Assessment of Heavy Metals in Mexican Dietary Supplements Using Total X-Ray Fluorescence Spectrometry and Health Risk Evaluation
Abstract
1. Introduction
1.1. Health Effects of Pb, As, and Cr in Vulnerable Populations
1.2. Heavy Metals in Dietary Supplements
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Sample Preparation
2.4. TXRF Analysis
2.5. Quality Control
2.6. Human Health Risk Assessment
2.6.1. Estimated Daily Intake (EDI)
2.6.2. Target Hazard Quotient (THQ) and Hazard Index (HI)
2.6.3. Cumulative Carcinogenic Risk (CCR)
2.7. Statistical Analysis
3. Results
3.1. Dietary Samples Characteristics
3.2. Quality Control
3.3. Application to Real Samples
3.3.1. Lead
3.3.2. Arsenic
3.3.3. Chromium
3.4. Regulatory Oversight of Dietary Supplement Production and Distribution in Mexico
3.5. Human Health Risk Assessment
3.5.1. Estimated Daily Intake
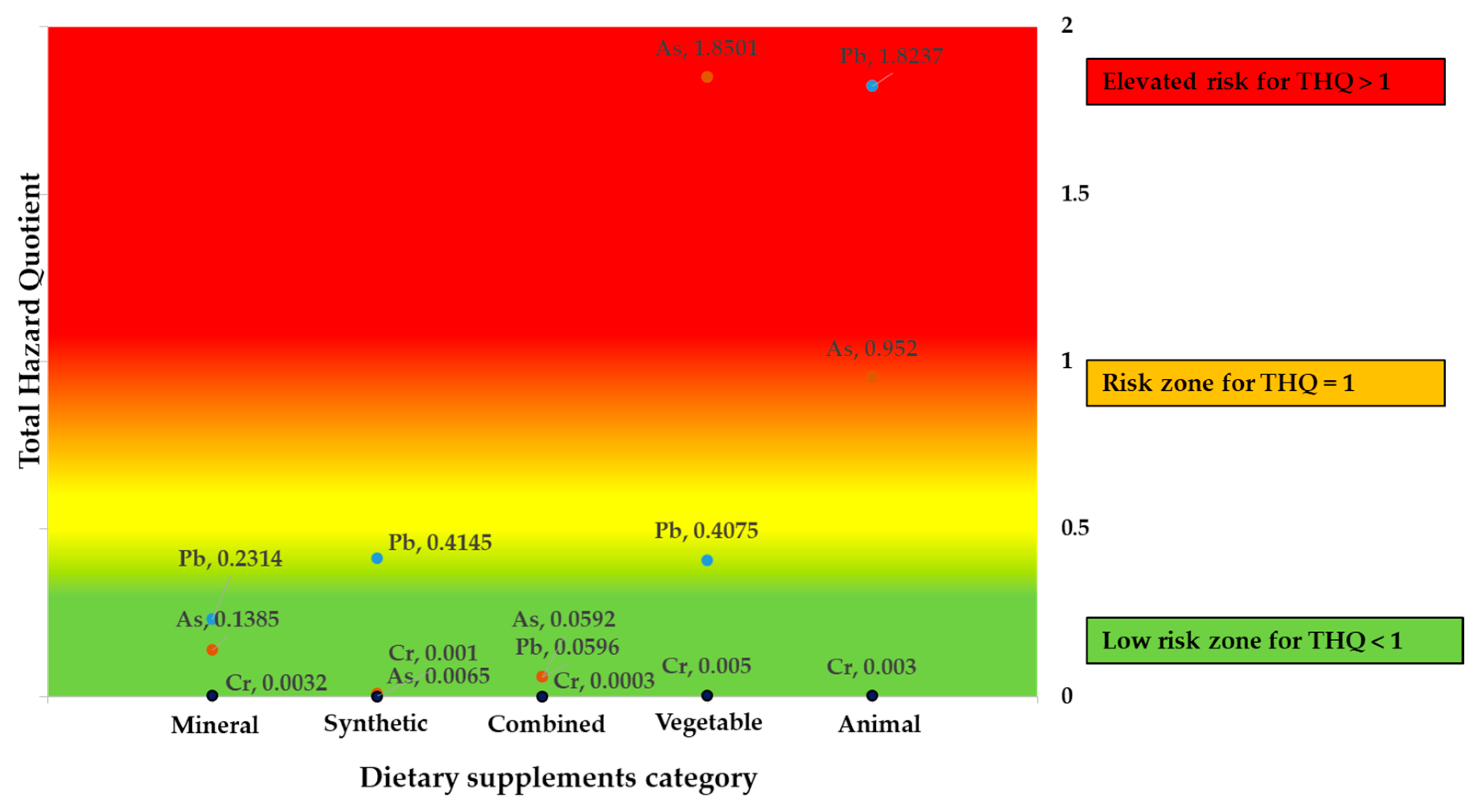
3.5.2. Total Hazard Quotient and Hazard Index
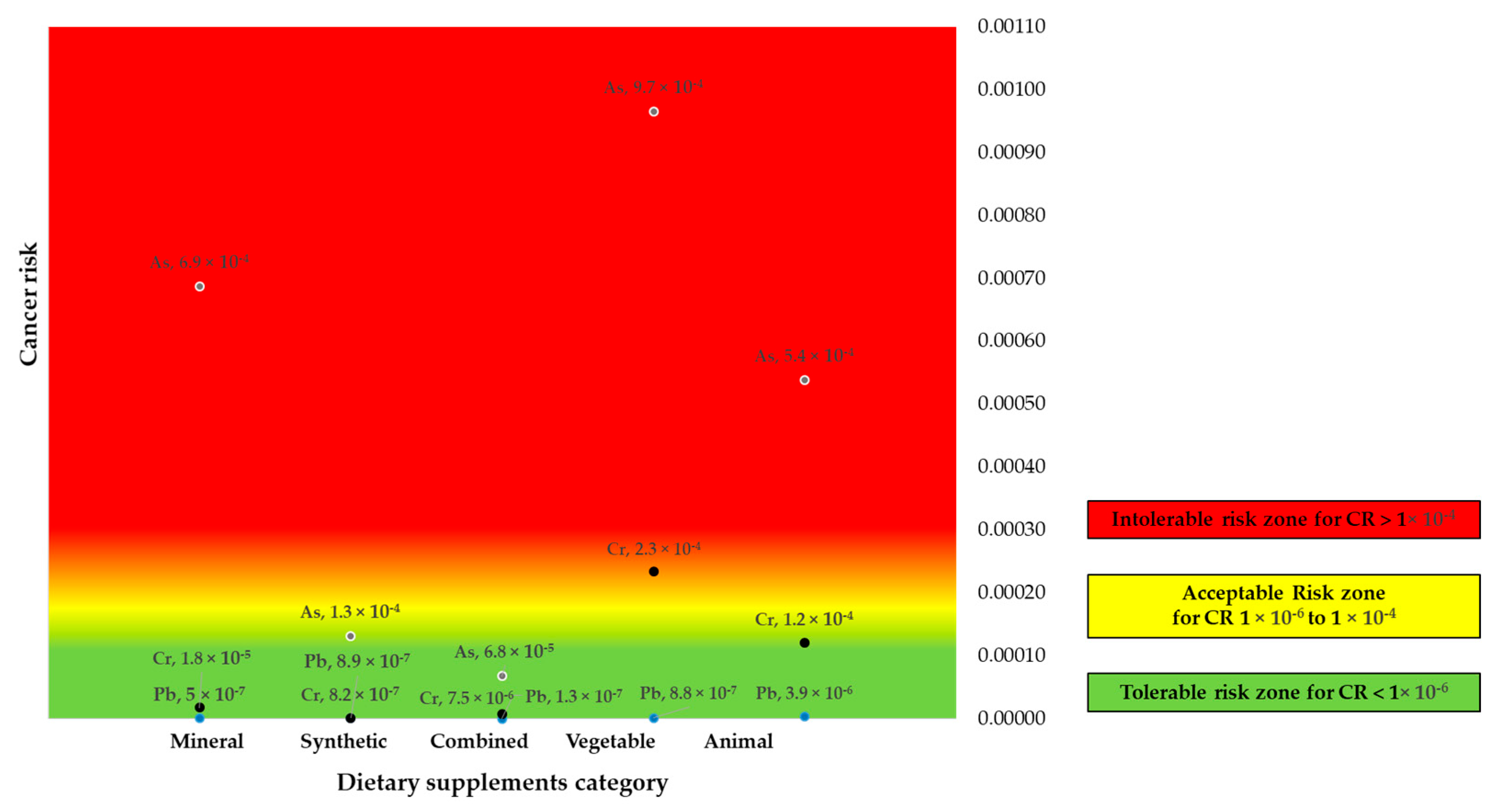
3.5.3. The Cumulative Cancer Risk (CCR)
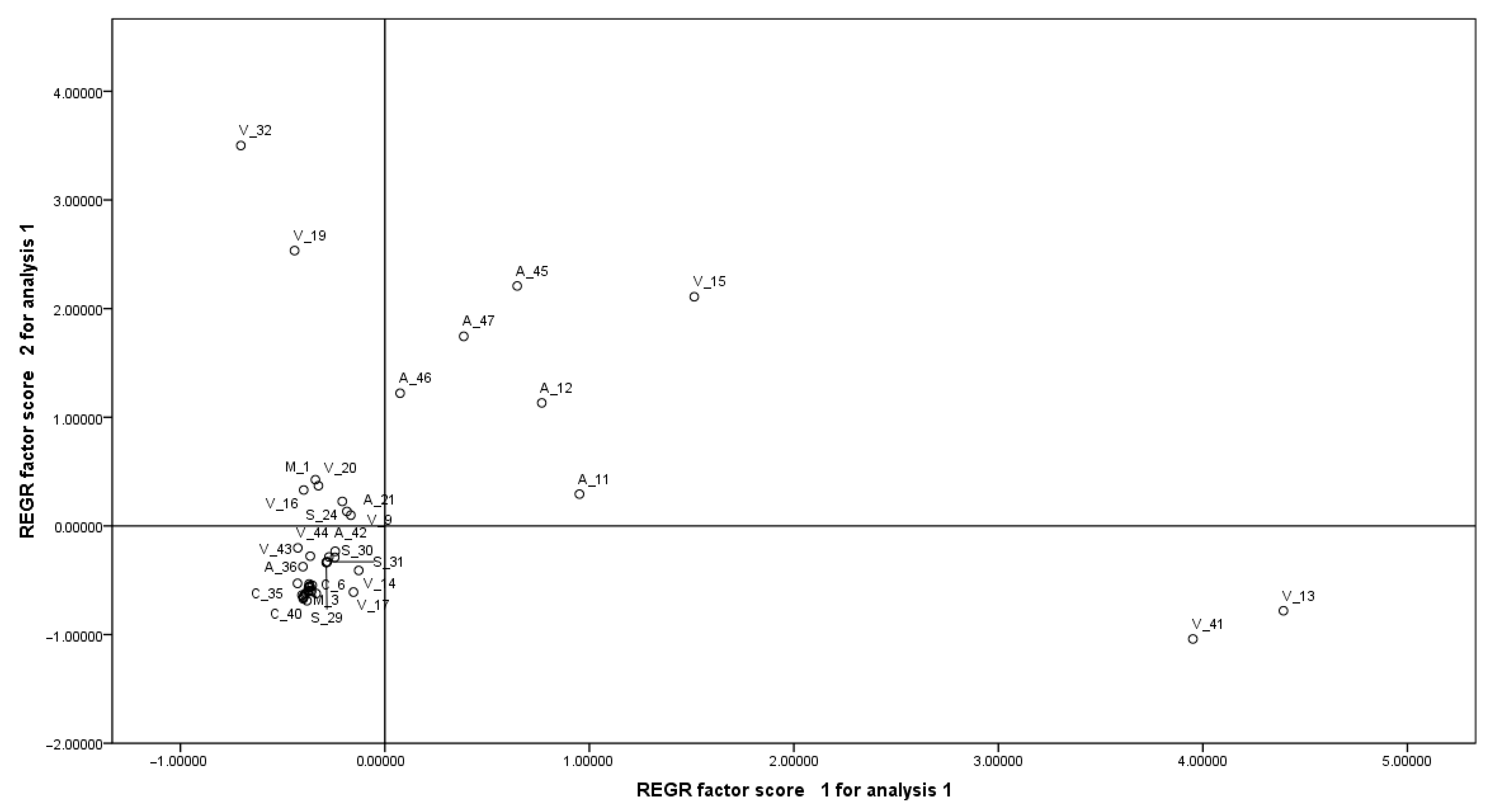
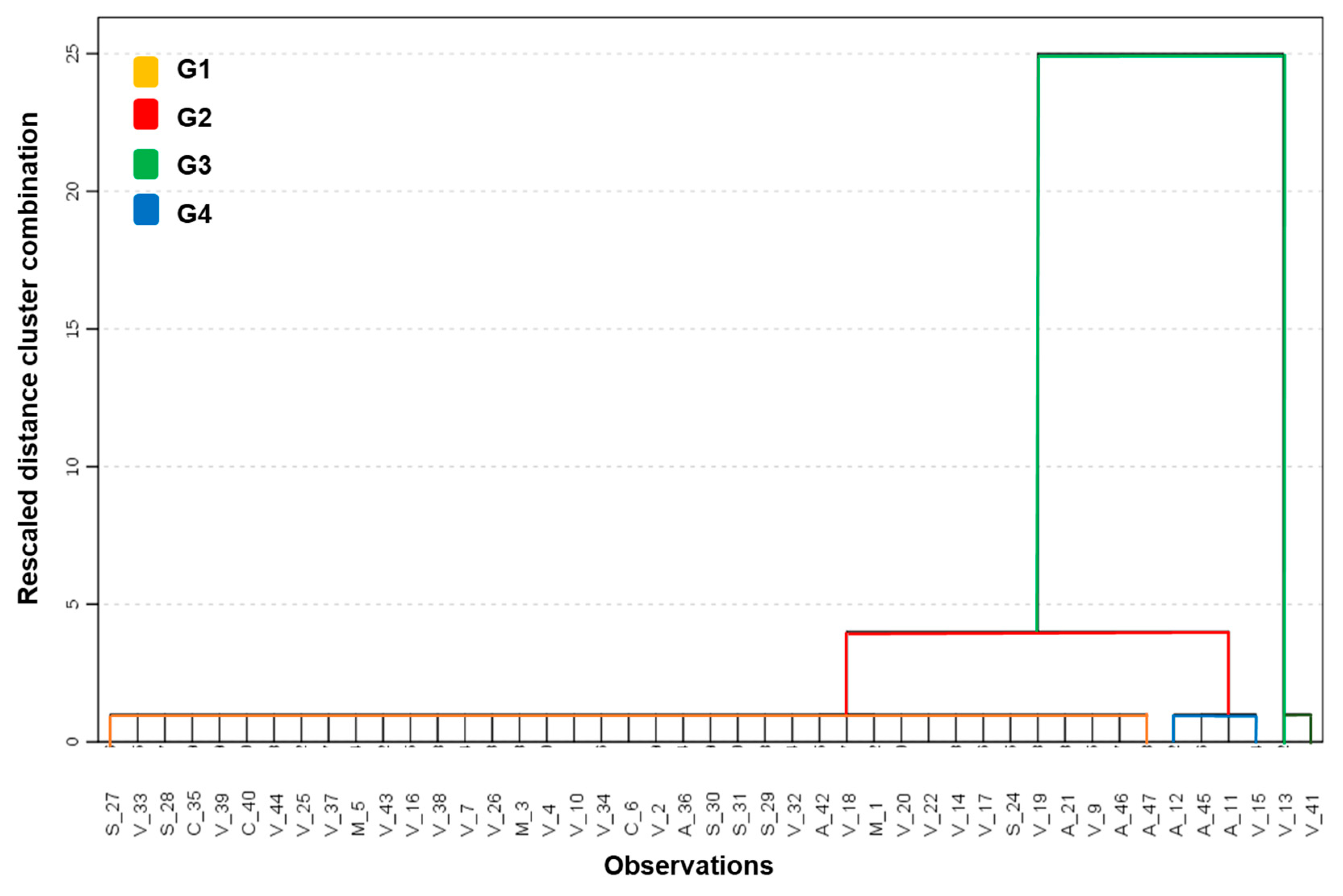
3.6. Multivariate Analysis
3.7. Novel Contributions of This Study
- Regional Knowledge Gap: This study provides the first comprehensive analysis of lead, arsenic, and chromium in dietary supplements from northeastern Mexico, filling a crucial geographic data gap in North American contamination patterns.
- Micronutrient Toxicity Focus: Novel measurement of chromium as both a needed nutrient and potential toxin, providing baseline data for a metal rarely tracked in supplement quality control despite its dual role.
- Methodological Innovation: First application of Total Reflection X-ray Fluorescence (TXRF) for simultaneous multi-element detection in Mexican dietary supplements, demonstrating comparable performance to ICP-MS with significant environmental and economic advantages.
- Regulatory Impact: Direct evidence supporting the urgent need to update Mexico’s 2009 regulatory framework, which currently lacks heavy metal limits, providing a scientific foundation for evidence-based policy development.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valencia, L.P.U.; Villarreal Ramírez, V.H.; Macías López, M.G.; Ortega Montes, F.; Terrazas Gómez, M.I. Estudio de Mercado Para Suplementos Alimenticios Orgánicos En Delicias, Chihuahua. Rev. Biológico Agropecu. Tuxpan 2021, 9, 16–29. [Google Scholar] [CrossRef]
- García-Rico, L.; Leyva-Perez, J.; Jara-Marini, M.E. Content and Daily Intake of Copper, Zinc, Lead, Cadmium, and Mercury from Dietary Supplements in Mexico. Food Chem. Toxicol. 2007, 45, 1599–1605. [Google Scholar] [CrossRef]
- Augustsson, A.; Qvarforth, A.; Engström, E.; Paulukat, C.; Rodushkin, I. Trace and Major Elements in Food Supplements of Different Origin: Implications for Daily Intake Levels and Health Risks. Toxicol. Rep. 2021, 8, 1067–1080. [Google Scholar] [CrossRef]
- Gajewska, M.J. Analysis of the Content of Selected Heavy Metals in Dietary Supplements Available on the Polish Market. Farm. Pol. 2023, 79, 529–536. [Google Scholar] [CrossRef]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Naz, M.; Ahmed, M.; Aftab, F.; Ali, M.A.; Sanaullah, M.; Ahmad, W.; Alshammari, A.H.; Khalid, K.; Wani, T.A.; Zargar, S. Contamination of Trace, Non-Essential/Heavy Metals in Nutraceuticals/Dietary Supplements: A Chemometric Modelling Approach and Evaluation of Human Health Risk upon Dietary Exposure. Food Chem. Toxicol. 2024, 190, 114806. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Shahwan, M.; Zyoud, S.H. Heavy Metal Contamination of Dietary Supplements Products Available in the UAE Markets and the Associated Risk. Sci. Rep. 2020, 10, 18824. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmed, M.; Ramzan, M.; Fatima, M.; Aftab, F.; Sanaullah, M.; Qamar, S.; Iftikhar, Z.; Wani, T.A.; Zargar, S. Appraisal of Potentially Toxic Metals Contamination in Protein Supplements for Muscle Growth: A Chemometric Approach and Associated Human Health Risks. J. Trace Elem. Med. Biol. 2024, 85, 127481. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, B.G.; Ramos-Sanchez, V.; Chávez-Flores, D.; Rodríguez-Maese, R.; Palacio, E. Total Reflection X-ray Fluorescence Spectroscopy (TXRF) Method Validation: Determination of Heavy Metals in Dietary Supplements. J. Chem. 2020, 2020, 8817393. [Google Scholar] [CrossRef]
- Korfali, S.I.; Hawi, T.; Mroueh, M. Evaluation of Heavy Metals Content in Dietary Supplements in Lebanon. Chem. Cent. J. 2013, 7, 10. [Google Scholar] [CrossRef]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of Regulatory Reference Values and Background Levels for Heavy Metals in the Human Diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Update of the Risk Assessment of Inorganic Arsenic in Food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for Chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Leskovac, A.; Petrović, S.; Mitić, M.; Lazarević-Pašti, T.; Novković, M.; Potkonjak, N. Metals on the Menu—Analyzing the Presence, Importance, and Consequences. Foods 2024, 13, 1890. [Google Scholar] [CrossRef]
- Kerna, N.A.; Holets, H.M.; Anderson II, J.; Flores, J.V.; Pruitt, K.D.; McKee, D.; Carsrud, N.D.V.; Ngwu, D.C.; Nnake, I.; Chawla, S.; et al. Heavy Metals and Human Health: From Neurological Disorders to Developmental Delays. Eur. J. Ecol. Biol. Agric. 2024, 1, 152–184. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Garcia, N.Y.; Cipriano Ramírez, A.I.; Juarez, K.; Brand Galindo, J.; Briceño, G.; Calderon Martinez, E. Maternal Exposure to Arsenic and Its Impact on Maternal and Fetal Health: A Review. Cureus 2023, 15, e49177. [Google Scholar] [CrossRef]
- Xia, W.; Hu, J.; Zhang, B.; Li, Y.; Wise, J.P.; Bassig, B.A.; Zhou, A.; Savitz, D.A.; Xiong, C.; Zhao, J.; et al. A Case-Control Study of Maternal Exposure to Chromium and Infant Low Birth Weight in China. Chemosphere 2016, 144, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Sazakli, E. Human Health Effects of Oral Exposure to Chromium: A Systematic Review of the Epidemiological Evidence. Int. J. Environ. Res. Public Health 2024, 21, 406. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Wesołowska, A.; Sobieraj, P.; Nawrocka, A.; Filipek, A.; Durkalec, M.; Katryńska, D.; Jedziniak, P. Essential and Non-Essential Element Concentrations in Human Milk Samples and the Assessment of Infants’ Exposure. Sci. Rep. 2024, 14, 8140. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, R.; Kumar, K.; Chayal, N.K.; Ali, M.; Srivastava, A.; Kumar, M.; Niraj, P.K.; Aryal, S.; Kumar, D.; et al. High Arsenic Contamination in the Breast Milk of Mothers Inhabiting the Gangetic Plains of Bihar: A Major Health Risk to Infants. Environ. Health. 2024, 23, 77. [Google Scholar] [CrossRef]
- Stanley, J.A.; Sivakumar, K.K.; Nithy, T.K.; Arosh, J.A.; Hoyer, P.B.; Burghardt, R.C.; Banu, S.K. Postnatal Exposure to Chromium through Mother’s Milk Accelerates Follicular Atresia in F1 Offspring through Increased Oxidative Stress and Depletion of Antioxidant Enzymes. Free Radic. Biol. Med. 2013, 61, 179–196. [Google Scholar] [CrossRef][Green Version]
- Capitão, C.; Martins, R.; Santos, O.; Bicho, M.; Szigeti, T.; Katsonouri, A.; Bocca, B.; Ruggieri, F.; Wasowicz, W.; Tolonen, H.; et al. Exposure to Heavy Metals and Red Blood Cell Parameters in Children: A Systematic Review of Observational Studies. Front. Pediatr. 2022, 10, 921239. [Google Scholar] [CrossRef]
- Vaidya, N.; Holla, B.; Heron, J.; Sharma, E.; Zhang, Y.; Fernandes, G.; Iyengar, U.; Spiers, A.; Yadav, A.; Das, S.; et al. Neurocognitive Analysis of Low-Level Arsenic Exposure and Executive Function Mediated by Brain Anomalies among Children, Adolescents, and Young Adults in India. JAMA Netw. Open 2023, 6, E2312810. [Google Scholar] [CrossRef]
- Caparros-Gonzalez, R.A.; Giménez-Asensio, M.J.; González-Alzaga, B.; Aguilar-Carduño, C.; Lorca-Marín, J.A.; Alguacil, J.; Gómez-Becerra, I.; Gómez-Ariza, J.L.; García-Barrera, T.; Hernandez, A.F.; et al. Childhood Chromium Exposure and Neuropsychological Development in Children Living in Two Polluted Areas in Southern Spain. Environ. Pollut. 2019, 252, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Halabicky, O.M.; Pinto-Martin, J.A.; Compton, P.; Liu, J. Early Childhood Lead Exposure and Adolescent Heart Rate Variability: A Longitudinal Cohort Study. Environ. Res. 2022, 205, 112551. [Google Scholar] [CrossRef]
- Liu, Y.; Téllez-Rojo, M.M.; Sánchez, B.N.; Zhang, Z.; Afeiche, M.C.; Mercado-García, A.; Hu, H.; Meeker, J.D.; Peterson, K.E. Early Lead Exposure and Pubertal Development in a Mexico City Population. Environ. Int. 2019, 125, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hou, Q.; Zhang, M.; Gao, E.; Wu, Y. Exposure to Arsenic and Cognitive Impairment in Children: A Systematic Review. PLoS ONE 2025, 20, e0319104. [Google Scholar] [CrossRef]
- Pathak, A.; Asediya, V.; Anjaria, P.; Singh, S.P. Health Risk Linked to Cr Toxicity in Food and Environment. In Environmental Science and Engineering; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2023; Volume Part F1975, pp. 217–252. [Google Scholar]
- Vig, E.K.; Hu, H. Lead Toxicity in Older Adults. J. Am. Geriatr. Soc. 2000, 48, 1501–1506. [Google Scholar] [CrossRef]
- Shih, R.A.; Glass, T.A.; Bandeen-Roche, K.; Carlson, M.C.; Bolla, K.I.; Todd, A.C.; Schwartz, B.S. Environmental Lead Exposure and Cognitive Function in Community-Dwelling Older Adults. Neurology 2006, 67, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Y.; Liu, H.; Sun, J.; Liu, Y.; Wu, J.; Li, D.; Sun, D. Assessment of Relationship on Excess Arsenic Intake from Drinking Water and Cognitive Impairment in Adults and Elders in Arsenicosis Areas. Int. J. Hyg. Environ. Health 2017, 220, 424–430. [Google Scholar] [CrossRef]
- Jiang, E.X.; Domingo-Relloso, A.; Abuawad, A.; Haack, K.; Tellez-Plaza, M.; Fallin, M.D.; Umans, J.G.; Best, L.G.; Zhang, Y.; Kupsco, A.; et al. Arsenic Exposure and Epigenetic Aging: The Association with Cardiovascular Disease and All-Cause Mortality in the Strong Heart Study. Environ. Health Perspect. 2023, 131, 127016. [Google Scholar] [CrossRef]
- Rojas, P.; Ruiz-Sánchez, E.; Ríos, C.; Ruiz-Chow, Á.; Reséndiz-Albor, A.A. A Health Risk Assessment of Lead and Other Metals in Pharmaceutical Herbal Products and Dietary Supplements Containing Ginkgo Biloba in the Mexico City Metropolitan Area. Int. J. Environ. Res. Public Health 2021, 18, 8285. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Ruiz-Sánchez, E.; Rojas, C.; García-Martínez, B.A.; López-Ramírez, A.M.; Osorio-Rico, L.; Ríos, C.; Reséndiz-Albor, A.A. Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City. Toxics 2023, 11, 801. [Google Scholar] [CrossRef]
- Mustatea, G.; Ungureanu, E.L.; Iorga, S.C.; Ciotea, D.; Popa, M.E. Risk Assessment of Lead and Cadmium in Some Food Supplements Available on the Romanian Market. Foods 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fan, X.; Zhou, J. Total Reflection X-ray Fluorescence Spectroscopy. OALib 2020, 7, e6671. [Google Scholar] [CrossRef]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Simbaña-Farinango, K.; Navarrete, H. Distribution, Contents, and Health Risk Assessment of Cadmium, Lead, and Nickel in Bananas Produced in Ecuador. Foods 2019, 8, 330. [Google Scholar] [CrossRef]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of Heavy Metals in Soil and Vegetables and Associated Health Risks in Mojo Area, Ethiopia. PLoS ONE 2020, 15, e0227883. [Google Scholar] [CrossRef]
- USEPA Integrated Risk Information System | US EPA. Available online: https://www.epa.gov/iris (accessed on 13 January 2025).
- USP-NF 233 Elemental Impurities—Limits| USP-NF. Available online: https://share.google/bIQWQDgL49GY5dVpt (accessed on 13 October 2025).
- USP-NF 232 Elemental Impurities—Limits| USP-NF. Available online: https://share.google/t7FxwzMNQlVkdKX5w (accessed on 13 October 2025).
- United States Pharmacopeia 2232 Elemental Contaminants in Dietary Supplements. Available online: https://share.google/0DEqNV3sGzuWcn5Ea (accessed on 13 October 2025).
- Ćwieląg-Drabek, M.; Piekut, A.; Szymala, I.; Oleksiuk, K.; Razzaghi, M.; Osmala, W.; Jabłońska, K.; Dziubanek, G. Health Risks from Consumption of Medicinal Plant Dietary Supplements. Food Sci. Nutr. 2020, 8, 3535–3544. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Budi, H.S.; Catalan Opulencia, M.J.; Afra, A.; Abdelbasset, W.K.; Abdullaev, D.; Majdi, A.; Taherian, M.; Ekrami, H.A.; Mohammadi, M.J. Source, Toxicity and Carcinogenic Health Risk Assessment of Heavy Metals. Rev. Environ. Health 2024, 39, 77–90. [Google Scholar]
- Łozak, A.; Sołtyk, K.; Kiljan, M.; Fijałek, Z.; Ostapczuk, P. Determination of Selected Trace Elements in Dietary Supplements Containing Plant Materials. Pol. J. Food Nutr. Sci. 2012, 62, 97–102. [Google Scholar] [CrossRef]
- Bandara, S.B.; Urban, A.; Liang, L.G.; Parker, J.; Fung, E.; Maier, A. Active Pharmaceutical Contaminants in Dietary Supplements: A Tier-Based Risk Assessment Approach. Regul. Toxicol. Pharmacol. 2021, 123, 104955. [Google Scholar] [CrossRef] [PubMed]
- Almela, C.; Algora, S.; Benito, V.; Clemente, M.J.; Devesa, V.; Súñer, M.A.; Vélez, D.; Montoro, R. Heavy Metal, Total Arsenic, and Inorganic Arsenic Contents of Algae Food Products. J. Agric. Food Chem. 2002, 50, 918–923. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Z.; Wang, Z.; Zhang, R.; Wu, Q.; Gao, H.; Wang, Y.; Shen, Z.; Lek, S.; Xiao, J. Species-Specific Bioaccumulation and Health Risk Assessment of Heavy Metal in Seaweeds in Tropic Coasts of South China Sea. Sci. Total Environ. 2022, 832, 155031. [Google Scholar] [CrossRef]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Helon, P.; Pietrzak, K.; Koch, W. In Vitro Evaluation of Bioavailability of Cr from Daily Food Rations and Dietary Supplements from the Polish Market. Nutrients 2024, 16, 1022. [Google Scholar] [CrossRef]
- Mihai, O.; Kawamoto, M.S.; LeBlanc, K.L.; Grinberg, P.; Nogueira, A.R.d.A.; Mester, Z. Determination of Chromium Picolinate and Trace Hexavalent Chromium in Multivitamins and Supplements by HPLC-ICP-QQQ-MS. J. Food Compos. Anal. 2020, 87, 103421. [Google Scholar] [CrossRef]
- Pillitteri, J.L.; Shiffman, S.; Rohay, J.M.; Harkins, A.M.; Burton, S.L.; Wadden, T.A. Use of Dietary Supplements for Weight Loss in the United States: Results of a National Survey. Obesity 2008, 16, 790–796. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of Chromium-Induced Toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Regulación | ANAISA. Available online: https://anaisa.mx/regulacion/ (accessed on 30 August 2025).
- Ley General de Salud | Secretaría de Salud | Gobierno | Gob.Mx. Available online: https://www.gob.mx/salud/articulos/ley-general-de-salud (accessed on 30 August 2025).
- Comisión Federal Para La Protección Contra Riesgos Sanitarios|Gobierno Gob.Mx. Available online: https://www.gob.mx/cofepris (accessed on 30 August 2025).
- Arcella, D.; Altieri, A.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; et al. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 2097. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, M.; Khan, M.A.; Sohail, A.; Sanaullah, M.; Ahmad, W.; Iqbal, D.N.; Khalid, K.; Wani, T.A.; Zargar, S. Assessment of Carcinogenic and Non-Carcinogenic Risk of Exposure to Potentially Toxic Elements in Tea Infusions: Determination by ICP-OES and Multivariate Statistical Data Analysis. J. Trace Elem. Med. Biol. 2024, 84, 127454. [Google Scholar] [CrossRef]
- Antoine, J.M.R.; Fung, L.A.H.; Grant, C.N. Assessment of the Potential Health Risks Associated with the Aluminium, Arsenic, Cadmium and Lead Content in Selected Fruits and Vegetables Grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
| Vulnerable Group | Metal Exposure | ||
|---|---|---|---|
| Lead | Arsenic | Chromium | |
| Pregnant Women | Fetal neurodevelopment damage, preterm birth, and low birth weight [15]. | Gestational diabetes, miscarriage risk, and DNA damage [16]. | Low birth weight risk (female infants), metabolic effects [17,18]. |
| Lactation mothers | Transfer via breast milk, infant neurotoxicity [19,20]. | Colostrum accumulation, immune system disruption [19]. | Transfer via breast milk, developmental effects [19,21]. |
| Children | Cognitive impairment, developmental delays, and academic performance [22]. | Attention/memory deficits, reasoning impairment [23]. | Neuropsychological development impairment [18,24]. |
| Adolescent population | Altered stress responses, cardiovascular implications [25,26]. | Reduced IQ, processing speed deficits, and language impairment [23,27]. | Genotoxicity, oxidative stress, DNA damage [28]. |
| Elderly people | Cognitive decline, hypertension, renal dysfunction [29,30]. | Cardiovascular aging, cognitive deterioration, and accelerated aging [31,32]. | Oxidative stress and biochemical alterations [18]. |
| Metal | TDIs (mg/kg bw/day) | Slope Factor, CSF (mg/kg bw/day)−1 | Reference |
|---|---|---|---|
| Pb | a 1.8 × 10−4 | 0.0085 | [6,11] |
| As | b 6 × 10−5 | 1.5 | [12,38] |
| Cr | c 0.3 | 0.5 | [13,38] |
| Sample ID | * Dosage per Day | Serving Weight (Kg) | IR (Kg/Day) | Therapeutic Indication |
|---|---|---|---|---|
| A11 | 6 | 0.0004 | 0.002 | Blood detoxifier |
| A12 | 1 | 0.03 | 0.03 | Muscle development |
| A21 | 2 | 0.015 | 0.030 | Improve the immune system |
| A36 | 6 | 0.0004 | 0.002 | blood detoxifier |
| A42 | 1 | 0.03 | 0.030 | Improve muscle development |
| A45 | 3 | 0.003 | 0.009 | Muscle activity and cell growth |
| A46 | 3 | 0.003 | 0.009 | Muscle activity and cell growth |
| A47 | 3 | 0.003 | 0.009 | Muscle activity and cell growth |
| C35 | 1 | 0.001 | 0.001 | Weight loss |
| C40 | 1 | 0.0005 | 0.0005 | Booster energy |
| C6 | 2 | 0.0008 | 0.002 | Weight loss |
| M1 | 2 | 0.001 | 0.002 | Booster energy |
| M3 | 3 | 0.0005 | 0.002 | Weight loss |
| M5 | 2 | 0.0005 | 0.001 | Booster energy |
| S24 | 1 | 0.040 | 0.040 | Relaxing of the blood vessels |
| S27 | 2 | 0.0005 | 0.001 | Regulation of the sleep cycle |
| S28 | 1 | 0.0006 | 0.0006 | Booster energy |
| S29 | 1 | 0.005 | 0.005 | Increased muscle mass |
| S30 | 1 | 0.005 | 0.005 | Increased muscle mass |
| S31 | 1 | 0.005 | 0.005 | Increased muscle mass |
| V10 | 3 | 0.0005 | 0.002 | Improve glucose level |
| V13 | 6 | 0.0005 | 0.003 | Improve the immune system |
| V14 | 6 | 0.0005 | 0.003 | Weight loss |
| V15 | 1 | 0.03 | 0.033 | Improve the immune system |
| V16 | 4 | 0.0005 | 0.002 | Weight loss |
| V17 | 2 | 0.0005 | 0.001 | Weight loss |
| V18 | 2 | 0.0005 | 0.001 | Antioxidant |
| V19 | 6 | 0.001 | 0.006 | Weight loss |
| V2 | 1 | 0.001 | 0.001 | Weight loss |
| V20 | 3 | 0.001 | 0.004 | Improve digestion |
| V22 | 1 | 0.025 | 0.025 | Improve the immune system |
| V25 | 1 | 0.002 | 0.002 | Improve digestion |
| V26 | 2 | 0.0005 | 0.001 | Antioxidant |
| V32 | 6 | 0.001 | 0.006 | Weight loss |
| V33 | 3 | 0.0005 | 0.002 | Improve glucose level |
| V34 | 2 | 0.0005 | 0.001 | Antioxidant |
| V37 | 3 | 0.001 | 0.003 | Weight loss |
| V38 | 6 | 0.0005 | 0.003 | Weight loss |
| V39 | 3 | 0.001 | 0.004 | Improve digestion |
| V4 | 1 | 0.002 | 0.002 | Improve digestion |
| V41 | 6 | 0.0005 | 0.003 | Improve the immune system |
| V43 | 3 | 0.001 | 0.003 | Improve digestion |
| V44 | 1 | 0.001 | 0.001 | Weight loss |
| V7 | 3 | 0.001 | 0.004 | Improve digestion |
| V9 | 3 | 0.001 | 0.003 | Improve digestion |
| a Sample ID | Standard Addition (µg/L) | b Metal Concentration (µg/L) | % Recovery | ||||
|---|---|---|---|---|---|---|---|
| Pb | Cr | As | Pb | Cr | As | ||
| V9 | 0 | 4.03 ± 0.17 | 28.94 ± 0.17 | 0.40 ± 0.09 | 86 | 104 | 96 |
| 25 | 25.52 ± 1.28 | 55.50 ± 0.40 | 25.02 ± 0.52 | ||||
| V10 | 0 | 2.16 ± 0.07 | 0.397 ± 0.001 | <LOD | 95 | 96 | 91 |
| 25 | 25.94 ± 0.56 | 24.51 ± 0.15 | 22.65 ± 0.37 | ||||
| A11 | 0 | 3.83 ± 0.23 | 73.07 ± 0.29 | 0.70 ± 0.13 | 86 | 96 | 98 |
| 25 | 25.29 ± 0.22 | 97.13 ± 0.44 | 25.21 ± 0.34 | ||||
| A12 | 0 | 1.77 ± 0.03 | 0.3894 ± 0.0002 | 0.47 ±0.04 | 92 | 95 | 103 |
| 25 | 24.77 ± 0.32 | 24.10 ± 0.13 | 26.23 ± 0.36 | ||||
| V14 | 0 | 3.82 ± 0.12 | 0.414 ± 0.001 | 2.26 ± 0.06 | 94 | 102 | 97 |
| 25 | 27.29 ± 0.84 | 26.04 ± 0.24 | 26.49 ± 0.64 | ||||
| V15 | 0 | 2.43 ± 0.09 | 0.72 ± 0.01 | <LOD | 97 | 101 | 104 |
| 25 | 26.57 ± 0.87 | 25.95 ± 0.14 | 26.03 ± 0.18 | ||||
| V16 | 0 | 3.34 ± 0.02 | 151.66 ± 0.09 | 0.08 ± 0.02 | 96 | 96 | 99 |
| 25 | 27.29 ± 0.24 | 175.61 ± 0.79 | 24.76 ± 0.61 | ||||
| S27 | 0 | 0.26 ± 0.02 | 1.85 ± 0.04 | 0.21 ± 0.04 | 96 | 89 | 100 |
| 25 | 24.26 ± 0.96 | 24.09 ± 0.40 | 25.29 ± 0.11 | ||||
| M1 | 0 | 5.60 ± 0.70 | 163.01 ± 0.39 | 0.26 ± 0.02 | 83 | 107 | 106 |
| 25 | 26.40 ± 0.20 | 189.70± 0.80 | 26.75 ± 0.36 | ||||
| C6 | 0 | 2.65 ± 0.07 | 12.88 ± 0.42 | 0.09 ± 0.02 | 94 | 93 | 104 |
| 25 | 26.15 ± 0.18 | 36.20 ± 0.20 | 26.10 ± 0.30 | ||||
| Category | n | Heavy Metal Concentration (mg/Kg) | ||
|---|---|---|---|---|
| Pb Mean ± SD (Range) | Cr Mean ± SD (Range) | As Mean ± SD (Range) | ||
| Vegetable | 25 | 1.72 ± 1.72 (<LOD–8.17) | 36.49 ± 60.18 (<LOD–233.20) | 3.63 ± 10.58 (<LOD–39.93) |
| Mineral | 3 | 2.41 ± 1.84 (1.08–4.51) | 49.9 ± 71.0 (2.15–131.46) | 0.67 ± 0.59 (0.21–1.34) |
| Animal | 8 | 3.70 ± 3.46 (0.39–9.03) | 14.75 ± 15.45 (<LOD–39.56) | 1.57 ± 3.98 (<LOD–11.41) |
| Synthetic | 6 | 1.25 ± 0.71 (0.29–1.81) | 1.12 ± 0.89 (0.42–2.44) | 0.04 ± 0.09 (<LOD–0.23) |
| Combined | 3 | 0.69 ± 0.92 (0.07–1.75) | 7.69 ± 7.25 (0.08–14.52) | 0.60 ± 0.83 (0.06–1.55) |
| Total | 45 | 1.99 ± 0.13 (0.07–9.03) | 26.88 ± 0.23 (0.08–233.20) | 2.39 ± 0.11 (0.03–39.93) |
| Variable | Origin | Mean | Standard Deviation | Min | Max | Variability Coefficient (%) |
|---|---|---|---|---|---|---|
| EDI_Pb | Animal | 4.61 × 10−4 | 3.26 × 10−4 | 1.4 × 10−5 | 9.25 × 10−4 | 70.68 |
| Combined | 1.50 × 10−5 | 2.30 × 10−5 | 1 × 10−6 | 4.10 × 10−5 | 150.95 | |
| Mineral | 5.80 × 10−5 | 6.10 × 10−5 | 2.3 × 10−5 | 1.29 × 10−4 | 104.23 | |
| Synthetic | 1.05 × 10−4 | 8.40 × 10−5 | 4 × 10−6 | 2.30 × 10−4 | 80.60 | |
| Vegetable | 1.03 × 10−4 | 2.09 × 10−4 | NA | 1.06 × 10−3 | 202.73 | |
| EDI_Cr | Animal | 1.08 × 10−3 | 8.33 × 10−4 | NA | 2.34 × 10−3 | 77.30 |
| Combined | 1.36 × 10−4 | 1.17 × 10−4 | 1 × 10−6 | 2.07 × 10−4 | 86.29 | |
| Mineral | 1.38 × 10−3 | 2.07 × 10−3 | 3 × 10−5 | 3.76 × 10−3 | 150.17 | |
| Synthetic | 2.64 × 10−4 | 5.68 × 10−4 | 4 × 10−6 | 1.42 × 10−3 | 215.35 | |
| Vegetable | 1.93 × 10−3 | 4.60 × 10−3 | NA | 2 × 10−2 | 237.99 | |
| EDI_As | Animal | 8 × 10−5 | 1.41 × 10−4 | NA | 3.91 × 10−4 | 175.28 |
| Combined | 5 × 10−5 | 5 × 10−6 | 1 × 10−6 | 1.10 × 10−5 | 106.36 | |
| Mineral | 1.20 × 10−5 | 7 × 10−6 | 6 × 10−6 | 1.90 × 10−5 | 57.91 | |
| Synthetic | 1 × 10−6 | 1 × 10−6 | NA | 3 × 10−6 | 244.95 | |
| Vegetable | 1.56 × 10−4 | 4.55 × 10−4 | NA | 1.71 × 10−3 | 291.74 |
| Variable | Origin | Mean | Standard Deviation | Min | Max | Variability Coefficient (%) |
|---|---|---|---|---|---|---|
| HI | Animal | 2.78 | 2.12 | 1.61 × 10−1 | 5.87 | 76.38 |
| Combined | 1.19 × 10−1 | 6.86 × 10−2 | 4.46 × 10−2 | 1.80 × 10−1 | 57.57 | |
| Mineral | 3.73 × 10−1 | 1.96 × 10−1 | 2.09 × 10−1 | 5.90 × 10−1 | 52.47 | |
| Synthetic | 4.22 × 10−1 | 3.26 × 10−1 | 5.20 × 10−2 | 9.12 × 10−1 | 77.34 | |
| Vegetable | 2.26 | 5.51 | NA | 2.08 × 101 | 243.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladiana, B.-P.B.; Eduardo, S.-E.; Octavio, A.-M.J.; Mariana, C.-M.; Paulina, A.-M.; Adriana, C.-C. Assessment of Heavy Metals in Mexican Dietary Supplements Using Total X-Ray Fluorescence Spectrometry and Health Risk Evaluation. Foods 2025, 14, 3534. https://doi.org/10.3390/foods14203534
Gladiana B-PB, Eduardo S-E, Octavio A-MJ, Mariana C-M, Paulina A-M, Adriana C-C. Assessment of Heavy Metals in Mexican Dietary Supplements Using Total X-Ray Fluorescence Spectrometry and Health Risk Evaluation. Foods. 2025; 14(20):3534. https://doi.org/10.3390/foods14203534
Chicago/Turabian StyleGladiana, Beltrán-Piña Blanca, Santellano-Estrada Eduardo, Acosta-Montes Jorge Octavio, Cardona-Mejía Mariana, Aguilar-Maldonado Paulina, and Chávez-Calderón Adriana. 2025. "Assessment of Heavy Metals in Mexican Dietary Supplements Using Total X-Ray Fluorescence Spectrometry and Health Risk Evaluation" Foods 14, no. 20: 3534. https://doi.org/10.3390/foods14203534
APA StyleGladiana, B.-P. B., Eduardo, S.-E., Octavio, A.-M. J., Mariana, C.-M., Paulina, A.-M., & Adriana, C.-C. (2025). Assessment of Heavy Metals in Mexican Dietary Supplements Using Total X-Ray Fluorescence Spectrometry and Health Risk Evaluation. Foods, 14(20), 3534. https://doi.org/10.3390/foods14203534







