O’Hare, T.J.; Wang, P.; Paterson, D.J.; de Jonge, M.D.; Bell, M.J. In situ analyses of inorganic nutrient distribution in sweetcorn and maize kernels using synchrotron-based X-ray fluorescence microscopy. Ann. Bot. 2019, 123, 543–556. [Google Scholar] [CrossRef]
- Institute for Reference Materials and Measurements. Certified Reference Material ERM—CD281; Rye Grass: Geel, Belgium, 2010; Available online: https://crm.jrc.ec.europa.eu/ (accessed on 15 October 2025).
- Oliveira, C.A.F.; Gonçalves, N.B.; Rosim, R.E.; Fernandes, A.M. Determination of Aflatoxins in Peanut Products in the Northeast Region of São Paulo, Brazil. Int. J. Mol. Sci. 2009, 10, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Algül, I.; Kara, D. Determination and chemometric evaluation of total aflatoxin, aflatoxin B1, ochratoxin A and heavy metals content in corn flours from Turkey. Food Chem. 2014, 157, 70–76. [Google Scholar] [CrossRef]
- Bressani, R.; Breuner, M.; Ortiz, M.A. Mineral content of maize. In Maize in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989; pp. 33–46. Available online: https://www.fao.org/4/t0395e/T0395E03.htm (accessed on 15 October 2025).
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana, R.M.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Conesa, E.; Ochoa, J.; Vicente, M.J.; Franco, J.A.; Bañon, S.; Martínez, J.J.; Fernández, J.A. The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination. Agronomy 2020, 10, 504. [Google Scholar] [CrossRef]
- White, P.J.; Bell, M.J.; Djalovi, I.; Hinsinger, P.; Rengel, Z. Potassium use efficiency of plants. In Improving Potassium Recommendations for Agricultural Crops; Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R., Thompson, M.L., Eds.; Springer: Cham, Switzerland, 2021; pp. 73–91. [Google Scholar] [CrossRef]
- Ahmadian, F.; Chaichi Nosrati, A.; Shahriari, A.; Faezi Ghasemi, M.; Shokri, S. Effects of zinc chelating nutrients on aflatoxin production in Aspergillus flavus. Food Chem. Toxicol. 2020, 111, 111180. [Google Scholar] [CrossRef]
- Su, Q.; Chi, S.-D.; He, Z.-M. The Development of Aspergillus flavus and Biosynthesis of Aflatoxin B1 are Regulated by the Golgi-Localized Mn2+ Transporter Pmr1. J. Agric. Food Chem. 2024, 72, 1276–1291. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Wildeman, A.S.; Culotta, V.C. Nutritional Immunity and Fungal Pathogens: A New Role for Manganese. Curr. Clin. Microbiol. Rep. 2024, 11, 70–78. [Google Scholar] [CrossRef]
- Pittman, J.K. Managing the Manganese: Molecular Mechanisms of Manganese Transport and Homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Pera, L.M.; Callieri, D.A. Influence of calcium on fungal growth, hyphal morphology and citric acid production in Aspergillus niger. Folia Microbiol. 1997, 42, 551–556. [Google Scholar] [CrossRef]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Armendáriz, C.; Gutiérrez, Á.J.; Gomes-Furtado, V.; González-Weller, D.; Revert, C.; Hardisson, A.; Paz, S. Essential metals and trace elements in cereals and their derivatives commercialized and consumed in Cape Verde. Biol. Trace Elem. Res. 2023, 201, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, L.; Poovaiah, B.W. Calcium signaling and biotic defense responses in plants. Plant Signal Behav. 2014, 9, e973818. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X.M.; Zhang, Y.R.; Cai, Y.Y.; Wang, J.Y.; Liu, M.Y.; Wang, J.Y.; Bao, J.D.; Lin, F.C. Research on the Molecular Interaction Mechanism between Plants and Pathogenic Fungi. Int. J. Mol. Sci. 2022, 23, 4658. [Google Scholar] [CrossRef]
- White, P.J. Long-Distance Transport in the Xylem and Phloem. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 49–70. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Morales-González, J.A.; Vargas-Mendoza, N.; Reyes-Ramírez, P.; Cruz-Jaime, S.; Sumaya-Martínez, T.; Pérez-Pastén, R.; Madrigal-Bujaidar, E. Antigenotoxic Studies of Different Substances to Reduce the DNA Damage Induced by Aflatoxin B1 and Ochratoxin A. Toxins 2010, 2, 738–757. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A Comprehensive Review of Mycotoxins: Toxicology, Detection, and Effective Mitigation Approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in Plant Defence-Signalling Pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Huber, D.M.; Graham, R.D. The Role of Nutrition in Crop Resistance and Tolerance to Disease. In Mineral Nutrition of Crops: Fundamental Mechanisms and Implications; Rengel, Z., Ed.; Food Product Press: New York, NY, USA, 1999; pp. 205–226. [Google Scholar]
- Leigh, R.A.; Wyn Jones, R.G. A Hypothesis Relating Critical Potassium Concentrations for Growth to the Distribution and Functions of This Ion in the Plant Cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Cakmak, I. Possible Roles of Zinc in Protecting Plant Cells from Damage by Reactive Oxygen Species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Kusur, F.; Memon, M. Metal Hyperaccumulator Plants and Their Role in Phytoremediation. In Phytoremediation for Environmental Sustainability; Prasad, R., Ed.; Springer: Singapore, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Ali, S.; Riaz, A.; Shafaat, S.; Sidra, S.K.; Sufyan, M.; Huzaifa, M.; Imtiaz, S.; Ur Rehman, H. Biofortification of cereals with iron through agronomic practices. Curr. Res. Agric. Farming. 2022, 3, 11–16. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Why it is important to toxicology and toxicologists. Environ. Toxicol. Chem. 2008, 27, 1451–1474. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Poole, R.K. (Ed.) Microbiology of Metal Ions, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128123867. [Google Scholar]
- Williamson, C. Whole grains and health. Nutr. Bull. 2007, 32, 412–413. [Google Scholar] [CrossRef]
- Gallo, A.; Catellani, A.; Ghilardelli, F.; Lapris, M.; Mastroeni, C. Strategies and Technologies in Preventing Regulated and Emerging Mycotoxin Co-Contamination in Forage for Safeguarding Ruminant Health. Animal 2024, 18 (Suppl. S2), 101280. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef]

| AFB1 (μg kg−1) | Cr (μg g−1) | Mn (μg g−1) | Co (μg g−1) | Ni (μg g−1) | Cu (μg g−1) | Zn (μg g−1) | Fe (μg g−1) | Ca (mg g−1) | K (mg g−1) | Mg (mg g−1) | Na (mg g−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inner fraction | Control | 0.75 ±0.016 | 6.88 ±0.031 | 0.15 ±0.001 | 2.34 ±0.03 | 7.7 ±0.053 | 9.61 ±0.112 | 27.64 ±0.382 | 1.46 ±0.105 | 3.43 ±0.189 | 1.5 ±0.021 | 0.33 ±0.027 |
| 6.75 | 0.79 ±0.004 | 6.33 ±0.053 | 0.14 ±0.001 | 1.51 ±0.02 | 5.18 ±0.072 | 8.38 ±0.045 | 18.61 ±0.35 | 1.67 ±0.136 | 3.08 ±0.047 | 1.4 ±0.002 | 0.35 ±0.041 | |
| 13.26 | 0.74 ±0.01 | 7.14 ±0.041 | 0.12 ±0 | 1.17 ±0.007 | 5.88 ±0.019 | 9.12 ±0.099 | 14.57 ±0.289 | 1.9 ±0.077 | 2.97 ±0.176 | 1.33 ±0.014 | 0.44 ±0.002 | |
| 17.07 | 0.88 ±0.008 | 6.54 ±0.092 | 0.13 ±0.001 | 1.06 ±0.012 | 5.96 ±0.099 | 9.97 ±0.043 | 14.27 ±0.075 | 1.73 ±0.058 | 2.95 ±0.161 | 1.45 ±0.008 | 0.46 ±0.045 | |
| 51.51 | 0.98 ±0.01 | 8.06 ±0.077 | 0.13 ±0.001 | 1.45 ±0.006 | 8.78 ±0.154 | 11 ±0.159 | 22.68 ±0.271 | 1.91 ±0.098 | 3.15 ±0.308 | 1.44 ±0.004 | 0.41 ±0.046 | |
| 61 | 1.01 ±0.028 | 9.76 ±0.115 | 0.13 ±0.001 | 1.57 ±0.025 | 7.08 ±0.034 | 13.69 ±0.043 | 22.03 ±0.203 | 1.79 ±0.069 | 2.76 ±0.261 | 1.41 ±0.005 | 0.4 ±0.032 | |
| 105 | 0.86 ±0.022 | 10.47 ±0.083 | 0.12 ±0.002 | 1.6 ±0.011 | 4.86 ±0.056 | 15.31 ±0.262 | 16.31 ±0.08 | 0.92 ±0.076 | 3.21 ±0.158 | 1.26 ±0.018 | 0.26 ±0.034 | |
| 151.94 | 0.87 ±0.023 | 8.12 ±0.037 | 0.13 ±0.001 | 1.93 ±0.043 | 5.45 ±0.037 | 16.42 ±0.081 | 12.13 ±0.032 | 0.98 ±0.081 | 2.97 ±0.201 | 1.17 ±0.026 | 0.25 ±0.009 | |
| 177.42 | 0.87 ±0.023 | 9.5 ±0.026 | 0.13 ±0.001 | 1.57 ±0.012 | 5.38 ±0.064 | 16.01 ±0.204 | 12.36 ±0.08 | 1.05 ±0.06 | 2.36 ±0.065 | 1.37 ±0.019 | 0.28 ±0.02 | |
| 248 | 0.87 ±0.003 | 7.93 ±0.213 | 0.13 ±0.001 | 1.42 ±0.011 | 5.5 ±0.025 | 15.16 ±0.046 | 12.06 ±0.222 | 1.02 ±0.171 | 2.63 ±0.094 | 1.32 ±0.018 | 0.23 ±0.019 | |
| 299 | 0.97 ±0.02 | 10.89 ±0.082 | 0.16 ±0 | 2.46 ±0.024 | 6.07 ±0.097 | 18.19 ±0.152 | 14.24 ±0.052 | 1.22 ±0.065 | 2.89 ±0.135 | 1.26 ±0.022 | 0.28 ±0.047 | |
| 308.13 | 1.3± 0.016 | 11.15 ±0.21 | 0.16 ±0 | 1.29 ±0.02 | 5.04 ±0.081 | 15.5 ±0.384 | 17.44 ±0.345 | 1.2 ±0.07 | 2.77 ±0.298 | 1.24 ±0.017 | 0.32 ±0.047 | |
| Outer fraction | Control | 0.68 ±0.013 | 4.19 ±0.054 | 0.14 ±0.001 | 1.61 ±0.011 | 5.87 ±0.028 | 2.88 ±0.029 | 11.13 ±0.101 | 1.85 ±0.068 | 1.42 ±0.08 | 0.68 ±0.011 | 0.42 ±0.011 |
| 6.75 | 0.66 ±0.005 | 3.25 ±0.031 | 0.12 ±0.001 | 0.71 ±0.029 | 3.91 ±0.014 | 2.27 ±0.088 | 7.28 ±0.158 | 1.18 ±0.199 | 1.14 ±0.224 | 0.54 ±0.002 | 0.28 ±0.058 | |
| 13.26 | 0.74 ±0.016 | 4.78 ±0.058 | 0.12 ±0.001 | 1.95 ±0.016 | 4.66 ±0.074 | 3.21 ±0.022 | 7.39 ±0.162 | 1.19 ±0.082 | 1.72 ±0.109 | 0.61 ±0.014 | 0.3 ±0.038 | |
| 17.07 | 0.83 ±0.013 | 4.9 ±0.081 | 0.15 ±0.001 | 1.7 ±0.028 | 6.45 ±0.09 | 5.56 ±0.098 | 10.73 ±0.128 | 2.03 ±0.048 | 1.37 ±0.217 | 0.82 ±0.006 | 0.46 ±0.02 | |
| 51.51 | 0.88 ±0.026 | 4.67 ±0.061 | 0.12 ±0.001 | 0.96 ±0.009 | 5.39 ±0.066 | 4.9 ±0.049 | 8.28 ±0.064 | 1.57 ±0.13 | 1.5 ±0.221 | 0.66 ±0.012 | 0.36 ±0.025 | |
| 61 | 0.88 ±0.006 | 4.75 ±0.103 | 0.15 ±0.001 | 1.48 ±0.018 | 4.17 ±0.018 | 8.64 ±0.108 | 7.67 ±0.193 | 0.78 ±0.093 | 1.18 ±0.242 | 0.46 ±0.007 | 0.23 ±0.03 | |
| 105 | 0.99 ±0.019 | 5.25 ±0.054 | 0.14 ±0.001 | 1.63 ±0.042 | 4.33 ±0.006 | 7.76 ±0.05 | 7 ±0.243 | 0.88 ±0.092 | 1.22 ±0.307 | 0.42 ±0.012 | 0.29 ±0.021 | |
| 151.94 | 1.01 ±0.018 | 7.54 ±0.042 | 0.15 ±0.001 | 1.87 ±0.016 | 6.08 ±0.059 | 13.83 ±0.275 | 9.65 ±0.322 | 0.92 ±0.026 | 2.24 ±0.115 | 0.73 ±0.008 | 0.3 ±0.021 | |
| 177 | 1± 0.03 | 6.47 ±0.072 | 0.16 ±0.001 | 2.6 ±0.035 | 6.08 ±0.012 | 10.47 ±0.061 | 8.21 ±0.144 | 1.31 ±0.024 | 1.26 ±0.19 | 0.69 ±0.007 | 0.34 ±0.033 | |
| 248 | 0.87 ±0.008 | 5.48 ±0.031 | 0.16 ±0.001 | 2.8 ±0.04 | 5.34 ±0.13 | 10.6 ±0.14 | 7.15 ±0.274 | 0.99 ±0.101 | 1.16 ±0.007 | 0.66 ±0.003 | 0.29 ±0.036 | |
| 299 | 0.99 ±0.017 | 7.07 ±0.074 | 0.16 ±0.002 | 4.37 ±0.055 | 6.57 ±0.149 | 13.2 ±0.027 | 10.85 ±0.114 | 1.25 ±0.037 | 1.7 ±0.11 | 0.65 ±0.003 | 0.35 ±0.015 | |
| 308.13 | 0.94 ±0.022 | 8.33 ±0.119 | 0.17 ±0.001 | 1.24 ±0.012 | 4.65 ±0.064 | 8.46 ±0.097 | 6.97 ±0.153 | 1.1 ±0.06 | 1.84 ±0.09 | 0.7 ±0.013 | 0.26 ±0.051 |
| Cr | Mn | Co | Ni | Cu | Zn | Fe | Ca | K | Mg | Na | |
| Cr | 1 | 0.702 | 0.564 | 0.433 | 0.150 | 0.822 | −0.194 | −0.410 | 0.054 | −0.191 | −0.245 |
| Mn | 0.696 | 1 | 0.749 | 0.479 | 0.293 | 0.727 | −0.039 | −0.194 | 0.571 | 0.263 | −0.182 |
| Co | 0.400 | 0.233 | 1 | 0.517 | 0.359 | 0.797 | 0.078 | −0.162 | 0.069 | 0.278 | −0.117 |
| Ni | −0.140 | 0.228 | 0.526 | 1 | 0.625 | 0.751 | 0.396 | −0.054 | 0.213 | 0.215 | 0.132 |
| Cu | −0.046 | −0.238 | −0.083 | 0.218 | 1 | 0.328 | 0.818 | 0.703 | 0.281 | 0.776 | 0.790 |
| Zn | 0.469 | 0.818 | 0.212 | 0.366 | −0.360 | 1 | −0.007 | −0.403 | 0.033 | −0.012 | −0.256 |
| Fe | 0.014 | −0.161 | 0.190 | 0.264 | 0.725 | −0.494 | 1 | 0.784 | 0.237 | 0.585 | 0.836 |
| Ca | −0.123 | −0.500 | −0.238 | −0.338 | 0.605 | −0.777 | 0.492 | 1 | 0.231 | 0.790 | 0.949 |
| K | −0.325 | −0.360 | 0.059 | 0.292 | 0.395 | −0.516 | 0.630 | 0.296 | 1 | 0.440 | 0.138 |
| Mg | −0.319 | −0.547 | −0.078 | −0.113 | 0.641 | −0.715 | 0.644 | 0.663 | 0.253 | 1 | 0.694 |
| Na | −0.060 | −0.452 | −0.264 | −0.488 | 0.455 | −0.739 | 0.347 | 0.936 | 0.223 | 0.607 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolić, D.; Baošić, R.; Mutić, J.; Stanković, M.; Mutavdžić, D.; Preradović, N.; Krstović, S.; Radotić, K. Associations Between Mineral Composition and Aflatoxin B1 in Maize (Zea mays L.) Seeds: Toward Contamination Indicators and Food Safety. Foods 2025, 14, 3552. https://doi.org/10.3390/foods14203552
Bartolić D, Baošić R, Mutić J, Stanković M, Mutavdžić D, Preradović N, Krstović S, Radotić K. Associations Between Mineral Composition and Aflatoxin B1 in Maize (Zea mays L.) Seeds: Toward Contamination Indicators and Food Safety. Foods. 2025; 14(20):3552. https://doi.org/10.3390/foods14203552
Chicago/Turabian StyleBartolić, Dragana, Rada Baošić, Jelena Mutić, Mira Stanković, Dragosav Mutavdžić, Nevena Preradović, Saša Krstović, and Ksenija Radotić. 2025. "Associations Between Mineral Composition and Aflatoxin B1 in Maize (Zea mays L.) Seeds: Toward Contamination Indicators and Food Safety" Foods 14, no. 20: 3552. https://doi.org/10.3390/foods14203552
APA StyleBartolić, D., Baošić, R., Mutić, J., Stanković, M., Mutavdžić, D., Preradović, N., Krstović, S., & Radotić, K. (2025). Associations Between Mineral Composition and Aflatoxin B1 in Maize (Zea mays L.) Seeds: Toward Contamination Indicators and Food Safety. Foods, 14(20), 3552. https://doi.org/10.3390/foods14203552







