Whole-Plant Rape Silage-Based Diets for Chongming White Goats: An Integrated Assessment of Growth Performance, Meat Quality and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Animals and Diets
2.3. Antioxidant Capacity Analysis
2.4. Growth and Slaughter Performances
2.5. Amino Acid Composition Analysis
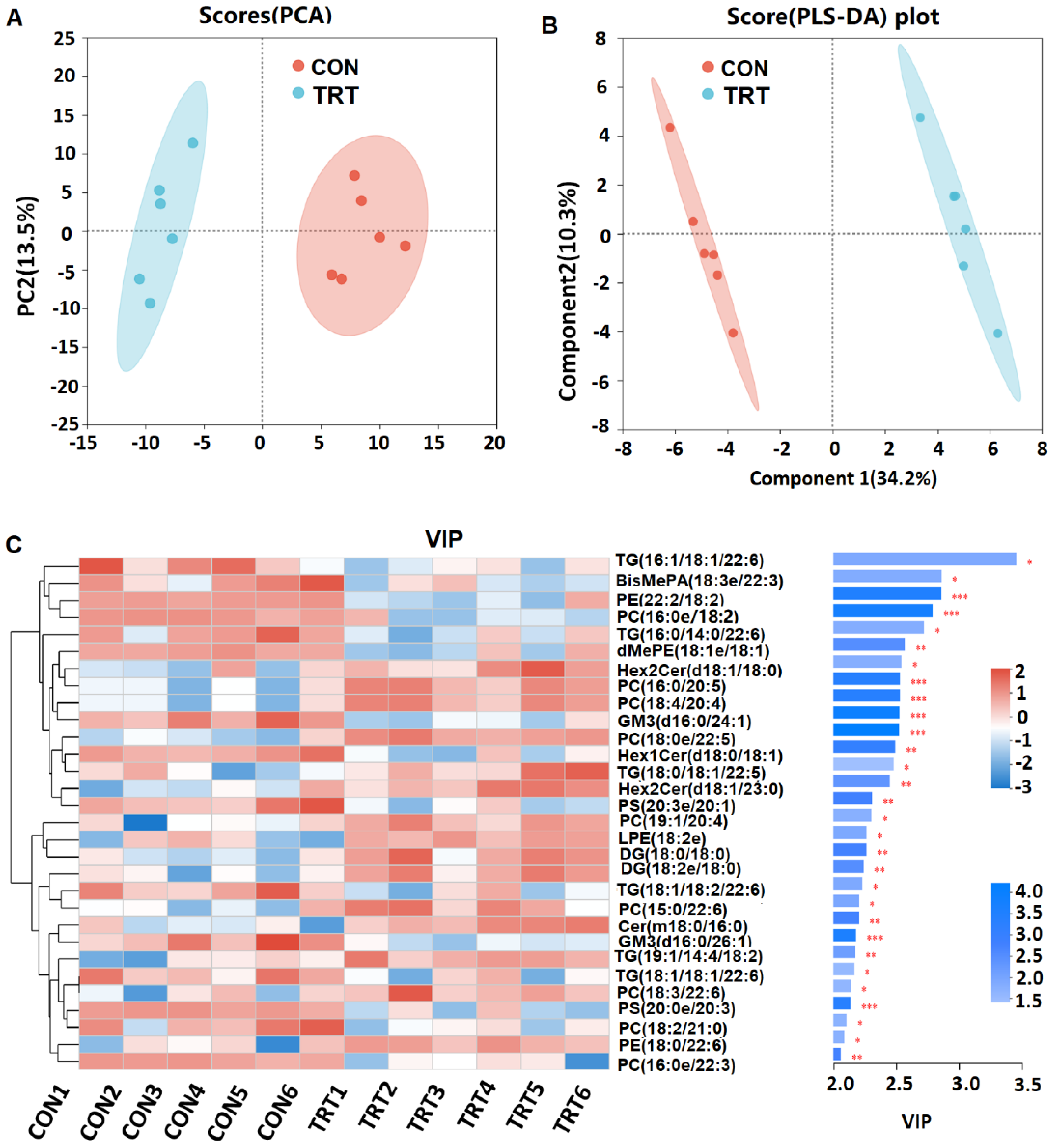
2.6. Untargeted Metabolomics
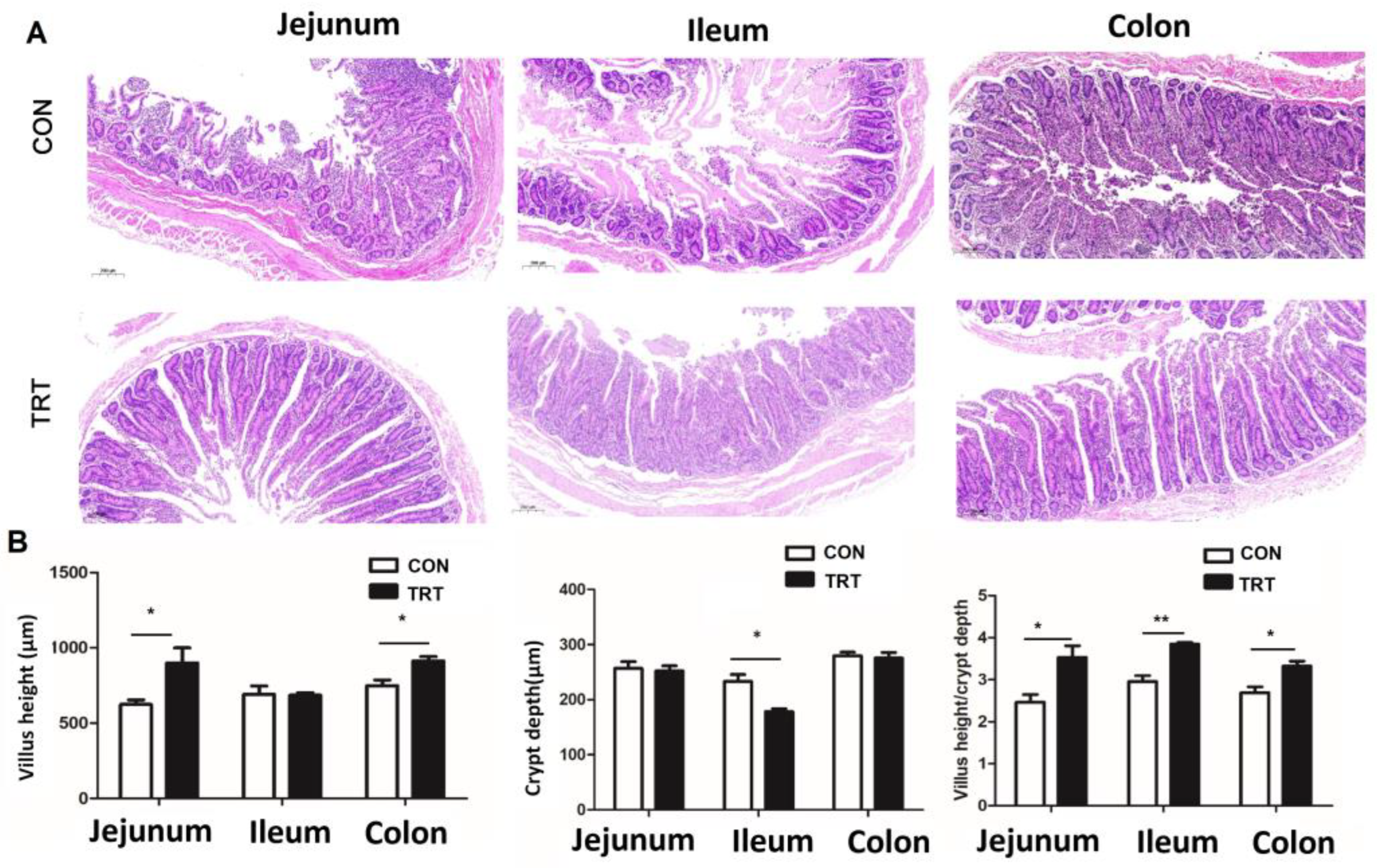
2.7. Morphology Determination
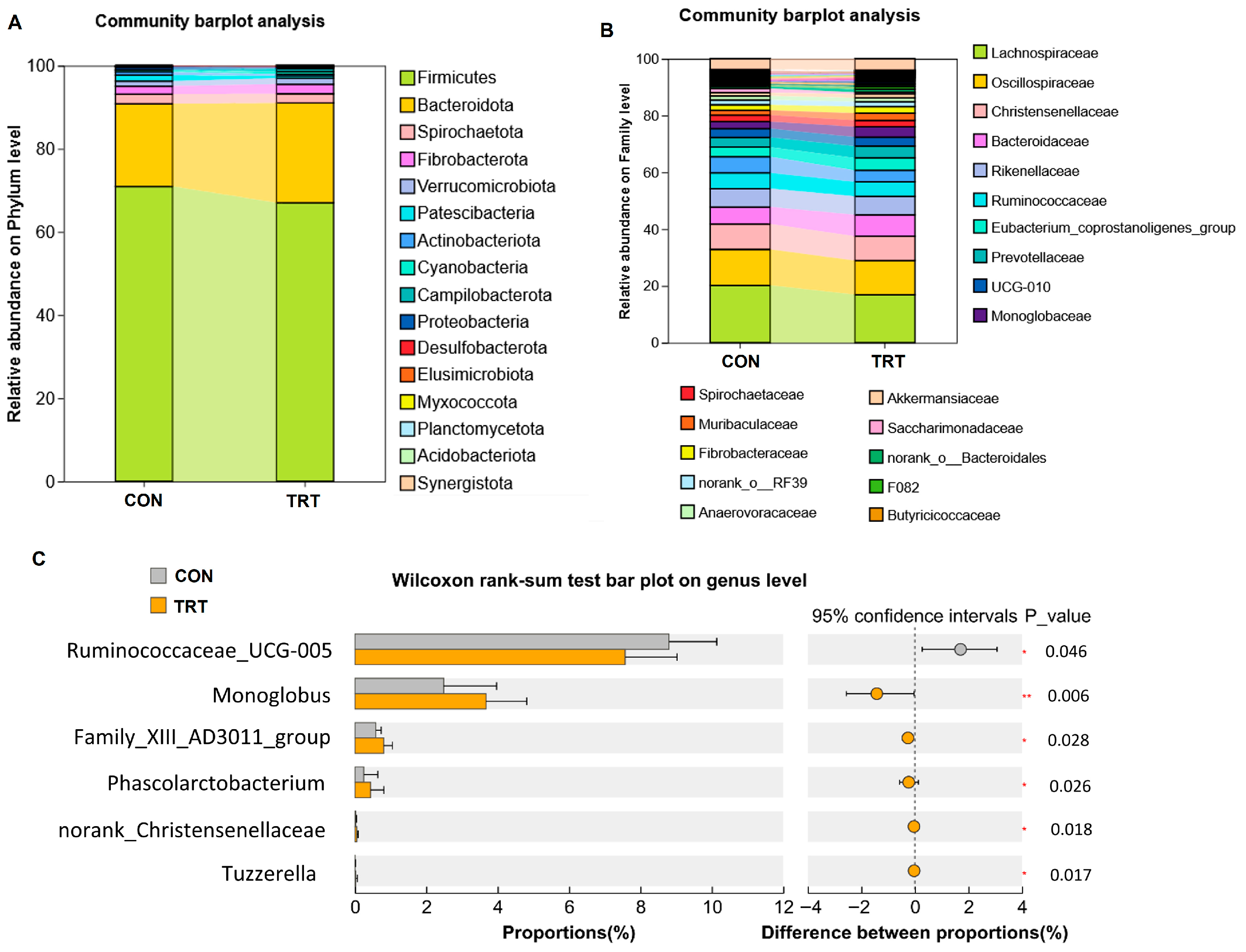
2.8. 16S rRNA Sequencing and Data Processing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Whole-Plant Rape Silage Does Not Adversely Affect the Growth and Slaughter Performance of Goats
3.2. Whole-Plant Rape Silage Relatively Improved Antioxidant Capacity of Goats
3.3. Whole-Plant Rape Silage Improved the Meat Quality of Chongming White Goats
3.4. Whole-Plant Rape Silage Promoted Intestinal Development
3.5. Whole-Plant Rape Silage Regulated Gut Microbiota Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawat, M.; Boland, M.; Singh, J.; Kaur, L. Goat Meat: Production and Quality Attributes. Foods 2023, 12, 3130. [Google Scholar] [CrossRef]
- Webb, E.C. Goat meat production, composition, and quality. Anim. Front. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Wang, H.H. The perspective of meat and meat-alternative consumption in China. Meat Sci. 2022, 194, 108982. [Google Scholar] [CrossRef]
- Moyo, M.; Adebayo, R.A.; Nsahlai, I.V. Effects of roughage quality, period of day and time lapse after meal termination on rumen digesta load in goats and sheep. Asian-Australas J. Anim. Sci. 2018, 31, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Ueno, R.K.; Neumann, M.; Marafon, F.; Basi, S.; Rosário, J.G. Dynamics of nutrients in the soil in areas intended for the production of forage maize (Zea mays L.). Appl. Res. Agrotechnol. 2012, 4, 181–202. [Google Scholar]
- Yang, B.; Na, N.; Wu, N.; Sun, L.; Li, Z.; Qili, M.; Han, H.; Xue, Y. Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage. Microorganisms 2024, 12, 1985. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Wang, W.; Lv, H.; Di, Z.; An, Z.; Wang, L.J.; Shaukat, A.; Bo, W.; Zhou, G.S.; Yang, L.G.; et al. Evaluating the effect of forage rape (Brassica napus) ensiling kinetics on degradability and milk performance as non-conventional forage for dairy buffalo. Front. Vet. Sci. 2022, 9, 926906. [Google Scholar] [CrossRef]
- Zhou, D.; Abdelrahman, M.; Zhang, X.; Yang, S.; Yuan, J.; An, Z.; Niu, K.; Gao, Y.; Li, J.; Wang, B.; et al. Milk production responses and digestibility of dairy buffaloes (Bubalus bubalis) partially supplemented with forage rape (Brassica napus) silage replacing corn silage. Animals 2021, 11, 2931. [Google Scholar] [CrossRef]
- Du, E.; Guo, W.; Zhao, N.; Chen, F.; Fan, Q.; Zhang, W.; Huang, S.; Zhou, G.; Fu, T.; Wei, J. Effects of diets with various levels of forage rape (Brassica napus) on growth performance, carcass traits, meat quality and rumen microbiota of Hu lambs. J. Sci. Food Agric. 2022, 102, 1281–1291. [Google Scholar] [CrossRef]
- Della Rosa, M.M.; Sandoval, E.; Reid, P.; Luo, D.; Pacheco, D.; Janssen, P.H.; Jonker, A. Substituting ryegrass-based pasture with graded levels of forage rape in the diet of lambs decreases methane emissions and increases propionate, succinate, and primary alcohols in the rumen. J. Anim. Sci. 2022, 100, skac223. [Google Scholar] [CrossRef]
- Kandel, M.; Macelline, S.P.; Toghyani, M.; Selle, P.H.; Liu, S.Y. The impact of canola meal and canola seed inclusions in broiler diets. Poult. Sci. 2025, 104, 105017. [Google Scholar] [CrossRef]
- Egila, N.S.H.; Dosoky, W.M.; Khisheerah, N.S.M.; Ahmed, M.H.; Zahran, S.M.; Almohmadi, N.H.; Abusudah, W.F.; Kamal, M.; Moustafa, M.; Tellez-Isaias, G.; et al. Does dietary linseed or canola oil affect lipid metabolism, immunity, and n-3 polyunsaturated fatty acids content in quail eggs? Poult. Sci. 2023, 102, 103116. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, L.; Dai, J.; Lv, Y.; Liao, R.; Shen, X.; Gao, J. Characterization and comparative analysis of whole-transcriptome sequencing in high- and low-fecundity Chongming White Goat ovaries during the estrus phase. Animals 2024, 14, 988. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Ruedt, C.; Gibis, M.; Weiss, J. Meat color and iridescence: Origin, analysis, and approaches to modulation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3366–3394. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Saker, H.D.; Jewad, A.M.; Hussein, M.G. Effects of hydroxyurea on the oxidative stress and markers of renal tissue damage in patients with sickle cell disease of adults in basra. Biochem. Cell. Arch. 2020, 20, 3927–3931. [Google Scholar]
- Surai, P.F.; Earle-Payne, K. Antioxidant defences and redox homeostasis in animals. Antioxidants 2022, 11, 1012. [Google Scholar] [CrossRef]
- Keim, J.P.; Daza, J.; Beltrán, I.; Balocchi, O.A.; Pulido, R.G.; Sepúlveda-Varas, P.; Pacheco, D.; Berthiaume, R. Milk production responses, rumen fermentation, and blood metabolites of dairy cows fed increasing concentrations of forage rape (Brassica napus ssp. Biennis). J. Dairy Sci. 2020, 103, 9054–9066. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Na, R.; Bai, X.; Ma, Y.; Yang, Y.; Ma, Y.; Wang, X. The Effect of replacing whole-plant corn silage with daylily on the growth performance, slaughtering performance, muscle amino acid composition, and blood composition of Tan sheep. Animals 2023, 13, 3493. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, V.; Holik, A.K.; Hölz, K.; Dingjan, T.; Hans, J.; Ley, J.P.; Krammer, G.E.; Niv, M.Y.; Somoza, M.M.; Somoza, V. Bitter-tasting amino acids l-arginine and l-isoleucine differentially regulate proton secretion via T2R1 signaling in human parietal cells in culture. J. Agric. Food Chem. 2020, 68, 3434–3444. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Duelund, L.; Petersen, M.A.; Hartmann, A.L.; Frøst, M.B. Umami taste, free amino acid composition, and volatile compounds of brown seaweeds. J. Appl. Phycol. 2019, 31, 1213–1232. [Google Scholar] [CrossRef]
- Kolev, H.M.; Kaestner, K.H. Mammalian intestinal development and differentiation-the state of the art. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 809–821. [Google Scholar] [CrossRef]
- Kai, Y. Intestinal villus structure contributes to even shedding of epithelial cells. Biophys. J. 2021, 120, 699–710. [Google Scholar] [CrossRef]
- Barzegar, M.; Zaghari, M.; Zhandi, M.; Sadeghi, M. Effects of zinc dosage and particle size on gut morphology, tight junctions and TNF-α expression in broiler breeder hens. J. Anim. Physiol. Anim. Nutr. 2022, 106, 772–782. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, J.; Liu, L.; Yaqoob, M.U.; Pei, X.; Tao, W.; Xiao, Z.; Sun, W.; Wang, M. Optimization for galactooligosaccharides synthesis: A potential alternative for gut health and immunity. Life Sci. 2020, 245, 117353. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Gui, H.; Wang, H.; Li, Y.; Zhang, J.; Cao, S.; Zhong, J.; Qian, Y.; Meng, C. Effect of garlic straw with silage corn stalks on Hu sheep rumen fermentation and microbial community in vitro. Metabolites 2023, 13, 1201. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; He, Z.; Li, H. Bacteroides and NAFLD: Pathophysiology and therapy. Front. Microbiol. 2024, 15, 1288856. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Tang, S.; Xin, Y.; Ma, Y.; Xu, X.; Zhao, S.; Cao, J. Screening of microbes associated with swine growth and fat deposition traits across the intestinal tract. Front. Microbiol. 2020, 11, 586776. [Google Scholar] [CrossRef]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Zhang, W.J.; Nie, C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. BioMed Res. Int. 2019, 2019, 7809171. [Google Scholar] [CrossRef]
- Wu, D.N.; Guan, L.; Jiang, Y.X.; Ma, S.H.; Sun, Y.N.; Lei, H.T.; Yang, W.F.; Wang, Q.F. Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc. Diagn. Ther. 2019, 9, 545–560. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Leslie, J.L.; Kitamoto, S.; Jin, C.; Thomsson, K.A.; Gillilland, M.G.; Kuffa, P.; Goto, Y.; Jenq, R.R.; Ishii, C.; et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 2020, 26, 608–617. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Song, S.; Zhang, M.; Zamaratskaia, G.; Xu, X.; Zhou, G.; Li, C. High-meat-protein high-fat diet induced dysbiosis of gut microbiota and tryptophan metabolism in wistar rats. J. Agric. Food Chem. 2020, 68, 6333–6346. [Google Scholar] [CrossRef]
- Kim, C.C.; Lunken, G.R.; Kelly, W.J.; Patchett, M.L.; Jordens, Z.; Tannock, G.W.; Sims, I.M.; Bell, T.J.; Hedderley, D.; Henrissat, B.; et al. Genomic insights from Monoglobus pectinilyticus: A pectin-degrading specialist bacterium in the human colon. ISME J. 2019, 13, 1437–1456. [Google Scholar] [CrossRef]
- Liu, M.; Fu, J.; Liu, Y.; Gou, W.; Yuan, W.; Shang, H. Pectin from comfrey roots alleviate DSS-induced ulcerative colitis in mice through modulating the intestinal barrier. Int. J. Biol. Macromol. 2024, 282, 137016. [Google Scholar] [CrossRef]
- Li, J.; Ma, G.; Xie, J.; Xu, K.; Lai, H.; Li, Y.; He, Y.; Yu, H.; Liao, X.; Wang, X.; et al. Differential gut microbiota, dietary intakes in constipation patients with or without hypertension. Mol. Nutr. Food Res. 2023, 67, e2300208. [Google Scholar] [CrossRef]

| Item | CON | TRT | p-Value |
|---|---|---|---|
| Initial BW (kg) | 31.45 ± 0.87 | 33.19 ± 0.96 | 0.187 |
| 30 d BW (kg) | 34.69 ± 0.99 | 36.13 ± 1.05 | 0.326 |
| 60 d BW (kg) | 38.15 ± 0.88 | 39.47 ± 1.23 | 0.391 |
| 90 d BW (kg) | 40.06 ± 1.07 | 43.04 ± 1.33 | 0.069 |
| 30 d ADG (g) | 108.03 ± 11.45 | 97.84 ± 9.38 | 0.496 |
| 60 d ADG (g) | 111.76 ± 8.77 | 104.61 ± 8.52 | 0.562 |
| 90 d ADG (g) | 90.56 ± 8.56 | 109.41 ± 7.29 | 0.234 |
| Carcass weight (kg) | 21.01 ± 0.77 b | 25.72 ± 1.08 a | 0.005 |
| Slaughter rate (%) | 55.01 ± 0.52 | 54.4 ± 0.63 | 0.473 |
| L* | 3.29 ± 0.48 | 4.64 ± 0.69 | 0.141 |
| a* | 5.76 ± 0.28 | 6.2 ± 0.58 | 0.508 |
| b* | 4.45 ± 0.21 | 4.61 ± 0.48 | 0.773 |
| pH | 6.19 ± 0.08 | 5.89 ± 0.11 | 0.064 |
| Time | Item | CON | TRT | p-Value |
|---|---|---|---|---|
| 60 days | SOD (U/mL) | 59.22 ± 1.94 | 62.44 ± 1.51 | 0.200 |
| GSH-Px (U/mL) | 1206.68 ± 30.85 | 1260.58 ± 33.68 | 0.246 | |
| TAC (nmol/mL) | 0.95 ± 0.05 | 1.19 ± 0.04 | 0.000 | |
| MDA (nmol/mL) | 2.60 ± 0.19 | 2.38 ± 0.14 | 0.356 | |
| 90 days | SOD (U/mL) | 45.71 ± 1.73 | 50.23 ± 1.81 | 0.063 |
| GSH-Px (U/mL) | 1010.84 ± 30.66 | 1099.57 ± 38.60 | 0.079 | |
| TAC (nmol/mL) | 0.32 ± 0.15 | 0.32 ± 0.02 | 0.887 | |
| MDA (nmol/mL) | 4.47 ± 0.39 | 4.97 ± 0.35 | 0.365 |
| Category of Amino Acids | CON | TRT | p-Value | |
|---|---|---|---|---|
| Non-essential amino acids | Alanine | 1054.26 ± 7.03 | 1069.79 ± 15.55 | 0.384 |
| Asparagine Anhydrous | 14.31 ± 2.49 | 8.05 ± 0.59 | 0.053 | |
| Glutamine | 7160.13 ± 2385.64 | 6239.03 ± 1646.68 | 0.757 | |
| Glutamic Acid | 8.74 ± 2.33 | 16.68 ± 4.57 | 0.153 | |
| Serine | 19.32 ± 2.23 | 14.71 ± 1.40 | 0.111 | |
| Hydroxyproline | 2.09 ± 0.23 | 2.37 ± 0.39 | 0.545 | |
| Proline | 15.90 ± 1.58 | 13.17 ± 0.67 | 0.141 | |
| Cysteine | 0.72 ± 0.29 | 0.29 ± 0.18 | 0.232 | |
| Aspartate | 8.56 ± 3.50 | 4.93 ± 3.34 | 0.471 | |
| Subtotal | 8284.04 ± 2392.64 | 7369.01 ± 1649.45 | 0.687 | |
| Semi-essential amino acid | Arginine | 75.91 ± 13.01 | 77.26 ± 10.10 | 0.936 |
| Glycine | 59.29 ± 6.97 | 61.58 ± 7.71 | 0.830 | |
| Tyrosine | 15.76 ± 0.42 a | 12.33 ± 0.48 b | 0.004 | |
| Subtotal | 150.96 ± 17.50 | 151.16 ± 13.89 | 0.565 | |
| Essential amino acids | Leucine | 21.42 ± 1.10 a | 16.01 ± 1.21 b | 0.011 |
| Isoleucine | 8.42 ± 0.24 a | 6.38 ± 0.48 b | 0.008 | |
| Threonine | 14.01 ± 1.40 | 9.62 ± 1.41 | 0.051 | |
| Phenylalanine | 8.36 ± 0.15 a | 6.42 ± 0.27 b | 0.000 | |
| Lysine | 141.85 ± 21.82 | 106.98 ± 15.87 | 0.225 | |
| Histidine | 126.71 ± 6.11 | 133 ± 12.81 | 0.667 | |
| Methionine | 4.85 ± 0.10 a | 3.58 ± 0.33 b | 0.007 | |
| Valine | 16.26 ± 0.88 a | 12.53 ± 0.89 b | 0.014 | |
| Tryptophan | 13.07 ± 0.65 a | 9.8 ± 1.03 b | 0.022 | |
| Subtotal | 354.94 ± 29.51 | 304.31 ± 18.57 | 0.111 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, R.; Xiao, C.; Lv, Y.; Liu, Y.; Lin, Y.; Zhu, L. Whole-Plant Rape Silage-Based Diets for Chongming White Goats: An Integrated Assessment of Growth Performance, Meat Quality and Gut Microbiota. Foods 2025, 14, 3512. https://doi.org/10.3390/foods14203512
Liao R, Xiao C, Lv Y, Liu Y, Lin Y, Zhu L. Whole-Plant Rape Silage-Based Diets for Chongming White Goats: An Integrated Assessment of Growth Performance, Meat Quality and Gut Microbiota. Foods. 2025; 14(20):3512. https://doi.org/10.3390/foods14203512
Chicago/Turabian StyleLiao, Rongrong, Changfeng Xiao, Yuhua Lv, Yue Liu, Yuexia Lin, and Lihui Zhu. 2025. "Whole-Plant Rape Silage-Based Diets for Chongming White Goats: An Integrated Assessment of Growth Performance, Meat Quality and Gut Microbiota" Foods 14, no. 20: 3512. https://doi.org/10.3390/foods14203512
APA StyleLiao, R., Xiao, C., Lv, Y., Liu, Y., Lin, Y., & Zhu, L. (2025). Whole-Plant Rape Silage-Based Diets for Chongming White Goats: An Integrated Assessment of Growth Performance, Meat Quality and Gut Microbiota. Foods, 14(20), 3512. https://doi.org/10.3390/foods14203512





