Design of Polymeric Delivery Systems for Lycium barbarum Phytochemicals: A Spray Drying Approach for Nutraceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Lycium Barbarum Extract
2.3. Preparation of Polymer Microparticles
2.4. Characterization of the Optimal Liposome Formulation
2.4.1. Production Yield
2.4.2. Particle Shape, Size, and Surface Properties
2.4.3. In Vitro Antioxidant and Antiradical Activities
Total Phenolic Content
DPPH Radical Scavenging Activity Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.4. Differential Scanning Calorimeter (DSC)
2.4.5. In Vitro Biocompatibility Studies
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Produced Microparticles
3.1.1. Production Yield
3.1.2. Microstructure Size
3.1.3. In Vitro Antioxidant and Antiradical Activities
3.1.4. Surface Morphology
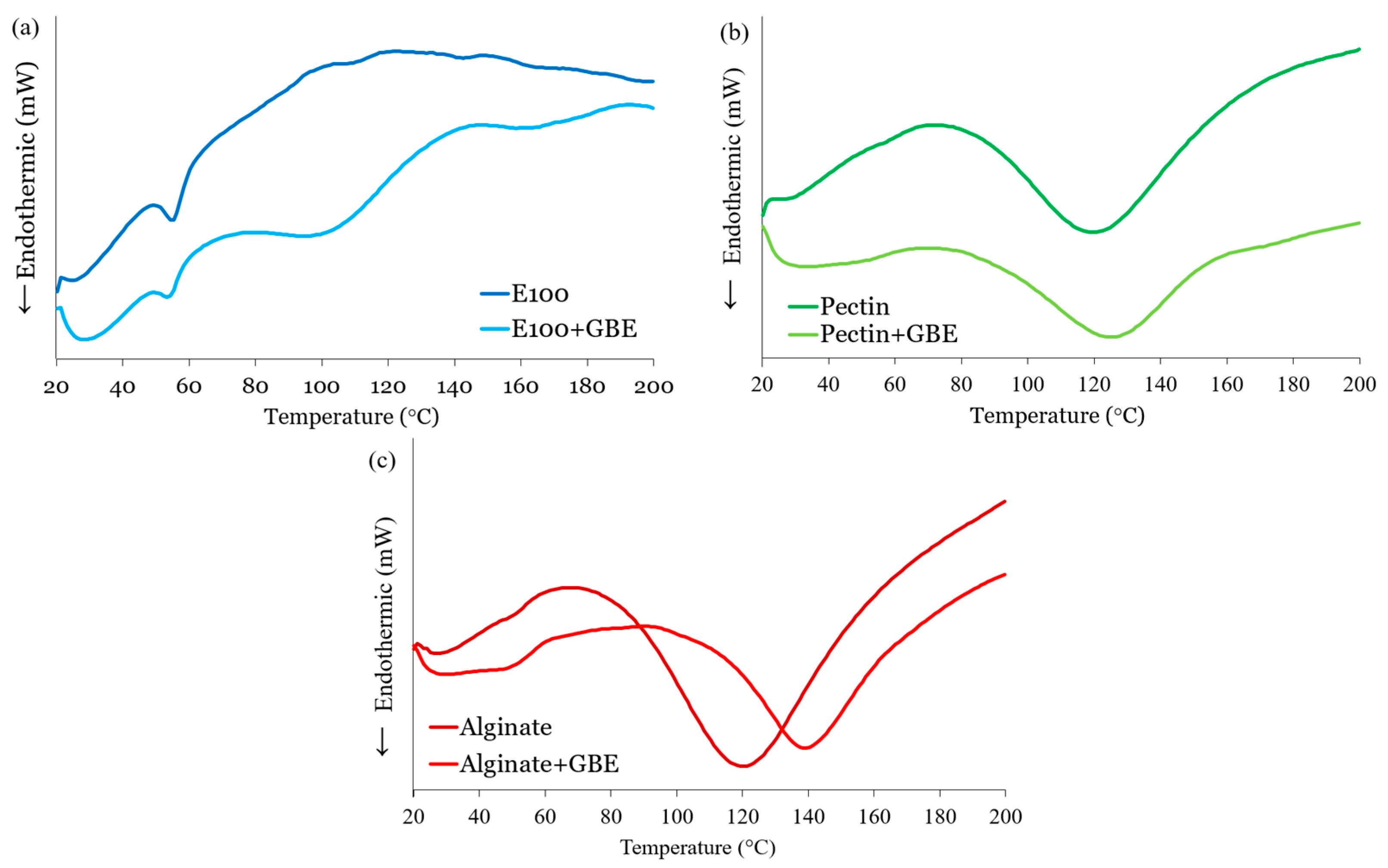
3.1.5. Thermal Behavior
3.2. In Vitro Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum Berries (Solanaceae) as Source of Bioactive Compounds for Healthy Purposes: A Review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.; Mellano, M.; Cerutti, A.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Shi, Y.-P. Comprehensive analysis of phenolic compounds in four varieties of goji berries at different ripening stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Vasantha Rupasinghe, H.P.; Nair, S.V.G.; Robinson, R.A. Chemopreventive Properties of Fruit Phenolic Compounds and Their Possible Mode of Actions. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–266. [Google Scholar]
- Teixeira, F.; Silva, A.M.; Sut, S.; Dall’ACqua, S.; Ramos, O.L.; Ribeiro, A.B.; Ferraz, R.; Delerue-Matos, C.; Rodrigues, F. Ultrasound-assisted extraction of bioactive compounds from goji berries: Optimization, bioactivity, and intestinal permeability assessment. Food Res. Int. 2024, 188, 114502. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Zheng, Y.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Appl. Sci. 2025, 15, 262. [Google Scholar] [CrossRef]
- Martinović, J.; Ambrus, R.; Planinić, M.; Perković, G.; Šelo, G.; Klarić, A.-M.; Bucić-Kojić, A. Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels 2025, 11, 130. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends in Foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; D’aMore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Al-hashimi, N.; Dahmash, E.Z.; Khoder, M.; Alany, R.G.; Elshaer, A. Systematic screening of particle engineered polymers for the preparation of multiparticulates embedded orally disintegrating tablets. J. Drug Deliv. Sci. Technol. 2024, 101, 106302. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Adomavičiūtė, E.; Jankauskaitė, V.; Marksa, M.; Barsteigienė, Z.; Bernatoniene, J. Formation and Investigation of Electrospun Eudragit E100/Oregano Mats. Molecules 2019, 24, 628. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Nagy, Y.I.; Shehabeldine, A.M.; Okba, M.M. Thymol-Loaded Eudragit RS30D Cationic Nanoparticles-Based Hydrogels for Topical Application in Wounds: In Vitro and In Vivo Evaluation. Pharmaceutics 2023, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Chaumun, M.; Goëlo, V.; Ribeiro, A.M.; Rocha, F.; Estevinho, B.N. In vitro evaluation of microparticles with Laurus nobilis L. extract prepared by spray-drying for application in food and pharmaceutical products. Food Bioprod. Process. 2020, 122, 124–135. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Sut, S.; Dall’acqua, S.; Delerue-Matos, C.; Estevinho, B.; Costa, P.C.; Rodrigues, F. Development and Characterization of Microparticles with Actinidia arguta Leaves Extract by Spray-Drying: A New Mind-Set Regarding Healthy Compounds for Oral Mucositis. Antioxidants 2023, 12, 1496. [Google Scholar] [CrossRef]
- Shikha, S.; Lee, Y.W.; Doyle, P.S.; Khan, S.A. Microfluidic Particle Engineering of Hydrophobic Drug with Eudragit E100─Bridging the Amorphous and Crystalline Gap. Mol. Pharm. 2022, 19, 4345–4356. [Google Scholar] [CrossRef]
- Li, T.; Wan, B.; Jog, R.; Costa, A.; Burgess, D.J. Pectin microparticles for peptide delivery: Optimization of spray drying processing. Int. J. Pharm. 2022, 613, 121384. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; Amboni, R.D.d.M.C. Encapsulation of stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation—Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and Oxidative Stability of PUFA-Rich Oil Microencapsulated by Spray Drying Using Pea Protein and Pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Almeida, H.H.S.; Barros, L.; Barreira, J.C.; Calhelha, R.C.; Heleno, S.A.; Sayer, C.; Miranda, C.G.; Leimann, F.V.; Barreiro, M.F.; Ferreira, I.C. Bioactive evaluation and application of different formulations of the natural colorant curcumin (E100) in a hydrophilic matrix (yogurt). Food Chem. 2018, 261, 224–232. [Google Scholar] [CrossRef]
- Flamminii, F.; Paciulli, M.; Di Michele, A.; Littardi, P.; Carini, E.; Chiavaro, E.; Pittia, P.; Di Mattia, C.D. Alginate-based microparticles structured with different biopolymers and enriched with a phenolic-rich olive leaves extract: A physico-chemical characterization. Curr. Res. Food Sci. 2021, 4, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J. Freeze-Dried Matrices Composed of Degradable Polymers with Surfactant-Loaded Microparticles Based on Pectin and Sodium Alginate. Materials 2021, 14, 3044. [Google Scholar] [CrossRef] [PubMed]
- Rosales, T.K.O.; Fabi, J.P. Pectin-based nanoencapsulation strategy to improve the bioavailability of bioactive compounds. Int. J. Biol. Macromol. 2023, 229, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Prezotti, F.G.; Boni, F.I.; Ferreira, N.N.; Silva, D.S.; Almeida, A.; Vasconcelos, T.; Sarmento, B.; Gremião, M.P.D.; Cury, B.S.F. Oral nanoparticles based on gellan gum/pectin for colon-targeted delivery of resveratrol. Drug Dev. Ind. Pharm. 2020, 46, 236–245. [Google Scholar] [CrossRef]
- San Hipólito-Luengo, Á.; Alcaide, A.; Ramos-González, M.; Cercas, E.; Vallejo, S.; Romero, A.; Talero, E.; Sánchez-Ferrer, C.F.; Motilva, V.; Peiró, C. Dual Effects of Resveratrol on Cell Death and Proliferation of Colon Cancer Cells. Nutr Cancer 2017, 69, 1019–1027. [Google Scholar] [CrossRef]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef]


| Production Yield (%) | Average Size (µm) | |
|---|---|---|
| E100 | 2.8 ± 0.1 | 3.3 ± 2.1 |
| E100 + GBE | 26.6 ± 8.0 | 2.9 ± 0.7 |
| Alginate | 13.9 ± 0.7 | 3.2 ± 1.0 |
| Alginate + GBE | 40.3 ± 7.0 | 3.6 ± 1.6 |
| Pectin | 21.7 ± 1.1 | 3.5 ± 2.7 |
| Pectin + GBE | 38.1 ± 3.0 | 4.1 ± 1.0 |
| RS30D | 1.3 ± 0.1 | 4.4 ± 2.0 |
| RS30D + GBE | 20.1 ± 6.5 | 1.9 ± 0.9 |
| TPC | FRAP | DPPH | |
|---|---|---|---|
| mg GAE/g | µmol FSE/g | mg TE/g | |
| GBE | 23.87 ± 1.16 e | 105.97 ± 8.20 f | 10.25 ± 0.81 d |
| E100 | 3.53 ± 0.58 a | 5.10 ± 0.99 a | 0.94 ± 0.27 a |
| E100 + GBE | 9.31 ± 1.18 c | 25.96 ± 7.04 b,c | 4.01 ± 1.38 c |
| Pectin | 3.60 ± 0.25 a | 18.50 ± 1.86 b | 1.05 ± 0.24 a |
| Pectin + GBE | 8.14 ± 0.50 b | 43.20 ± 5.26 d | 4.14 ± 1.74 c |
| Alginate | 2.61 ± 0.47 a | 20.87 ± 3.51 b,c | 1.06 ± 0.49 a |
| Alginate + GBE | 7.85 ± 0.46 b | 42.16 ± 6.16 d | 3.33 ± 0.80 b,c |
| RS30D | 9.61 ± 0.81 c | 26.59 ± 3.30 c | 1.77 ± 0.49 a,b |
| RS30D + GBE | 15.51 ± 1.16 d | 59.83 ± 7.96 e | 3.50 ± 1.78 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, F.; Rut, A.; Costa, P.C.; Rodrigues, F.; Estevinho, B.N. Design of Polymeric Delivery Systems for Lycium barbarum Phytochemicals: A Spray Drying Approach for Nutraceuticals. Foods 2025, 14, 3504. https://doi.org/10.3390/foods14203504
Teixeira F, Rut A, Costa PC, Rodrigues F, Estevinho BN. Design of Polymeric Delivery Systems for Lycium barbarum Phytochemicals: A Spray Drying Approach for Nutraceuticals. Foods. 2025; 14(20):3504. https://doi.org/10.3390/foods14203504
Chicago/Turabian StyleTeixeira, Filipa, Angelina Rut, Paulo C. Costa, Francisca Rodrigues, and Berta Nogueiro Estevinho. 2025. "Design of Polymeric Delivery Systems for Lycium barbarum Phytochemicals: A Spray Drying Approach for Nutraceuticals" Foods 14, no. 20: 3504. https://doi.org/10.3390/foods14203504
APA StyleTeixeira, F., Rut, A., Costa, P. C., Rodrigues, F., & Estevinho, B. N. (2025). Design of Polymeric Delivery Systems for Lycium barbarum Phytochemicals: A Spray Drying Approach for Nutraceuticals. Foods, 14(20), 3504. https://doi.org/10.3390/foods14203504






