Preparation, Stability and In Vitro Antineoplastic Function of Lecithin–Chitosan–Polyethylene Glycol Nanoparticles Loaded with Bioactive Peptides Derived from Phycocyanin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Preparation of Lecithin NPs (LEC NPs)
2.3. The Encapsulation Efficiency Detection of PCPs in NPs
2.4. The Preparation of Polyethylene Glycol–Chitosan–Lecithin NPs (LEC–CS–PEG NPs)
2.5. The Particle Size, PDI and Zeta Potential Measurements
2.6. Fourier Transform Infrared (FTIR) Spectroscopy Detection
2.7. The Thermal Stability Assessment of the Prepared NPs
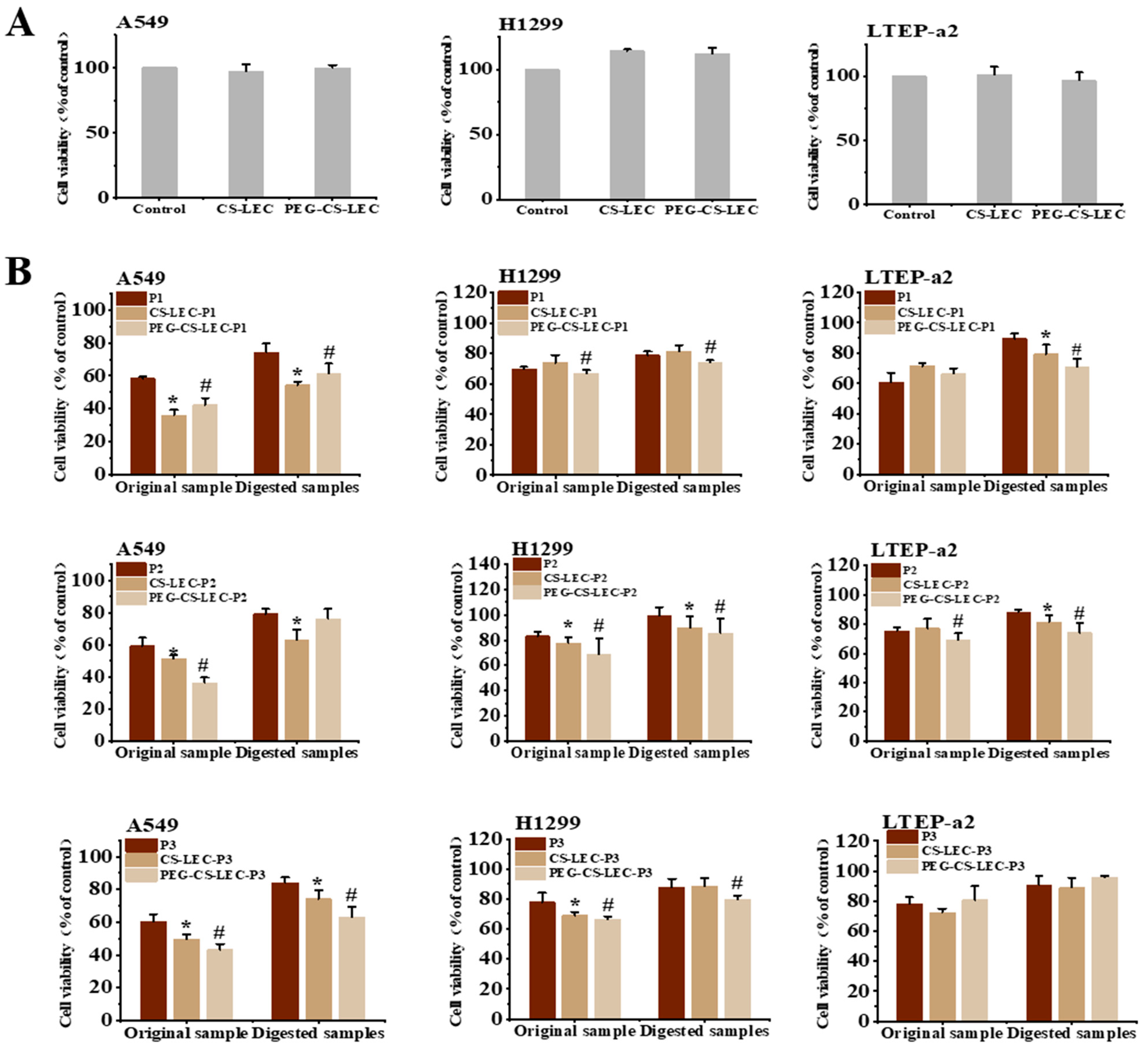
2.8. Cell Viability Assays
2.9. Transmission Electron Microscope (TEM) Observation
2.10. Statistical Analysis
3. Results
3.1. Preparation of PCP-Loaded LEC–CS NPs
3.2. Effects of PEG-2000 on the Stabilities of PCPs@LEC–CS
3.3. Transmission Electron Microscope Observation of the Micromorphology of NPs
3.4. Fourier Transform Infrared Spectroscopy Detection of PCPs@LEC–CS–PEG
3.5. The Thermal, pH and Storage Stability Analysis of PCPs@LEC–CS–PEG NPs
3.6. Detection of the Release Rate of PCPs from NPs in Simulated Gastric Digestion In Vitro
3.7. The In Vitro Effects of PCPs@LEC–CS–PEG NPs on Growth of NSCLC Cells
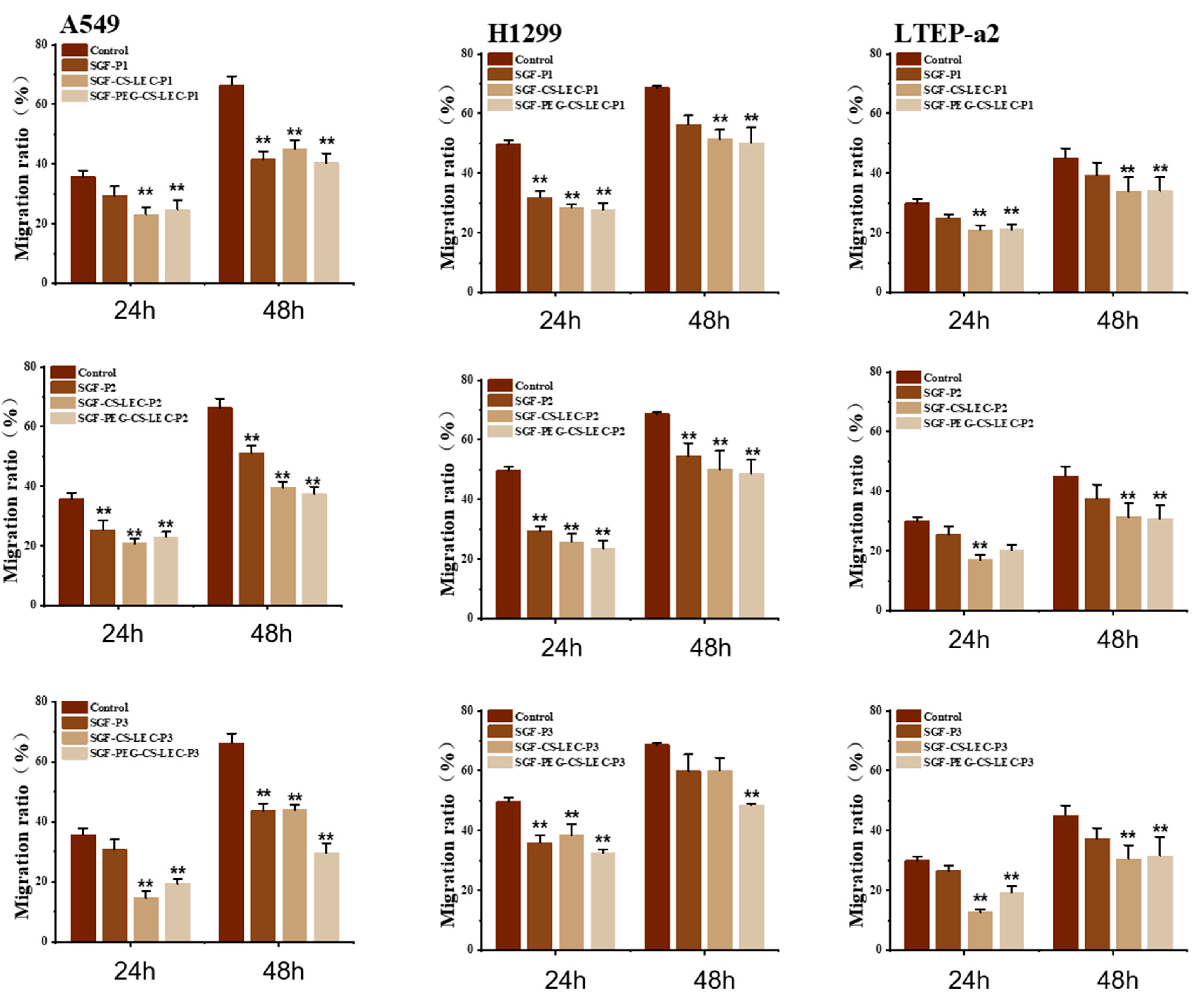
3.8. The In Vitro Effects of PCPs@LEC–CS–PEG NPs on the Migration of NSCLC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| PCPs | Phycocyanin-derived peptides |
| NPs | Nanoparticles |
| CS | Chitosan |
| LEC | Lecithin |
| PEG | Polyethylene glycol |
| FTIR | Fourier transform infrared |
| IBD | Inflammatory bowel disease |
| ACE | Angiotensin I converting enzyme |
| PC | Phycocyanin |
References
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a Super Functional Ingredient from Algae; Properties, Purification Characterization, and Applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and Bioactivities of Phycocyanin. Crit. Rev. Food Sci. Nutr. 2016, 57, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Chaowen, H.; Dongxuan, H.; Dongsheng, H.; Jianfeng, P.; Fan, Y.; Yahui, C.; Xiaohua, L. C-Phycocyanin Suppresses Cell Proliferation and Promotes Apoptosis by Regulating the AMPK Pathway in NCL-H292 Non-Small Cell Lung Cancer Cells. Folia Biol. 2022, 68, 16–24. [Google Scholar]
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Yan, Y.; Wu, T.; Zhang, J.; Wang, C. C-Phycocyanin Suppresses the In Vitro Proliferation and Migration of Non-Small-Cell Lung Cancer Cells Through Reduction of RIPK1/NF-κB Activity. Mar. Drugs 2019, 17, 362. [Google Scholar] [CrossRef]
- Soror, A.-F.S.; Ahmed, M.W.; Hassan, A.E.A.; Alharbi, M.; Alsubhi, N.H.; Al-Quwaie, D.A.; Alrefaei, G.I.; Binothman, N.; Aljadani, M.; Qahl, S.H.; et al. Evaluation of Green Silver Nanoparticles Fabricated by Spirulina Platensis Phycocyanin as Anticancer and Antimicrobial Agents. Life 2022, 12, 1493. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Li, Q.; Peng, W.; Zhang, Z.; Pei, X.; Sun, Z.; Ou, Y. A Phycocyanin Derived Eicosapeptide Attenuates Lung Fibrosis Development. Eur. J. Pharmacol. 2021, 908, 174356. [Google Scholar] [CrossRef]
- Wu, B.; Cheng, H.; Li, X.; Yang, Q.; Hao, S.; Wang, C.; Sun, B. Identification and Functional Analysis of Phycocyanin-Derived Bioactive Peptides with Non-Small Cell Lung Cancer Cell Inhibition. Algal Res. 2024, 79, 103467. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Wen, Y.; Linhardt, R.J.; Zong, M.-H.; Zou, Y.-X.; Wu, H. Targeted Delivery of Phycocyanin for the Prevention of Colon Cancer Using Electrospun Fibers. Food Funct. 2019, 10, 1816–1825. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, H.; Zhou, M.; Fu, P.; Zhao, W. Identification and Molecular Docking of Tyrosinase Inhibitory Peptides from Allophycocyanin in Spirulina Platensis. J. Sci. Food Agric. 2024, 104, 3648–3653. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef]
- Boutrou, R.; Jardin, J.; Blais, A.; Tomé, D.; Léonil, J. Glycosylations of Kappa-Casein-Derived Caseinomacropeptide Reduce Its Accessibility to Endo- but Not Exointestinal Brush Border Membrane Peptidases. J. Agric. Food Chem. 2008, 56, 8166–8173. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Craik, D.J. Thermal, Chemical, and Enzymatic Stability of the Cyclotide Kalata B1: The Importance of the Cyclic Cystine Knot. Biochemistry 2004, 43, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J. Acetylation of Peptides Inhibits Their Degradation by Rumen Micro-Organisms. Br. J. Nutr. 1992, 68, 365–372. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; McClements, D.J.; Taghizadeh, M.S.; Niazi, A.; Garcia-Vaquero, M. Strategies for Oral Delivery of Bioactive Peptides with Focus on Debittering and Masking. NPJ Sci. Food 2023, 7, 22. [Google Scholar]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential Oil-Loaded Lipid Nanoparticles for Wound Healing. Int. J. Nanomed. 2017, 13, 175–186. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.H.; Kwon, S. Enhanced Nasal Drug Delivery Efficiency by Increasing Mechanical Loading Using Hypergravity. Sci. Rep. 2018, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.-J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan Encapsulation Modulates the Effect of Capsaicin on the Tight Junctions of MDCK Cells. Sci. Rep. 2015, 5, 10048. [Google Scholar] [CrossRef]
- Bernocchi, B.; Carpentier, R.; Lantier, I.; Ducournau, C.; Dimier-Poisson, I.; Betbeder, D. Mechanisms Allowing Protein Delivery in Nasal Mucosa Using NPL Nanoparticles. J. Control. Release 2016, 232, 42–50. [Google Scholar] [CrossRef]
- Dartora, V.F.C.; Passos, J.S.; Osorio, B.; Hung, R.-C.; Nguyen, M.; Wang, A.; Panitch, A. Chitosan Hydrogels with MK2 Inhibitor Peptide-Loaded Nanoparticles to Treat Atopic Dermatitis. J. Control. Release 2023, 362, 591–605. [Google Scholar] [CrossRef]
- Xu, C.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The Immunostimulatory Effects of Hydroxypropyltrimethyl Ammonium Chloride Chitosan-Carboxymethyl Chitosan Nanoparticles. Int. J. Biol. Macromol. 2021, 181, 398–409. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wang, Q.; Liu, R.; Lu, J.; Lu, W.; Qin, S. Therapeutic Effect of Phycocyanin on Chronic Obstructive Pulmonary Disease in Mice. J. Adv. Res. 2024, 66, 285–301. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Qin, L.; Xu, X.; Wang, X.; Zhang, G.; Liu, Z.; Wang, B.; Li, B.; Chu, X.-M. The Peptide from C-Phycocyanin Alleviates Myocardial Ischemia-Reperfusion Injury by Suppressing Ferroptosis via Upregulating UCHL3. Free Radic. Biol. Med. 2025, 237, 160–175. [Google Scholar]
- Holsæter, A.M.; Wizgird, K.; Karlsen, I.; Hemmingsen, J.F.; Brandl, M.; Škalko-Basnet, N. How Docetaxel Entrapment, Vesicle Size, Zeta Potential and Stability Change with Liposome Composition—A Formulation Screening Study. Eur. J. Pharm. Sci. 2022, 177, 106267. [Google Scholar] [CrossRef]
- Federer, C.; Spleis, H.V.; Summonte, S.; Friedl, J.D.; Wibel, R.; Bernkop-Schnürch, A. Preparation and Evaluation of Charge Reversal Solid Lipid Nanoparticles. J. Pharm. Sci. 2022, 111, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized Chitosan for Cancer Nano Drug Delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Hayee, R.; Iqtedar, M.; Albekairi, N.A.; Alshammari, A.; Makhdoom, M.A.; Islam, M.; Ahmed, N.; Rasool, M.F.; Li, C.; Saeed, H. Levofloxacin Loaded Chitosan and Poly-Lactic-Co-Glycolic Acid Nano-Particles Against Resistant Bacteria: Synthesis, Characterization and Antibacterial Activity. J. Infect. Public Health 2024, 17, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ni, C.; Zhang, L. Preparation of Complex Nano-Particles Based on Alginic Acid/Poly [(2-Dimethylamino) Ethyl Methacrylate] and a Drug Vehicle for Doxorubicin Release Controlled by Ionic Strength. Eur. J. Pharm. Sci. 2012, 45, 43–49. [Google Scholar] [CrossRef]
- Izumi, K.; Ji, J.; Koiwai, K.; Kawano, R. Long-Term Stable Liposome Modified by PEG-Lipid in Natural Seawater. ACS Omega 2024, 9, 10958–10966. [Google Scholar] [PubMed]
- Aiello, G.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A.; Vistoli, G.; Lammi, C. Behavior of Three Hypocholesterolemic Peptides from Soy Protein in an Intestinal Model Based on Differentiated Caco-2 Cell. J. Funct. Foods 2018, 45, 363–370. [Google Scholar] [CrossRef]
- Salem, R.B.S.-B.; Ktari, N.; Bkhairia, I.; Nasri, R.; Mora, L.; Kallel, R.; Hamdi, S.; Jamoussi, K.; Boudaouara, T.; El-Feki, A.; et al. In Vitro and In Vivo Anti-Diabetic and Anti-Hyperlipidemic Effects of Protein Hydrolysates from Octopus Vulgaris in Alloxanic Rats. Food Res. Int. 2018, 106, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; O’Brien, N.; FitzGerald, R.J. Angiotensin Converting Enzyme and Dipeptidyl Peptidase-IV Inhibitory Activities of Transglutaminase Treated Sodium Caseinate Hydrolysates. Int. Dairy J. 2018, 78, 85–91. [Google Scholar] [CrossRef]
- Paz, S.M.; Rodriguez-Martin, N.M.; Villanueva, A.; Pedroche, J.; Cruz-Chamorro, I.; Millan, F.; Millan-Linares, M.C. Evaluation of Anti-Inflammatory and Atheroprotective Properties of Wheat Gluten Protein Hydrolysates in Primary Human Monocytes. Foods 2020, 9, 854. [Google Scholar] [CrossRef]
- Xu, F.; Yang, F.; Qiu, Y.; Wang, C.; Zou, Q.; Wang, L.; Li, X.; Jin, M.; Liu, K.; Zhang, S.; et al. The Alleviative Effect of C-Phycocyanin Peptides Against TNBS-Induced Inflammatory Bowel Disease in Zebrafish via the MAPK/Nrf2 Signaling Pathways. Fish Shellfish Immunol. 2024, 145, 109351. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Huang, C.-Y.; Shieh, T.-M.; Kao, C.; Chang, F.-K.; Huang, T.-C.; Ali, M.; Chang, H.-Y.; Hong, Y.-H.; Hsia, S.-M. Spirulina Phycocyanin Extract and Its Active Components Suppress Epithelial-Mesenchymal Transition Process in Endometrial Cancer via Targeting TGF-Beta1/SMAD4 Signaling Pathway. Biomed. Pharmacother. 2022, 152, 113219. [Google Scholar]
- Sun, X.; Liang, X.; Wang, Y.; Ma, P.; Xiong, W.; Qian, S.; Cui, Y.; Zhang, H.; Chen, X.; Tian, F.; et al. A Tumor Microenvironment-Activatable Nanoplatform with Phycocyanin-Assisted in-Situ Nanoagent Generation for Synergistic Treatment of Colorectal Cancer. Biomaterials 2023, 301, 122263. [Google Scholar] [CrossRef]
- Sanders, M.R.; Clifton, L.A.; Frazier, R.A.; Green, R.J. Role of Lipid Composition on the Interaction Between a Tryptophan-Rich Protein and Model Bacterial Membranes. Langmuir 2016, 32, 2050–2057. [Google Scholar] [CrossRef]
- Gianfranceschi, G.L.; Gianfranceschi, G.; Quassinti, L.; Bramucci, M. Biochemical Requirements of Bioactive Peptides for Nutraceutical Efficacy. J. Funct. Foods 2018, 47, 252–263. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of Bioactive Peptides: A Strategy to Improve the Stability, Protect the Nutraceutical Bioactivity and Support Their Food Applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, B.; Yin, Q.-F.; Wang, Y.-J. Carboxymethyl Chitosan Nanoparticles Coupled with CD59-Specific Ligand Peptide for Targeted Delivery of C-Phycocyanin to HeLa Cells. Tumor Biol. 2017, 39, 1010428317692267. [Google Scholar]
- Sun, N.; Liu, Y.; Liu, K.; Wang, S.; Liu, Q.; Lin, S. Gastrointestinal Fate of Food Allergens and Its Relationship with Allergenicity. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3376–3404. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Toldrá, F. Stability of ACE Inhibitory Ham Peptides Against Heat Treatment and In Vitro Digestion. Food Chem. 2014, 161, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, Q.; Liu, Y.; Li, F.; Yang, Q.; Wang, J.; Wang, C. Insulin Receptor Substrate 1 Is Involved in the Phycocyanin-Mediated Antineoplastic Function of Non-Small Cell Lung Cancer Cells. Molecules 2021, 26, 4711. [Google Scholar]
- Hao, S.; Li, S.; Wang, J.; Yan, Y.; Ai, X.; Zhang, J.; Ren, Y.; Wu, T.; Liu, L.; Wang, C. Phycocyanin Exerts Anti-Proliferative Effects Through Down-Regulating TIRAP/NF-κB Activity in Human Non-Small Cell Lung Cancer Cells. Cells 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Jia, B.; Li, X.; Li, Y.; Wu, B.; Yang, Q.; Wang, C.; Sun, B.; Hao, S. Preparation, Stability and In Vitro Antineoplastic Function of Lecithin–Chitosan–Polyethylene Glycol Nanoparticles Loaded with Bioactive Peptides Derived from Phycocyanin. Foods 2025, 14, 3487. https://doi.org/10.3390/foods14203487
Cheng H, Jia B, Li X, Li Y, Wu B, Yang Q, Wang C, Sun B, Hao S. Preparation, Stability and In Vitro Antineoplastic Function of Lecithin–Chitosan–Polyethylene Glycol Nanoparticles Loaded with Bioactive Peptides Derived from Phycocyanin. Foods. 2025; 14(20):3487. https://doi.org/10.3390/foods14203487
Chicago/Turabian StyleCheng, Haozhe, Binyang Jia, Xinran Li, Yali Li, Boxiong Wu, Qi Yang, Chengtao Wang, Baoguo Sun, and Shuai Hao. 2025. "Preparation, Stability and In Vitro Antineoplastic Function of Lecithin–Chitosan–Polyethylene Glycol Nanoparticles Loaded with Bioactive Peptides Derived from Phycocyanin" Foods 14, no. 20: 3487. https://doi.org/10.3390/foods14203487
APA StyleCheng, H., Jia, B., Li, X., Li, Y., Wu, B., Yang, Q., Wang, C., Sun, B., & Hao, S. (2025). Preparation, Stability and In Vitro Antineoplastic Function of Lecithin–Chitosan–Polyethylene Glycol Nanoparticles Loaded with Bioactive Peptides Derived from Phycocyanin. Foods, 14(20), 3487. https://doi.org/10.3390/foods14203487






