Chill or Thrill? The Effect of Storage Temperature Regime on Listeria Growth in Fresh-Cut Fruit Cocktails
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Experiment
- -
- Treatment 1: 4 °C for eight days;
- -
- Treatment 2: 8 °C for eight days;
- -
- Treatment 3: 4 °C for one day followed by 8 °C for the remaining storage time totaling eight days (dynamic temperature regime).
2.3. Analyses
2.3.1. Microbiological Analyses
2.3.2. Water Activity
2.3.3. Biochemical Analyses
2.3.4. Calculation
- •
- Number of generations (n).
- •
- Generation time (g).
- •
- Specific growth rate (k).
- •
- Division rate (v).
- •
- Growth potential (δ).
3. Results
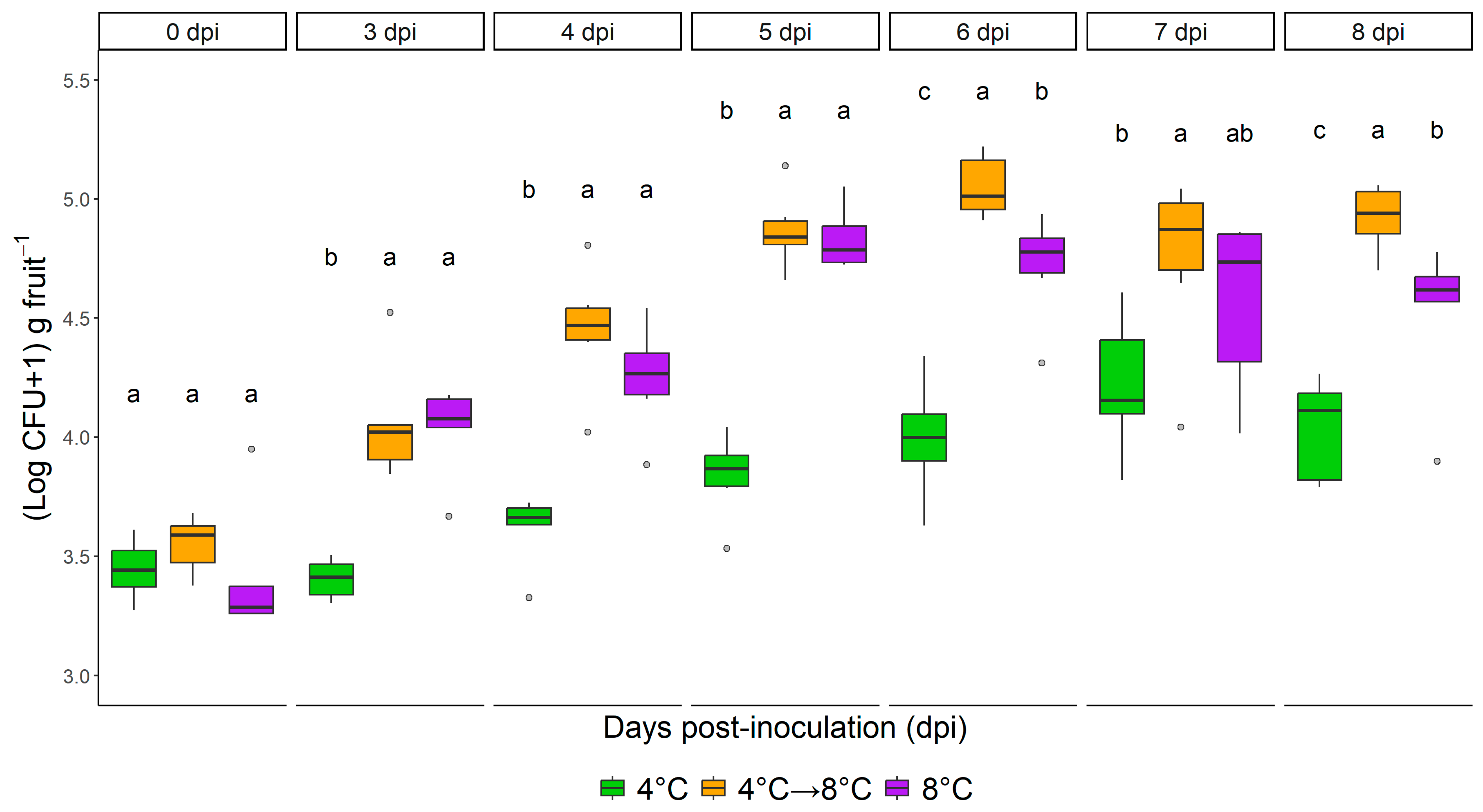
3.1. Impact of Temperature Regime on L. monocytogenes
3.2. Impact of Temperature Regime on Heterotrophic Organisms
3.3. Abiotic Factors
4. Discussion
4.1. Effect of Temperature Increase (4 °C to 8 °C)
4.2. The Impact of Dynamic Temperature Regime
4.3. The Effect of Shelf-Life Extension (From Four to Eight Days of Storage)
4.4. Growth Mechanisms Under Adverse Conditions
4.5. Microbial Behavior and Food Safety Concerns
5. Conclusions and Practical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Martín-Belloso, O. New advances in extending the shelf-life of fresh-cut fruits: A review. Trends Food Sci. Technol. 2003, 14, 341–353. [Google Scholar] [CrossRef]
- Siddiqui, M.W. Technologies and Mechanisms for Safety Control: Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2020; p. 385. [Google Scholar]
- Mulaosmanovic, E.; Windstam, S.T.; Vågsholm, I.; Alsanius, B.W. Size matters: Biological and food safety relevance of leaf damage for colonization of Escherichia coli O157:H7gfp+. Front. Microbiol. 2021, 11, 608086. [Google Scholar] [CrossRef]
- Wing, E.J.; Gregory, S.H. Listeria monocytogenes: Clinical and experimental update. J. Infect. Dis. 2002, 185, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, Y.; Li, H.; Liu, Z.; Jia, X.; Li, W.; Jia, H.; Li, X. Constant temperature during postharvest storage delays fruit ripening and enhances the antioxidant capacity of mature green tomato. J. Food Process. Preserv. 2020, 44, e14831. [Google Scholar] [CrossRef]
- Ferrante, A.; Maggiore, T. Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharvest Biol. Technol. 2007, 45, 73–80. [Google Scholar] [CrossRef]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M. The commercial storage of fruits, vegetables, and florist and nursery stock. In Vol. Agriculture Handbook 66; Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2016. [Google Scholar]
- Huang, J.; Luo, Y.; Zhou, B.; Zheng, J.; Nou, X. Growth and survival of Salmonella enterica and Listeria monocytogenes on fresh-cut produce and their juice extracts: Impacts and interactions of food matrices and temperature abuse conditions. Food Control 2019, 100, 300–304. [Google Scholar] [CrossRef]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate outbreak of listeriosis associated with cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Söderqvist, K.; Lambertz, L.T.; Vågsholm, I.; Fernström, L.L.; Alsanius, B.W.; Mogren, L.; Boqvist, S. Fate of Listeria monocytogenes, pathogenic Yersinia enterocolitica, and Escherichia coli O157:H7 gfp+ in ready-to-eat salad during cold storage: What is the risk to consumers? J. Food Prot. 2017, 80, 204–212. [Google Scholar] [CrossRef]
- Beaufort, A.H.; Bergis, H.; Lardeux, A.-L.; Polet, M.; Botteldoorn, N.; Papageorgiou, G.; Andersen, J.K.; Boel, J.; Hickey, B.; Prencipe, V.; et al. EURL Lm Technical Guidance Document for Conducting Shelf-Life Studies on Listeria monocytogenes in Ready-To-Eat Foods; European Union Reference Laboratory for Listeria monocytogenes: Maisons-Alfort, France, 2014; p. 47. [Google Scholar]
- Mulaosmanovic, E.; Lindblom, T.; Windstam, S.; Bengtsson, M.; Rosberg, A.; Mogren, L.; Alsanius, B. Processing of leafy vegetables matter: Damage and microbial community structure from field to bag. Food Control 2021, 125, 107894. [Google Scholar] [CrossRef]
- Ali, L.; Alsanius, B.W.; Rosberg, A.K.; Svensson, B.; Nielsen, T.; Olsson, M. Effects of nutrition strategy on the levels of nutrients and bioactive compounds in blackberries. Eur. J. Food Res. Technol. 2012, 234, 33–44. [Google Scholar]
- Pepper, I.; Gerba, C.P.; Gentry, T.; Microbiology, E. Environmental Microbiology; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Iturralde-García, R.D.; Cinco-Moroyoqui, R.X.; Martinez-Cruz, F.; Ruiz-Cruz, O.; Wong-Corral, S.; Borboa-Flores, F.J.; Cornejo-Ramirez, J.I.; Bernal-Mercado, Y.T.; Del-Toro-Sanchez, A.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Bonaventura, D.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Ru, S.; Stephan, R.; Guldimann, C. Growth potential of Listeria monocytogenes in six different RTE fruit products: Impact of food matrix, storage temperature and shelf life. Ital. J. Food Saf. 2018, 7, 7581. [Google Scholar] [CrossRef]
- Alegre, I.; Abadias, M.; Anguera, M.; Oliveira, M.; Vinas, I. Factors affecting growth of foodborne pathogens on minimally processed apples. Food Microbiol. 2010, 27, 70–76. [Google Scholar] [CrossRef]
- Cai, S.; Worobo, R.W.; Snyder, A.B. Combined Effect of Storage Condition, Surface Integrity, and Length of Shelf Life on the Growth of Listeria monocytogenes and Spoilage Microbiota on Refrigerated Ready-to-Eat Products. J. Food Prot. 2019, 82, 1423–1432. [Google Scholar] [CrossRef]
- Nyarko, E.; Kniel, K.E.; Millner, P.D.; Luo, Y.; Handy, E.T.; Reynnells, R.; East, C.; Sharma, M. Survival and growth of Listeria monocytogenes on whole cantaloupes is dependent on site of contamination and storage temperature. Int. J. Food Microbiol. 2016, 234, 65–70. [Google Scholar] [CrossRef]
- Gu, G.; Kroft, B.; Lichtenwald, M.; Luo, Y.; Millner, P.; Patel, J.; Nou, X. Dynamics of Listeria monocytogenes and the microbiome on fresh-cut cantaloupe and romaine lettuce during storage at refrigerated and abusive temperatures. Int. J. Food Microbiol. 2022, 364, 109531. [Google Scholar] [CrossRef]
- Hong, Y.K.; Yoon, W.B.; Huang, L.; Yuk, H.G. Predictive Modeling for Growth of Non- and Cold-adapted Listeria monocytogenes on Fresh-cut Cantaloupe at Different Storage Temperatures. J. Food Sci. 2014, 79, M1168–M1174. [Google Scholar] [CrossRef]
- Huang, J.; Luo, Y.; Nou, X. Growth of Salmonella enterica and Listeria monocytogenes on Fresh-Cut Cantaloupe under Different Temperature Abuse Scenarios. J. Food Prot. 2015, 78, 1125–1131. [Google Scholar] [CrossRef]
- Kroft, B.; Gu, G.; Bolten, S.; Micallef, S.A.; Luo, Y.; Millner, P.; Nou, X. Effects of temperature abuse on the growth and survival of Listeria monocytogenes on a wide variety of whole and fresh-cut fruits and vegetables during storage. Food Control 2022, 137, 108919. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Fett, W. Behavior of Listeria monocytogenes Inoculated on Cantaloupe Surfaces and Efficacy of Washing Treatments to Reduce Transfer from Rind to Fresh-Cut Pieces. J. Food Prot. 2002, 65, 924–930. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Olanya, M.; Geveke, D.J.; Sommers, C.H. Effect of Native Microflora, Waiting Period, and Storage Temperature on Listeria monocytogenes Serovars Transferred from Cantaloupe Rind to Fresh-Cut Pieces during Preparation. J. Food Prot. 2012, 75, 1912–1919. [Google Scholar] [CrossRef]
- Moreira Calix, J.F. Effect of st ect of storage temper age temperature on the sur e on the survival or gr al or growth of Listeria owth of Listeria monocytogenes on whole and fresh-cut produce. In The School of Nutrition and Food Sciences; Louisiana State University: Baton Rouge, LA, USA, 2019. [Google Scholar]
- Russo, S.; Cosciani-Cunico, E.; Dalzini, E.; Daminelli, P.; Ricchi, M.; Arrigoni, N.; Cammi, G. Evaluation of maximum growth rate of Listeria monocytogenes in ready-to-eat fresh-cut papaya and melon. Int. Food Res. J. 2023, 30, 953–963. [Google Scholar] [CrossRef]
- Bolten, S.; Belias, A.; Weigand, K.A.; Pajor, M.; Qian, C.; Ivanek, R.; Wiedmann, M. Population dynamics of Listeria spp. Salmonella spp. and Escherichia coli on fresh produce: A scoping review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4537–4572. [Google Scholar] [CrossRef]
- Gorski, L.; Noriega, A.A.; Carter, M.Q. Listeria monocytogenes serotype 4b strains demonstrate a fitness advantage over strains of serotypes 1/2a and 4b-v1 on cantaloupe rind and flesh at different temperatures. Microbiol. Spectr. 2025, 13, e0125225. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Risk assessment of Listeria monocytogenes in ready-to-eat foods. In Microbiological Risk Assessment Series; Food and Agriculture Organization of the United Nations and World Health Organization: Rome, Italy, 2004. [Google Scholar]
- Sant’Ana, A.S.; Barbosa, M.S.; Destro, M.T.; Landgraf, M.; Franco, B.D.G.M. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. Food Microbiol. 2012, 157, 52–58. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Fang, T.; Liu, Y.; Huang, L. Growth kinetics of Listeria monocytogenes and spoilage microorganisms in fresh-cut cantaloupe. Food Microbiol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Gahan, C.G.M.; Hill, C. Gastrointestinal phase of Listeria monocytogenes infection. J. Appl. Microbiol. 2005, 98, 1345–1353. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cefola, M.; Renna, M.; Pace, B. Marketability of ready-to-eat cactus pear as affected by temperature and modified atmosphere. J. Food Sci. Technol. 2011, 51, 25–33. [Google Scholar] [CrossRef]
- Manthou, E.; Coeuret, G.; Chaillou, S.; Nychas, G.J. Evolution of fungal community associated with ready-to-eat pineapple during storage under different temperature conditions. Food Microbiol. 2021, 97, 103736. [Google Scholar] [CrossRef]
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 333, 108855. [Google Scholar] [CrossRef]
- Althaus, D.; Hofer, E.; Corti, S.; Julmi, A.; Stephan, R. Bacteriological survey of ready-to-eat lettuce, fresh-cut fruit, and sprouts collected from the Swiss market. J. Food Prot. 2012, 75, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Truchado, P.; Tudela, J.A.; Allende, A. Environmental monitoring of three fresh-cut processing facilities reveals harborage sites for Listeria monocytogenes. Food Control 2023, 155, 110093. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 59, 411–422. [Google Scholar] [CrossRef]

| Parameter | Day | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Listeria monocytogenes | • | • | • | • | • | • | • |
| Heterotrophic microbiota | • | • | |||||
| Yeasts | • | ||||||
| pH | • | • | |||||
| Total soluble solids | • | • | |||||
| Titrable acidity | • | • | |||||
| Water activity | • | • | • | • | • | • | • |
| Microbiological Medium | Incubation | |
|---|---|---|
| Temperature | Length | |
| Tryptic Soy Agar | 25 | 48 |
| Harlequin Agar | 37 | 24 |
| Yeast malt Agar | 25 | 72 |
| Temperature Regime | n | g | k | v |
|---|---|---|---|---|
| L. monocytogenes | ||||
| 4 °C | 1.96 | 10.20 | 0.03 | 0.10 |
| 4 °C → 8 °C | 4.55 | 4.39 | 0.07 | 0.23 |
| 8 °C | 3.69 | 5.42 | 0.06 | 0.18 |
| Heterotrophic bacteria in non-inoculated fresh-cut fruit cocktails | ||||
| 4 °C | 9.5 | 2.11 | 0.14 | 0.48 |
| 4 °C → 8 °C | 14.28 | 1.40 | 0.22 | 0.71 |
| 8 °C | 13.15 | 1.52 | 0.20 | 0.66 |
| Heterotrophic bacteria in L. monocytogenes-inoculated fresh-cut fruit cocktails | ||||
| 4 °C | 8.21 | 2.44 | 0.12 | 0.41 |
| 4 °C → 8 °C | 12.79 | 1.56 | 0.19 | 0.64 |
| 8 °C | 13.22 | 1.51 | 0.20 | 0.66 |
| Parameter | 4 °C | 4 °C → 8 °C | 8 °C | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 8 | Day 0 | Day 8 | Day 0 | Day 8 | |
| pH | 3.85 ± 0.19 A 1 | 3.91 ± 0.22 A | 3.89 ± 0.06 A | 3.95 ± 0.09 A | 3.96 ±0.08 A | 3.92 ± 0.10 A |
| aw | 0.982 ± 0.003 A | 0.985 ± 0.004 A | 0.980 ± 0.002 A | 0.980 ± 0.003 A | 0.980 ± 0.002 A | 0.980 ± 0.003 A |
| Titrable acidity | 0.56 ± 0.06 A | 0.54 ± 0.08 A | 0.55 ± 0.02 B | 0.63 ± 0.05 A | 0.60 ± 0.04 A | 0.62 ± 0.06 A |
| Total soluble solids | 14.14 ± 0.64 A | 13.70 ± 0.72 A | 14.09 ± 0.27 A | 13.26 ± 0.54 B | 14.46 ± 0.55 A | 13.70 ± 0.55 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsanius, B.W.; Windstam, S.; Mulaosmanovic, E. Chill or Thrill? The Effect of Storage Temperature Regime on Listeria Growth in Fresh-Cut Fruit Cocktails. Foods 2025, 14, 3523. https://doi.org/10.3390/foods14203523
Alsanius BW, Windstam S, Mulaosmanovic E. Chill or Thrill? The Effect of Storage Temperature Regime on Listeria Growth in Fresh-Cut Fruit Cocktails. Foods. 2025; 14(20):3523. https://doi.org/10.3390/foods14203523
Chicago/Turabian StyleAlsanius, Beatrix W., Sofia Windstam, and Emina Mulaosmanovic. 2025. "Chill or Thrill? The Effect of Storage Temperature Regime on Listeria Growth in Fresh-Cut Fruit Cocktails" Foods 14, no. 20: 3523. https://doi.org/10.3390/foods14203523
APA StyleAlsanius, B. W., Windstam, S., & Mulaosmanovic, E. (2025). Chill or Thrill? The Effect of Storage Temperature Regime on Listeria Growth in Fresh-Cut Fruit Cocktails. Foods, 14(20), 3523. https://doi.org/10.3390/foods14203523







