Structural Properties and Anti-Inflammatory Activity of GLP-P, a Kefir-Derived Neutral Glycopeptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Extraction, Separation, and Purification of GLP-P
2.3. Structural Identification
2.3.1. Molecular Weight Measurement
2.3.2. Monosaccharide Composition Analysis
2.3.3. Amino Acid Composition Analysis
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. Nuclear Magnetic Resonance
2.3.6. Atomic Force Microscope (AFM)
2.4. Cell Experiments
2.4.1. Cell Culture
2.4.2. Cell Viability Assay
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. NO Measurement
2.4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.6. Real-Time Quantitative PCR (RT-qPCR)
2.4.7. Western Blotting Analysis
2.4.8. Immunofluorescence Staining
2.5. Statistical Analysis
3. Results
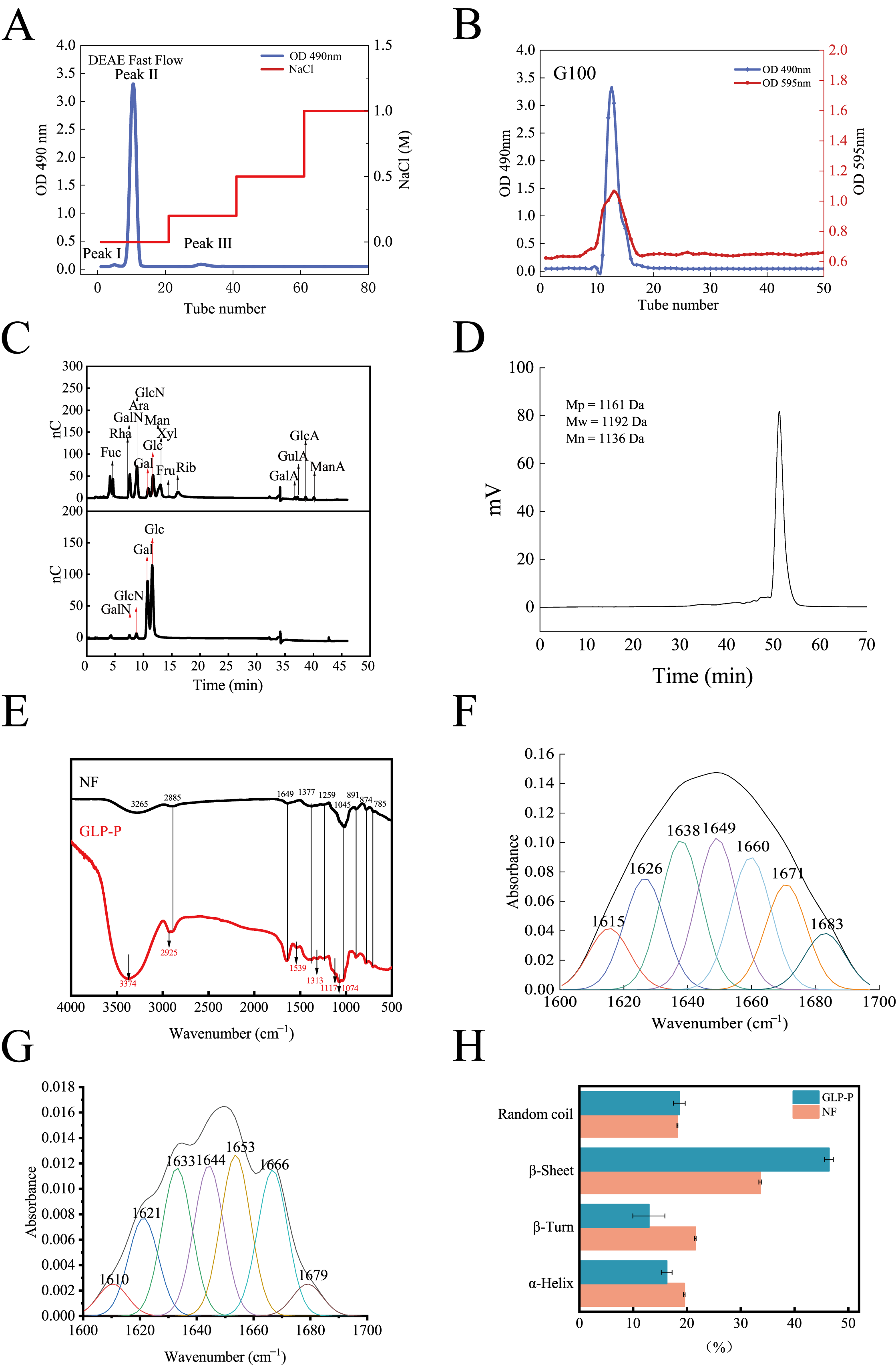
3.1. Gel Column Separation and Purification Results
3.2. Structural Characterization and Analysis
3.2.1. Analysis of Monosaccharides and Amino Acids Composition
3.2.2. Molecular Weight Distribution and Infrared Spectroscopy Analysis
3.2.3. Methylation Analysis
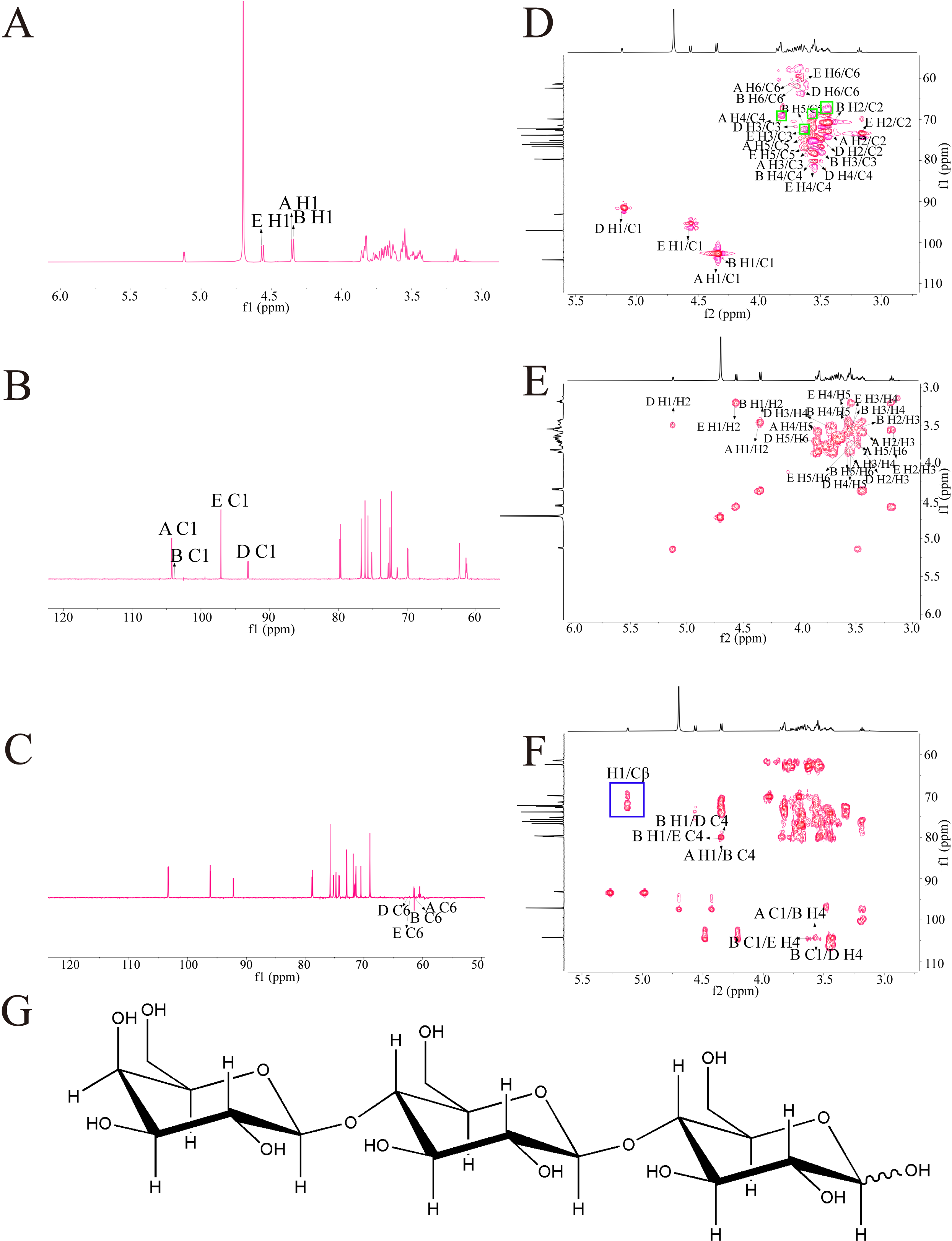
3.2.4. NMR Analysis
3.3. Conformational Analysis
3.4. Anti-Inflammatory Activity of GLP-P
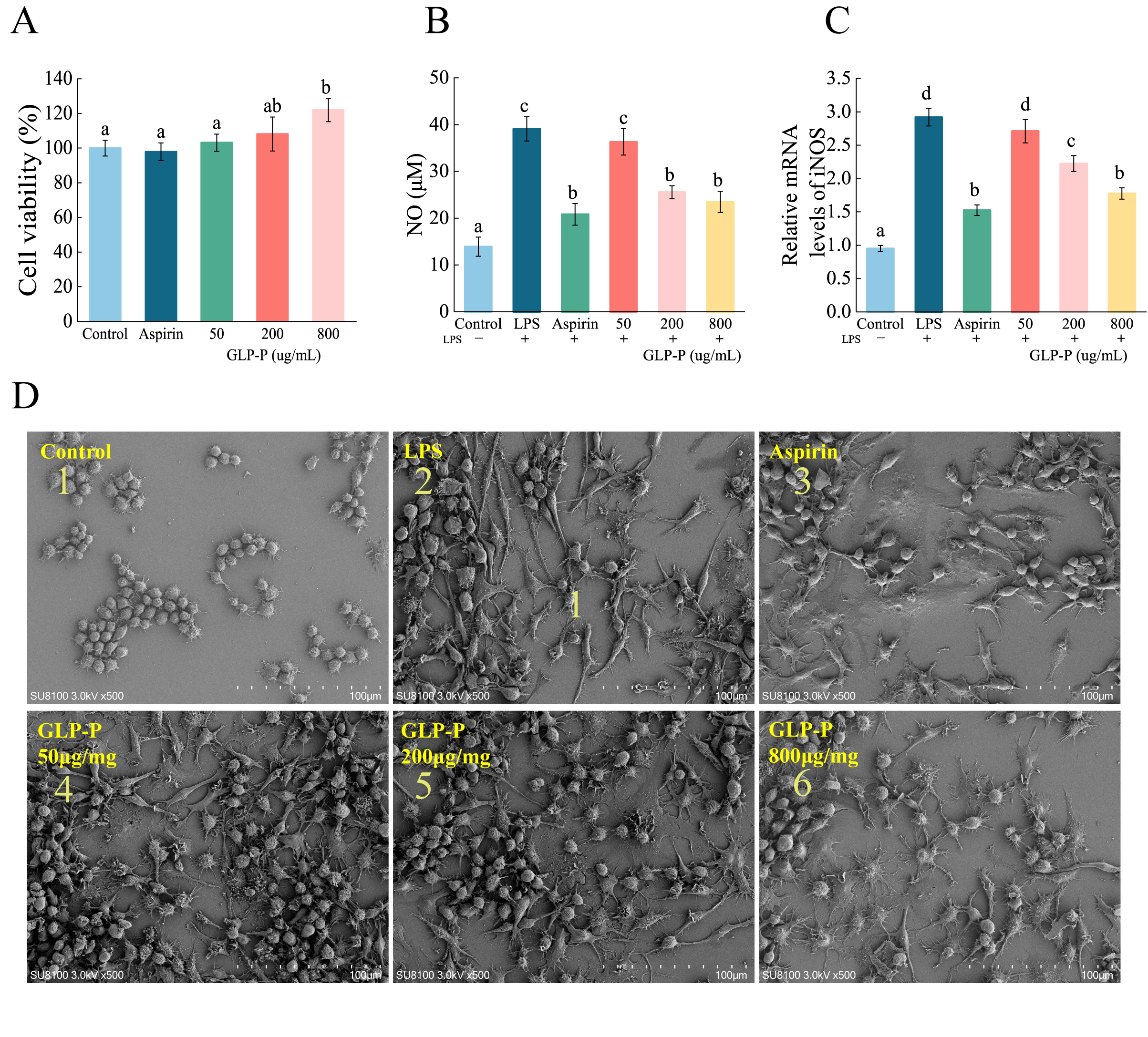
3.4.1. Effects of GLP-P on Cytotoxicity
3.4.2. Effects of GLP-P on LPS-Induced NO Production and iNOS Expression
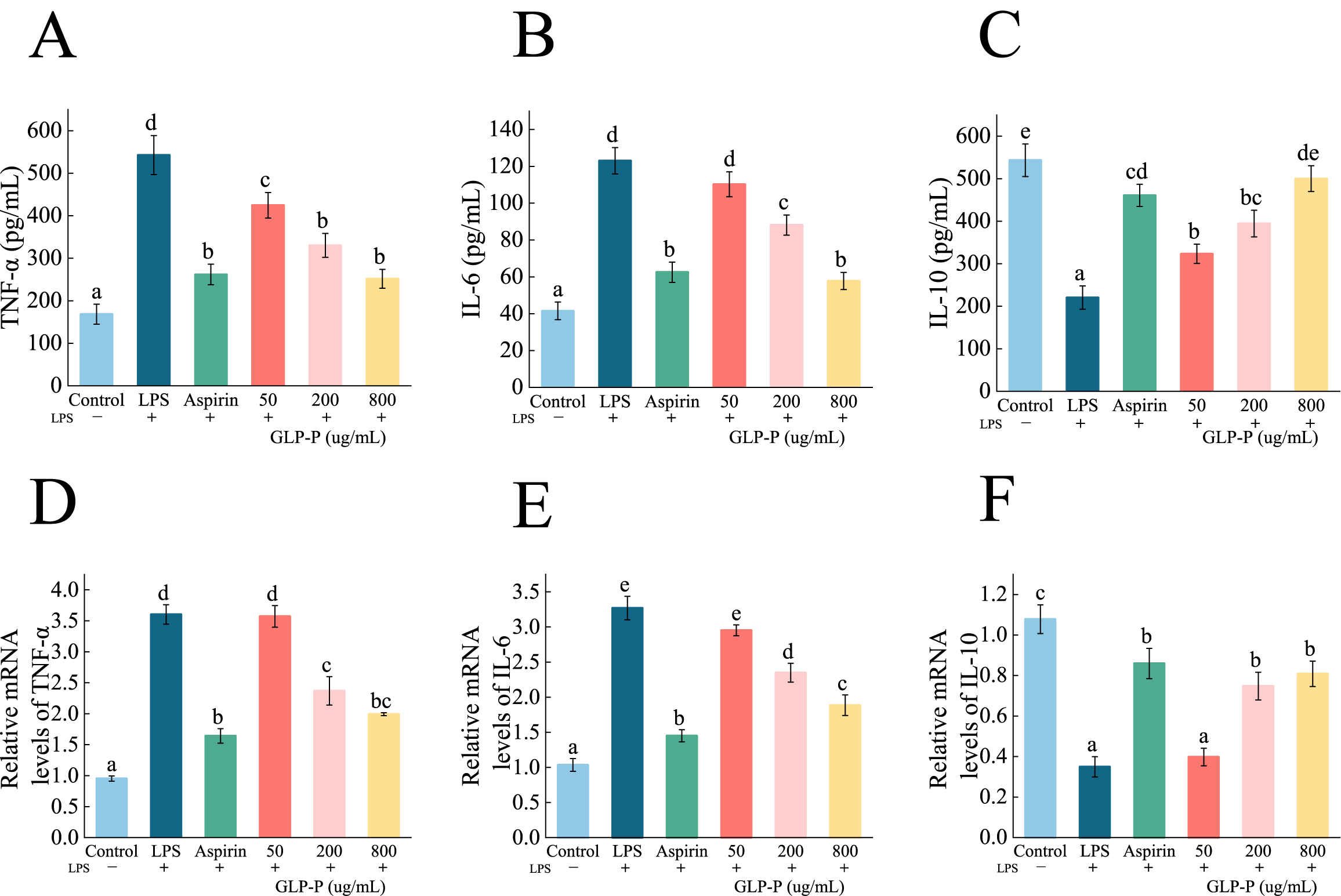
3.4.3. The Effect of GLP-P on Cytokine Secretion
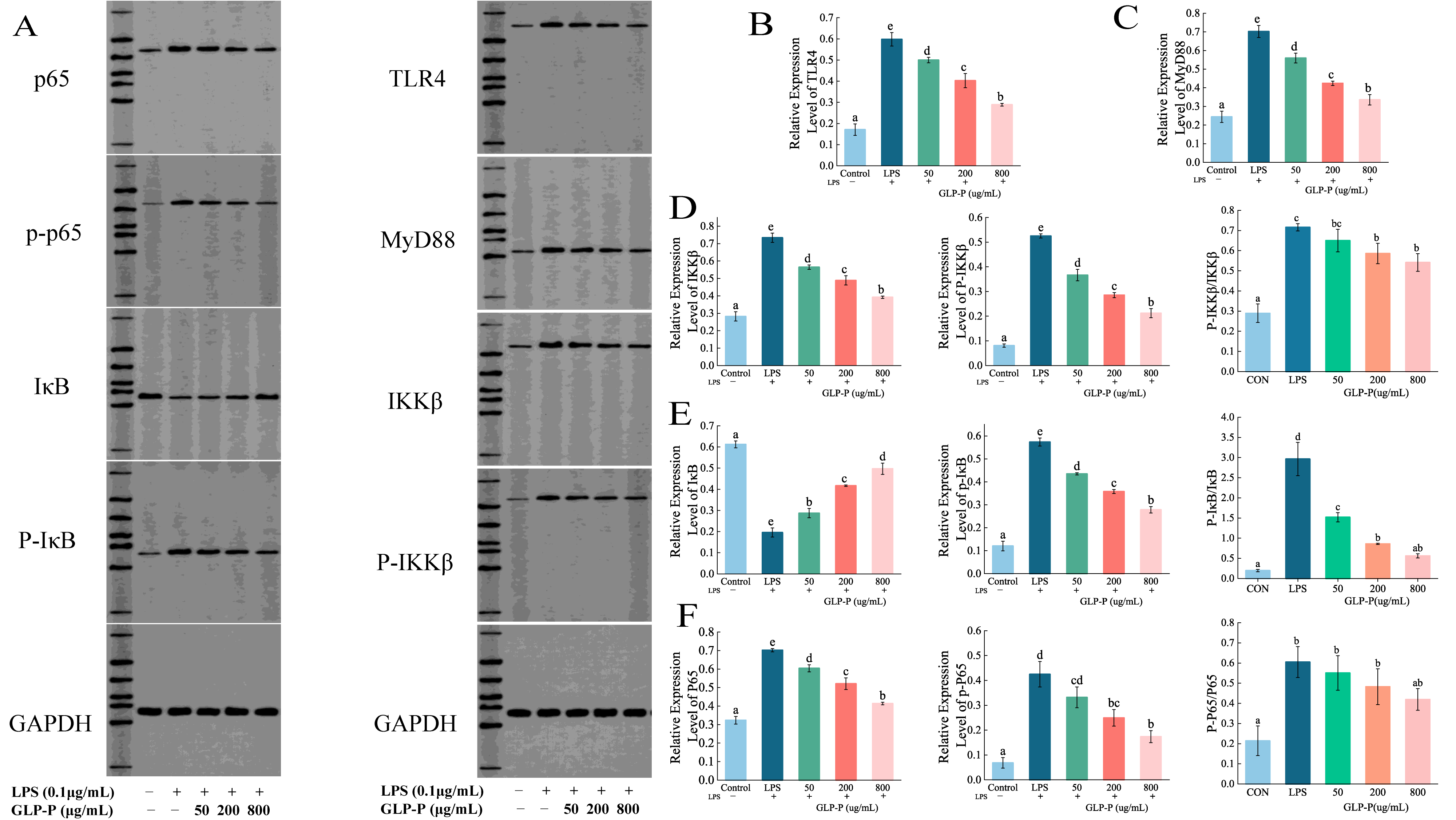
3.4.4. Effects of GLP-P on Protein Levels in the TLR4/MyD88/NF-κB Pathway
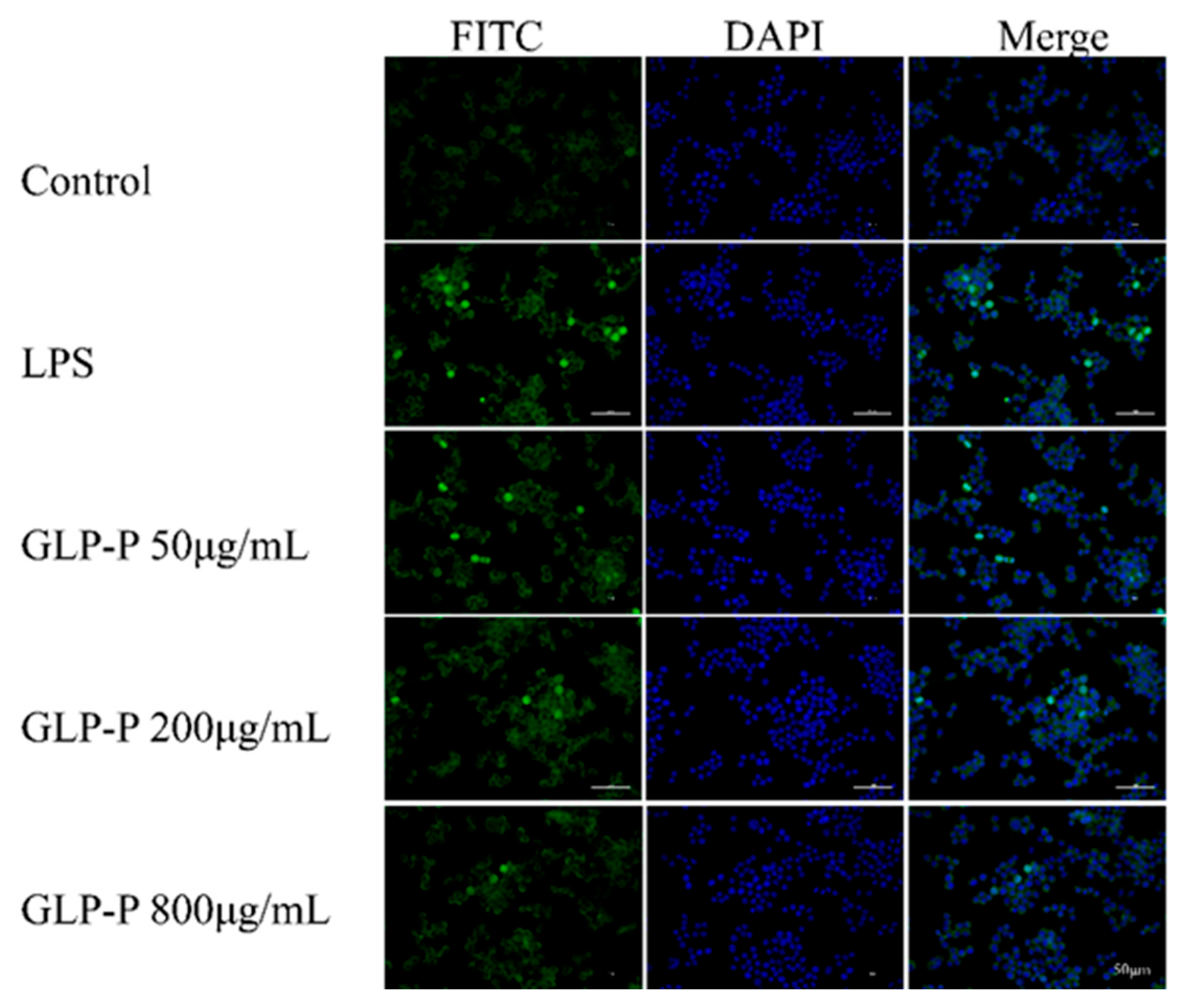
3.4.5. Effects of GLP-P on NF-κB Nuclear Translocation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GLP-P | kefir glycopeptide |
| LPS | lipopolysaccharide |
| TLR4 | toll-like receptor 4 |
| NF-κB | nuclear factor-κB |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| IL-10 | interleukin-10 |
| iNOS | inducible nitric oxide synthase |
| NO | nitric oxide. |
| IκB | inhibitor of NF-κB |
| IKK | IκB kinase |
| MyD88 | myeloid differentiation factor 88 |
References
- Cui, Y.; Wang, X.; Yue, Y.; Du, G.; Chen, H.; Ning, M.; Yuan, Y.; Yue, T. Metagenomic Features of Tibetan Kefir Grains and Its Metabolomics Analysis during Fermentation. LWT 2023, 175, 114502. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A Comparison of Milk Kefir and Water Kefir: Physical, Chemical, Microbiological and Functional Properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Fiorda, F.A.; Pereira, G.V.d.M.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; Vandenberghe, L.P.d.S.; Soccol, C.R. Microbiological, Biochemical, and Functional Aspects of Sugary Kefir Fermentation—A Review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Chuang, K.-C.; Lai, Y.-W.; Ko, C.-H.; Yen, C.-C.; Chen, H.-L.; Lan, Y.-W.; Chen, C.-F.; Chen, W.; Chen, C.-M. Therapeutic Effects of Kefir Peptides on Adjuvant-Induced Arthritis in Rats through Anti-Inflammation and Downregulation of Matrix Metalloproteinases. Life Sci. 2023, 317, 121411. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jing, C.; Yue, Y.; Ning, M.; Chen, H.; Yuan, Y.; Yue, T. Kefir Ameliorates Alcohol-Induced Liver Injury Through Modulating Gut Microbiota and Fecal Bile Acid Profile in Mice. Mol. Nutr. Food Res. 2024, 68, 2300301. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Wu, H.-S.; Ho, M.-H.; Chong, K.-Y.; Chen, C.-M. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol. Nutr. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef]
- Lai, J.-C.; Chang, G.R.-L.; Tu, M.-Y.; Cidem, A.; Chen, I.-C.; Chen, C.-M. Potential of Kefir-Derived Peptides, Probiotics, and Exopolysaccharides for Osteoporosis Management. Curr. Osteoporos. Rep. 2025, 23, 18. [Google Scholar] [CrossRef]
- Pereira, T.M.C.; Côco, L.Z.; Ton, A.M.M.; Meyrelles, S.S.; Campos-Toimil, M.; Campagnaro, B.P.; Vasquez, E.C. The Emerging Scenario of the Gut–Brain Axis: The Therapeutic Actions of the New Actor Kefir against Neurodegenerative Diseases. Antioxidants 2021, 10, 1845. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Chen, Y.-C.; Yen, C.-C.; Chen, H.-L.; Tung, M.-C.; Fan, H.-C.; Chen, C.-M. Kefir Peptides Mitigate Bleomycin-Induced Pulmonary Fibrosis in Mice through Modulating Oxidative Stress, Inflammation and Gut Microbiota. Biomed. Pharmacother. 2024, 174, 116431. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Liu, M.; Tian, Q.; Hui, M.; Shi, X.; Hou, X. Physical and Chemical Properties, Structural Characterization and Nutritional Analysis of Kefir Yoghurt. Front. Microbiol. 2023, 13, 1107092. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R.; Sharma, A.; Lee, H.-J. Kefir: A Fermented Plethora of Symbiotic Microbiome and Health. J. Ethn. Foods 2024, 11, 35. [Google Scholar] [CrossRef]
- McGovern, C.J.; González-Orozco, B.D.; Jiménez-Flores, R. Evaluation of Kefir Grain Microbiota, Grain Viability, and Bioactivity from Fermenting Dairy Processing by-Products. J. Dairy Sci. 2024, 107, 4259–4276. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, B.; Ren, L.; Jiang, Y.; Meng, Y.; Ma, R.; Wang, S.; Li, X.; Cui, F.; Li, T.; et al. Structure, Sources, Functional Mechanisms, and Applications of Bioactive Glycopeptides in Food: A Comprehensive Review. Trends Food Sci. Technol. 2025, 157, 104899. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Ding, R.; Yao, W.; Gao, X. Inflammatory Modulation Effect of Glycopeptide from Ganoderma capense (Lloyd) Teng. Mediat. Inflamm. 2014, 2014, 691285. [Google Scholar] [CrossRef]
- He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-Peptide from Trametes Versicolor: The Potential Medicine for Colorectal Cancer Treatment. Biomedicines 2022, 10, 2841. [Google Scholar] [CrossRef]
- Li, R.; Qu, S.; Qin, M.; Huang, L.; Huang, Y.; Du, Y.; Yu, Z.; Fan, F.; Sun, J.; Li, Q.; et al. Immunomodulatory and Antiviral Effects of Lycium barbarum Glycopeptide on Influenza a Virus Infection. Microb. Pathog. 2023, 176, 106030. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Dong, J.; Li, W.; Li, Z.; Gao, R.; Liu, X.; Wang, J.; Su, Q.; Wen, B.; Ouyang, W.; et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv. Sci. 2023, 10, e2301244. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, C.; Gao, P.; Zhou, W.; Xiao, R.; Zhang, Z.; Liao, W.; Ding, K. A Novel Pectin-Like Glycopeptide Isolated from the Fruit of Fructus mori Impedes Aggregation and Production of Aβ42. J. Agric. Food Chem. 2022, 70, 9908–9918. [Google Scholar] [CrossRef]
- Acosta, N.; Costabel, L.; Campos, S.; Cuatrin, A.; Olivares, M. Increase of Milk Heat Stability by Addition of Casein Glycomacropeptide. Int. Dairy J. 2022, 139, 105559. [Google Scholar] [CrossRef]
- Saleem, K.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Hussain, M.; Raza, A.; Rasheed, A.; Asghar, A.; Shah, M.A. Nutritional and Functional Properties of Kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Baars, T.; Esch, B.v.; Diks, M.; van Ooijen, L.; Zhang, Z.; Dekker, P.; Boeren, S.; Garssen, J.; Hettinga, K.; Kort, R. Bacterial Diversity, Bioactive Peptides, and Enhanced Immunomodulatory Effects in Raw Milk Kefir Made with Defined Starter Cultures versus Backslopping. Int. Dairy J. 2025, 164, 106202. [Google Scholar] [CrossRef]
- Ibacache, C.; González-Pizarro, K.; Charifeh, M.; Canales, C.; Díaz Viciedo, R.; Schmachtenberg, O.; Dinamarca, M. Metagenomic and Functional Characterization of Two Chilean Kefir Beverages Reveals a Dairy Beverage Containing Active Enzymes, Short-Chain Fatty Acids, Microbial β-Amyloids, and Bio-Film Inhibitors. Foods 2022, 11, 900. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Vieira, C.P.; Rosario, A.I.L.S.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-Junior, C.A. Bioactive Compounds from Kefir and Their Potential Benefits on Health: A Systematic Review and Meta-Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 9081738. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-W. Structural Characterization and Physicochemical Properties of Kefiran and Their Interactions with Milk Protein. Master’s Thesis, Inner Mongolia Agricultural University, Inner Mongolia, China, 15 June 2023. [Google Scholar]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural Studies of a Mannoglucan from Cremastra appendiculata (Orchidaceae) by Chemical and Enzymatic Methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, C.; Li, J.; Shao, X.; Wu, J.; Zhang, M.; Yao, H.; Wu, X. Characterization of Purified Mulberry Leaf Glycoprotein and Its Immunoregulatory Effect on Cyclophosphamide-Treated Mice. Foods 2022, 11, 2034. [Google Scholar] [CrossRef]
- Feng, L.; Han, N.; Han, Y.-B.; Shang, M.-W.; Liang, T.-W.; Liu, Z.-H.; Li, S.-K.; Zhai, J.-X.; Yin, J. Structural Analysis of a Soluble Polysaccharide GSPA-0.3 from the Root of Panax ginseng C. A. Meyer and Its Adjuvant Activity with Mechanism Investigation. Carbohydr. Polym. 2024, 326, 121591. [Google Scholar] [CrossRef]
- Phan, U.T.T.; Nguyen, H.D.; Nguyen, T.K.O.; Tran, T.H.; Le, T.H.; Tran, T.T.P. Anti-Inflammatory Effect of Piper longum L. Fruit Methanolic Extract on Lipopolysaccharide-Treated RAW 264.7 Murine Macrophages. Heliyon 2024, 10, e26174. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Jiang, X.; Peng, K.; Yi, Y.; Meng, Y.; Wang, H. Structural Characterization and Immunoregulatory Mechanism of a Low-Molecular-Weight Polysaccharide from Lotus Root. Int. J. Biol. Macromol. 2024, 280, 135957. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Li, Y.; Zhang, X.; Shen, L.; Zhao, X.; Beta, T.; Li, B.; Chen, R.; Huang, W. Immune Regulation and Inflammation Inhibition of Arctium lappa L. Polysaccharides by TLR4/NF-κB Signaling Pathway in Cells. Int. J. Biol. Macromol. 2024, 254, 127700. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Q.; Lin, B.; Sun, M.; Zheng, Q.; Huang, J.; Lai, G. Structural Characterization of a Soluble Polysaccharide SSPS1 from Soy Whey and Its Immunoregulatory Activity in Macrophages. Int. J. Biol. Macromol. 2022, 217, 131–141. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, J.; Li, H.; Du, Y.; Li, S.; Li, A.; Suo, X.; Wang, Y.; Sun, Q. Anti-Inflammatory Activity of the Water Extract of Chloranthus serratus Roots in LPS-Stimulated RAW264.7 Cells Mediated by the Nrf2/HO-1, MAPK and NF-κB Signaling Pathways. J. Ethnopharmacol. 2021, 271, 113880. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Xu, H.; Li, Y.; Peng, F.; Qiu, W.; Tang, C. Lycium barbarum Glycopeptide Ameliorates Motor and Visual Deficits in Autoimmune Inflammatory Diseases. Phytomedicine 2024, 129, 155610. [Google Scholar] [CrossRef]
- M’hir, S.; Ayed, L.; De Pasquale, I.; Fanizza, E.; Tlais, A.Z.A.; Comparelli, R.; Verni, M.; Latronico, R.; Gobbetti, M.; Di Cagno, R.; et al. Comparison of Milk Kefirs Obtained from Cow’s, Ewe’s and Goat’s Milk: Antioxidant Role of Microbial-Derived Exopolysaccharides. Antioxidants 2024, 13, 335. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Liu, H.; Wu, Q.; Yang, S.; Song, S.; Yu, L.; Bi, Y. Neutral Oligosaccharides from Ginseng (Panax ginseng) Residues vs. Neutral Ginseng Polysaccharides: A Comparative Study of Structure Elucidation and Biological Activity. Food Chem. 2025, 464, 141674. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Woźniak, Ł.; Zou, L.; Cao, H.; Xiao, J.; et al. A Neutral Polysaccharide with a Triple Helix Structure from Ginger: Characterization and Immunomodulatory Activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef]
- Waeytens, J.; De Meutter, J.; Goormaghtigh, E.; Dazzi, A.; Raussens, V. Determination of Secondary Structure of Proteins by Nanoinfrared Spectroscopy. Anal. Chem. 2023, 95, 621–627. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Javed, A.; Song, B.-R.; Lee, C.H.; Alam, M.B.; Kim, S.L.; Lee, S.-H. Glycoprotein from Sargassum fusiforme Exhibiting Anti-Inflammatory Responses in Vitro and in Vivo via Modulation of TLR4/MyD88 and NF-κB Signaling. Int. J. Biol. Macromol. 2024, 272, 132574. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.-M.; Qin, G.-Y. Structure Characterization and Antioxidant Activity of Polysaccharides from Chinese Quince Seed Meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Yang, X.; Li, Y.; Yao, Y.; Lui, E.M.K.; Ren, G. Structural and Anti-Inflammatory Characterization of a Novel Neutral Polysaccharide from North American ginseng (Panax Quinquefolius). Int. J. Biol. Macromol. 2015, 74, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Tintor, Đ.; Ninković, K.; Milošević, J.; Polović, N.Đ. Gaining Insight into Protein Structure via ATR-FTIR Spectroscopy. Vib. Spectrosc. 2024, 134, 103726. [Google Scholar] [CrossRef]
- Perticaroli, S.; Nickels, J.D.; Ehlers, G.; Sokolov, A.P. Rigidity, Secondary Structure, and the Universality of the Boson Peak in Proteins. Biophys. J. 2014, 106, 2667–2674. [Google Scholar] [CrossRef]
- Derenne, A.; Derfoufi, K.-M.; Cowper, B.; Delporte, C.; Butré, C.I.; Goormaghtigh, E. Analysis of Glycoproteins by ATR-FTIR Spectroscopy: Comparative Assessment. Methods Mol. Biol. 2021, 2271, 361–374. [Google Scholar] [CrossRef]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A Review of NMR Analysis in Polysaccharide Structure and Conformation: Progress, Challenge and Perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Cao, M.-X.; Xie, X.-D.; Wang, X.-R.; Hu, W.-Y.; Zhao, Y.; Chen, Q.; Ji, L.; Wei, Y.-Y.; Yu, M.-L.; Hu, T.-J. Separation, Purification, Structure Analysis, In Vitro Antioxidant Activity and circRNA-miRNA-mRNA Regulatory Network on PRV-Infected RAW264.7 Cells of a Polysaccharide Derived from Arthrospira Platensis. Antioxidants 2021, 10, 1689. [Google Scholar] [CrossRef]
- Conibear, A.C.; Rosengren, K.J.; Becker, C.F.W.; Kaehlig, H. Random Coil Shifts of Posttranslationally Modified Amino Acids. J. Biomol. NMR 2019, 73, 587–599. [Google Scholar] [CrossRef]
- Shi, D.; Xu, X.; Wang, J.; Bu, T.; Sun, P.; Yang, K.; Cai, M. Synergistic Anti-Inflammatory Effects of Ganoderma Lucidum Polysaccharide and Ganoderic Acid A on LPS-Induced RAW264.7 Cells by Inhibition of TLR4/NF-κB Activation. Int. J. Biol. Macromol. 2025, 309, 143074. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Liu, M.; Liu, M.; Zhao, Z. Structural Characterization of Alkali-Extracted Jujube Polysaccharides and Their Effects on the Fecal Microbiota in Vitro. LWT 2023, 184, 115087. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Shao, J.; Shu, X.; Jia, J.; Ren, X.; Guan, Y. Macrophage Immunomodulatory Activity of the Polysaccharide Isolated from Collybia radicata Mushroom. Int. J. Biol. Macromol. 2018, 108, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.; Oerlemans, F.T.J.J.; Wijers, M.; Willemse, M.; Fransen, J.A.M.; Wieringa, B. Glucose Controls Morphodynamics of LPS-Stimulated Macrophages. PLoS ONE 2014, 9, e96786. [Google Scholar] [CrossRef] [PubMed]
- Kleveta, G.; Borzęcka, K.; Zdioruk, M.; Czerkies, M.; Kuberczyk, H.; Sybirna, N.; Sobota, A.; Kwiatkowska, K. LPS Induces Phosphorylation of Actin-Regulatory Proteins Leading to Actin Reassembly and Macrophage Motility. J. Cell. Biochem. 2012, 113, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, L.; Guo, Z.; Liu, C.; Hu, B.; Li, M.; Gu, Z.; Xin, Y.; Sun, H.; Guan, Y.; et al. Yak Bone Collagen-Derived Anti-Inflammatory Bioactive Peptides Alleviate Lipopolysaccharide-Induced Inflammatory by Inhibiting the NF-κB Signaling Pathway and Nitric Oxide Production. Food Biosci. 2023, 52, 102423. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural Characterization and Anti-Inflammatory Activity of Polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Song, X.; Zhu, H.; Chen, Z.; Wang, Y.; Zhang, J.; Wang, Y.; Rong, P.; Wang, J. Transcutaneous Auricular Vagus Nerve Stimulation Alleviates Inflammation-Induced Depression by Modulating Peripheral-Central Inflammatory Cytokines and the NF-κB Pathway in Rats. Front. Immunol. 2025, 16, 1536056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.; Wang, Z.; Li, Z.; Geng, Y. Effects of Pine Pollen Polysaccharides and Sulfated Polysaccharides on Ulcerative Colitis and Gut Flora in Mice. Polymers 2023, 15, 1414. [Google Scholar] [CrossRef]
- Samuel, D.; Kumar, T.; Jayaraman, G.; Yang, P.W.C.; Yu, C. Proline Is a Protein Solubilizing Solute. IUBMB Life 1997, 41, 235–242. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological Properties and Chain Conformation of Soy Hull Water-Soluble Polysaccharide Fractions Obtained by Gradient Alcohol Precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Zhan, Q.; Wang, Y.; Meng, K.; Hu, Q.; Zhao, L. Studying on the Structure-Activity Relationship of Flammulina velutipes Polysaccharides via Ultrasonic Degradation: Insights into Molecular Weight, Chain Conformation, and Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2025, 302, 140480. [Google Scholar] [CrossRef]
- Lee, Q.; Xue, Z.; Luo, Y.; Lin, Y.; Lai, M.; Xu, H.; Liu, B.; Zheng, M.; Lv, F.; Zeng, F. Low Molecular Weight Polysaccharide of Tremella fuciformis Exhibits Stronger Antioxidant and Immunomodulatory Activities than High Molecular Weight Polysaccharide. Int. J. Biol. Macromol. 2024, 281, 136097. [Google Scholar] [CrossRef]
- Lee, Q.; Han, X.; Zheng, M.; Lv, F.; Liu, B.; Zeng, F. Preparation of Low Molecular Weight Polysaccharides from Tremella fuciformis by Ultrasonic-Assisted H2O2-Vc Method: Structural Characteristics, in Vivo Antioxidant Activity and Stress Resistance. Ultrason. Sonochemistry 2023, 99, 106555. [Google Scholar] [CrossRef] [PubMed]
- Wongkuna, S.; P.K, V.P.; Holmberg, S.M.; Bjørnshave, A.; Schroeder, B.O. Milk-Derived Casein Glycomacropeptide Improves Colonic Mucus Function under Western-Style Diet Feeding in a Sialylation-Dependent Manner. Food Res. Int. 2025, 221, 117206. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Jia, S.; Zeng, H.; Zheng, B. Structural Characterization and Antioxidant Activity of a Glycoprotein Isolated from Shiitake Mushrooms. Food Biosci. 2023, 53, 102608. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Dastagir, N.; Uddin, N.; Rehman, K.; Azim, M.K. Characterization of Immunomodulatory Activities of Honey Glycoproteins and Glycopeptides. J. Agric. Food Chem. 2015, 63, 177–184. [Google Scholar] [CrossRef]


| Elution Peak Ⅰ | Elution Peak Ⅱ | Elution Peak Ⅲ | |
|---|---|---|---|
| DEAE Sepharose Fast Flow | 4.78 ± 0.16% | 68.98 ± 0.11% | 8.83 ± 0.27% |
| Sephadex G-100 | \ | 85.28 ± 2% | \ |
| Total Yield | \ | 58.83 ± 1.47% | \ |
| Amino Acid | Concentration (μg/mg) | Amino Acid | Concentration (μg/mg) |
|---|---|---|---|
| arginine | 6.55 ± 0.02 | threonine | 8.71 ± 0.04 |
| lysine | 157.77 ± 0.03 | glycine | 1.37 ± 0.02 |
| asparagine | 30.89 ± 0.01 | serine | 9.5 ± 0.08 |
| proline | 10.89 ± 0.04 | isoleucine | 1.52 ± 0.04 |
| Wavenumber (cm−1) | Functional Group |
|---|---|
| 3374, 3265 | O-H stretching [37] |
| 2925, 2885 | C-H stretching [37] |
| 1648 | C=O stretching [38] (amide I) |
| 1539 | C=O stretching [38] (amide II) |
| 1377 | C–H bending [39] |
| 1313, 1259 | N–H bending + C–N stretching (Amide III) [40] |
| 1117, 1074 | C-O-C Symmetric C–O–C stretching [41] |
| 1045 | C-OH [41] |
| 890, 874 | C–H bending of α- and β-glycosidic bonds [42] |
| 784 | symmetric ring stretching vibration of the pyran ring [30] |
| Retention Time (min) | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Type of Linkage |
|---|---|---|---|---|
| 36.910 | 2,3,4,6-Me4-Galp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 0.522 | Galp-(1→ |
| 48.409 | 2,3,6-Me3-Glcp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 0.478 | →4)-Glcp-(1→ |
| Glycosyl Residues | Chemical Shift δH/C (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a/C6 | H6b | |
| β-D-Galp-(1→ | 4.35 | 3.44 | 3.56 | 3.84 | 3.57 | 3.67 | 3.65 |
| A | 104.27 | 72.32 | 76.63 | 70.08 | 73.93 | 61.45 | |
| →4)-β-D-Glcp-(1→ | 4.34 | 3.43 | 3.53 | 3.56 | 3.58 | 3.68 | 3.66 |
| B | 104.25 | 72.31 | 76.62 | 79.85 | 73.94 | 61.46 | |
| →4)-α-D-Glcp | 5.12 | 3.47 | 3.71 | 3.54 | 3.85 | 3.68 | 3.62 |
| D | 93.10 | 75.15 | 71.50 | 79.54 | 72.19 | 62.40 | |
| →4)-β-D-Glcp | 4.57 | 3.17 | 3.53 | 3.56 | 3.50 | 3.70 | 3.64 |
| E | 97.06 | 75.72 | 72.29 | 79.89 | 76.22 | 62.29 | |
| X | Y | Glycosidic Bond Linkage Patterns |
|---|---|---|
| AH1/BC4 | AC1/BH4 | β-D-Galp-(1→4)-β-D-Glcp-(1→ |
| BH1/DC4 | BC1/DH4 | →4)-β-D-Glcp-(1→4)-α-D-Glcp |
| BH1/EC4 | BC1/EH4 | →4)-β-D-Glcp-(1→4)-β-D-Glcp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Z.; Bai, Y. Structural Properties and Anti-Inflammatory Activity of GLP-P, a Kefir-Derived Neutral Glycopeptide. Foods 2025, 14, 3509. https://doi.org/10.3390/foods14203509
Yang Y, Zhang Z, Bai Y. Structural Properties and Anti-Inflammatory Activity of GLP-P, a Kefir-Derived Neutral Glycopeptide. Foods. 2025; 14(20):3509. https://doi.org/10.3390/foods14203509
Chicago/Turabian StyleYang, Yuejiao, Zhiying Zhang, and Ying Bai. 2025. "Structural Properties and Anti-Inflammatory Activity of GLP-P, a Kefir-Derived Neutral Glycopeptide" Foods 14, no. 20: 3509. https://doi.org/10.3390/foods14203509
APA StyleYang, Y., Zhang, Z., & Bai, Y. (2025). Structural Properties and Anti-Inflammatory Activity of GLP-P, a Kefir-Derived Neutral Glycopeptide. Foods, 14(20), 3509. https://doi.org/10.3390/foods14203509






