Acute Toxicity and Neuroprotective Effect of “RJ6601”, a Newly Formulated Instant Soup, in Geriatric Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of “RJ6601” Instant Soup
2.2. Animals and Treatment
2.2.1. Acute Toxicity Test
2.2.2. Study Design for an Evaluation of the Neuroprotective Effect
2.3. Biochemical Determinations
2.3.1. Oxidative Stress Marker Assessment
2.3.2. Assessment of the Cholinergic and Monoaminergic Functions
2.4. Histological Procedure and Nissl Staining
2.5. Statistical Analysis
3. Results
3.1. Acute Toxicity of the Novel Instant Soup RJ6601
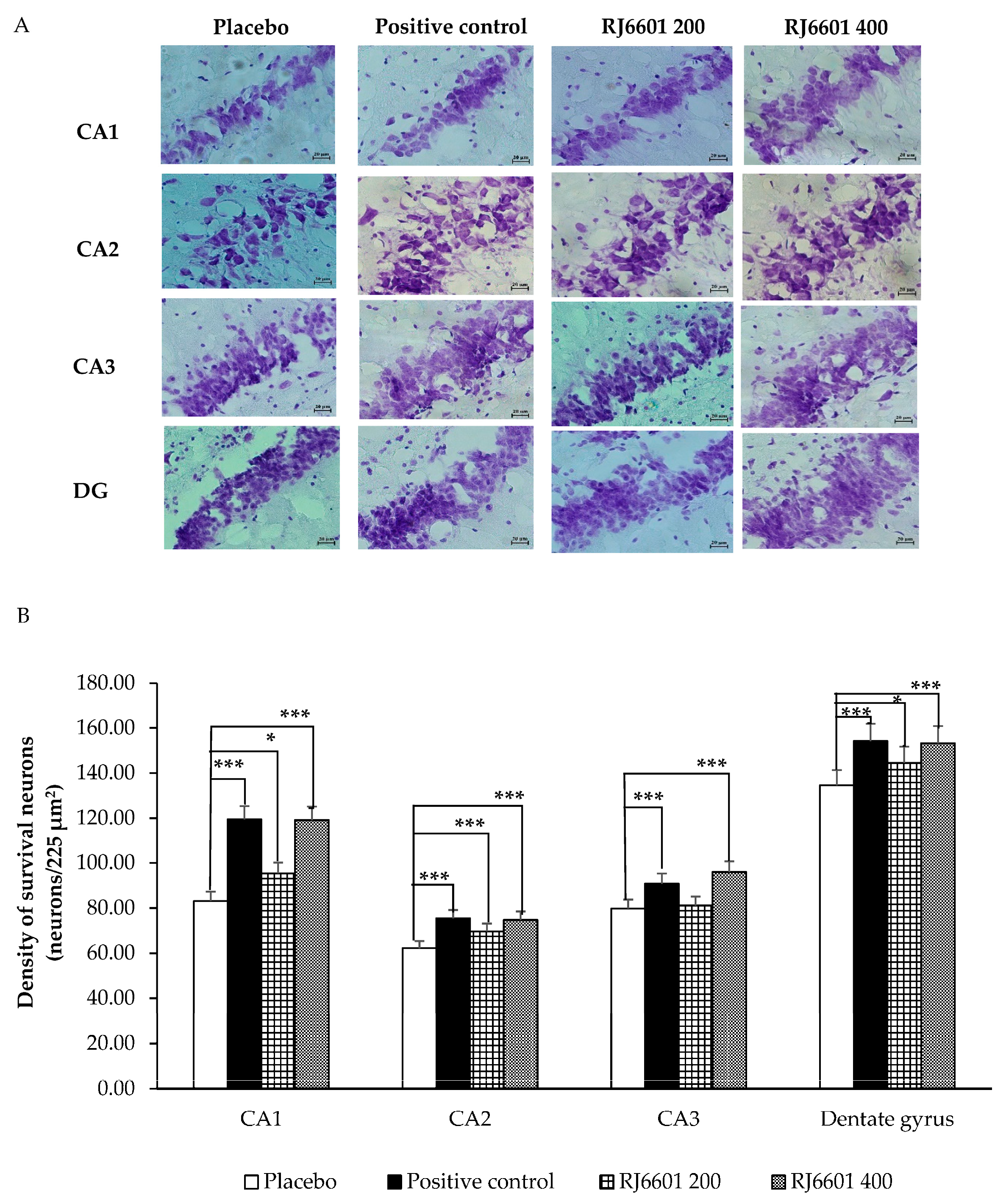
3.2. Changes in Hippocampal Neuron Density
3.3. Biochemical Changes
3.3.1. Neurotransmitter Changes
3.3.2. Oxidative Stress Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, J.Y.; Lee, E.; Jung, H.-W.; Jang, I.-Y. Geriatrics fact sheet in Korea 20. Ann. Geriatr. Med. Res. 2021, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.-J. Normal Aging Induces changes in the brain and neurodegeneration progress: Review of the structural, biochemical, metabolic, cellular, and molecular changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Liu, J.-K. Antiaging Agents: Safe Interventions to Slow Aging and Healthy Life Span Extension. Nat. Prod. Bioprospecting 2022, 12, 18. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. BioMed Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Mebarek, K.; Bouanane, S.; Merzouk, H.; Ahmed, F.Z.B.; Bensalah, M.; Karaouzene, N.S.; Nacer, W.; Reguig, S.B. Effect of dietary fiber on oxidative status and lipid profile in aged diabetic rats. Arch. Cardiovasc. Dis. Suppl. 2019, 11, e359–e360. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, A.; Xie, K.; Yu, Y. Dietary supplementation with high fiber alleviates oxidative stress and inflammatory responses caused by severe sepsis in mice without altering microbiome diversity. Front. Physiol. 2018, 9, 1929. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Duan, W.; Maswood, N. How does the brain control lifespan? Ageing Res. Rev. 2002, 1, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ourry, V.; Gonneaud, J.; Landeau, B.; Moulinet, I.; Touron, E.; Dautricourt, S.; Le Du, G.; Mézenge, F.; André, C.; Bejanin, A.; et al. Association of quality of life with structural, functional and molecular brain imaging in community-dwelling Older Adults. NeuroImage 2021, 231, 117819. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. Brain neurotransmitters in aging and dementia: Similar changes across diagnostic dementia groups. Gerontology 1987, 33, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Terribilli, D.; Schaufelberger, M.S.; Duran, F.L.S.; Zanetti, M.V.; Curiati, P.K.; Menezes, P.R.; Scazufca, M.; Amaro, E.; Leite, C.C.; Busatto, G.F. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol. Aging 2011, 32, 354–368. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, A.; Cohen, R.A.; Porges, E.C.; Nissim, N.R.; Woods, A.J. Cognitive aging and the hippocampus in older adults. Front. Aging Neurosci. 2016, 8, 298. [Google Scholar] [CrossRef]
- Saraçlı, Ö.; Akca, A.S.D.; Atasoy, N.; Önder, Ö.; Şenormancı, Ö.; Kaygisız, İ.; Atik, L. The relationship between quality of life and cognitive functions, anxiety and depression among hospitalized elderly patients. Clin. Psychopharmacol. Neurosci. 2015, 13, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.A.; Walsh, E.I.; Shaw, M.E.; Abhayaratna, W.P.; Anstey, K.J.; Sachdev, P.S.; Cherbuin, N. Longitudinal trajectories of hippocampal volume in middle to older age community dwelling individuals. Neurobiol. Aging 2021, 97, 97–105. [Google Scholar] [CrossRef]
- Chen, Z.L.; Strickland, S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 1997, 91, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult hippocampal neurogenesis in aging and alzheimer’s disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Sabandal, P.R.; Saldes, E.B.; Han, K.-A. Acetylcholine deficit causes dysfunctional inhibitory control in an aging-dependent manner. Sci. Rep. 2022, 12, 20903. [Google Scholar] [CrossRef]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in acute toxicity testing: Strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef]
- Chaisanam, R.; Wattanathorn, J.; Thukham-Mee, W.; Piyavhatkul, N.; Paholpak, P. Anxiolytic, Antidepression, and Memory-Enhancing Effects of the Novel Instant Soup RJ6601 in the Middle-Aged of Female Rats. Foods. 2024, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Engel, K.H.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; et al. Guidance on the scientific requirements for a notification and application for authorisation of traditional foods from third countries in the context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8966. [Google Scholar]
- Organisation for Economic Co-Operation and Development Test No. 420: Acute Oral Toxicity: Fixed Dose Procedure. Available online: https://www.oecd.org/en/publications/test-no-420-acute-oral-toxicity-fixed-dose-procedure_9789264070943-en.html (accessed on 18 November 2024).
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Phytosome loading the combined extract of mulberry fruit and ginger protects against cerebral ischemia in metabolic syndrome rats. Oxid. Med. Cell. Longev. 2020, 2020, 5305437. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Palachai, N.; Thukham-Mee, W.; Muchimapura, S. Memory-enhancing effect of a phytosome containing the combined extract of mulberry fruit and ginger in an animal model of ischemic stroke with metabolic syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 3096826. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Janke, K.L.; Cominski, T.P.; Kuzhikandathil, E.V.; Servatius, R.J.; Pang, K.C.H. Investigating the role of hippocampal BDNF in anxiety vulnerability using classical eyeblink conditioning. Front. Psychiatry 2015, 6, 106. [Google Scholar] [CrossRef]
- Lee, A.L.; Ogle, W.O.; Sapolsky, R.M. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002, 4, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Thukham-Mee, W.; Wattanathorn, J.; Paholpak, P.; Ransikachi, P.; Piyavhatkul, N. The Positive Modulation Effect of a 6-Week Consumption of an Anthocyanin-Rich Mulberry Milk on Working Memory, Cholinergic, and Monoaminergic Functions in Healthy Working-Age Adults. Oxid. Med. Cell. Longev. 2021, 2021, 5520059. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press Inc.: London, UK, 2013. [Google Scholar]
- Sriwat, J.; Sarntikasem, A.; Lomtong, T. General Food Safety Regulations in Thailand. Available online: https://kku.world/05omxp (accessed on 24 August 2024).
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Guidance on the scientific requirements for an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. Eur. Food Saf. Auth. 2024, 22, e8961. [Google Scholar]
- Pieters, M.N.; Kramer, H.J.; Slob, W. A no-observed-adverse-effect level of 1000 mg/kg in a 28-day repeated-dose study as a limit value for acute toxicity testing. Int. J. Toxicol. 1998, 17, 23–33. [Google Scholar] [CrossRef]
- Langa, K.M. Cognitive Aging, dementia, and the future of an aging population. In Future Directions for the Demography of Aging: Proceedings of a Workshop; Majmundar, M.K., Hayward, M.D., Eds.; National Academies Press: Washington, DC, USA, 2018; Available online: https://kku.world/sjdma1 (accessed on 24 January 2024).
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef]
- Levone, B.R.; Cryan, J.F.; O’Leary, O.F. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress 2015, 1, 147–155. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxid. Basel Switz. 2023, 12, 517. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Reiter, R.J. Antioxidant actions of melatonin. Adv. Pharmacol. 1997, 38, 103–117. [Google Scholar]
- Yenilmez, A.; Isikli, B.; Aral, E.; Degirmenci, I.; Sutken, E.; Baycu, C. Antioxidant effects of melatonin and coenzyme Q10 on oxidative damage caused by single-dose ochratoxin A in rat kidney. Chin. J. Physiol. 2010, 53, 310–317. [Google Scholar] [CrossRef]
- Rieckmann, A.; Nyberg, L. Cognitive Aging: The role of neurotransmitter systems. In The Cambridge Handbook of Cognitive Aging: A Life Courseperspective; Thomas, A.K., Gutchess, A., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 82–100. ISBN 978-1-108-42834-7. [Google Scholar]
- Trang, A.; Khandhar, P.B. Physiology, acetylcholinesterase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://kku.world/yq4zec (accessed on 10 January 2024).
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [PubMed]

| Direct Observation Parameters | 30 min | 2 h | 4 h | 6 h | Day 2–14 |
|---|---|---|---|---|---|
| Alertness | Normal | Normal | Normal | Normal | Normal |
| Grooming | Absent | Absent | Absent | Absent | Absent |
| Hyperactivity | Absent | Absent | Absent | Absent | Absent |
| Tremors | Absent | Absent | Absent | Absent | Absent |
| Convulsion | Absent | Absent | Absent | Absent | Absent |
| Salivation | Normal | Normal | Normal | Normal | Normal |
| Fur | Normal | Normal | Normal | Normal | Normal |
| Eyes | Normal | Normal | Normal | Normal | Normal |
| Diarrhea | Absent | Absent | Absent | Absent | Absent |
| Lethargy | Absent | Absent | Absent | Absent | Absent |
| Sleep and coma | Normal | Normal | Normal | Normal | Normal |
| Injury | Absent | Absent | Absent | Absent | Absent |
| Pain response | Absent | Absent | Absent | Absent | Absent |
| Signs of illness | Absent | Absent | Absent | Absent | Absent |
| Hematological Parameters | Female Wistar Rats | ||
|---|---|---|---|
| Reference Value | Control (Mean ± SEM) | RJ6601 (2000 mg/kg BW) (Mean ± SEM) | |
| Red blood cells (106/μL) | 7.16–9.24 (106/μL) | 5.84 ± 1.37 | 7.98 ± 0.25 |
| Hemoglobin (g/dL) | 13.7–17.2 (g/dL) | 14.57 ± 0.72 | 15.13 ± 0.43 |
| Hematocrit (%) | 38.5–49.2 (%) | 45.13 ± 1.49 | 48.40 ± 1.56 |
| White blood cells (103/μL) | 0.96–7.88 (103/μL) | 3.02 ± 0.53 | 2.00 ± 0.20 |
| Platelet count (103/μL) | 599–1144 (103/μL) | 748.33 ± 12.77 | 752.33 ± 15.41 |
| Neutrophils (%) | 20.1–44.5% | 25.70 ± 1.02 | 29.33 ± 1.14 |
| Lymphocytes (%) | 43.7–70.9% | 41.43 ± 5.24 | 68.13 ± 1.19 |
| Monocytes (%) | 3.4–9.8% | 1.60 ± 0.35 | 1.37 ± 0.33 |
| Eosinophil (%) | 0.7–9.2% | 0.80 ± 0.26 | 1.00 ± 0.23 |
| Basophil (%) | 0.0–22.6% | 0.27 ± 0.07 | 0.25 ± 0.00 |
| Mean corpuscular volume (fL) | 60–100 (fL) | 67.27 ± 2.95 | 60.63 ± 0.33 |
| Mean corpuscular Hemoglobin (pg) | 18–26 (pg) | 19.13 ± 0.27 | 18.97 ± 0.33 |
| Mean corpuscular Hemoglobin concentration (g/dL) | 32–36 (g/dL) | 31.23 ± 0.59 | 31.27 ± 0.17 |
| Red blood cell distribution width (%) | 12.2 to 16.1 (%) | 5.84 ± 1.37 | 7.98 ± 0.25 |
| Female Wistar Rats | |||
|---|---|---|---|
| Blood Biochemical Parameters | Reference Value | Control (Mean ± SEM) | RJ6601 (2000 mg/kg BW) (Mean ± SEM) |
| BUN (mg/dL) | 7.0–22.0 | 20.67 ± 0.91 | 19.47 ± 0.75 |
| Creatinine (mg/dL) | 0.50–1.50 | 0.64 ± 0.03 | 0.46 ± 0.05 |
| HDL-C (mg/dL) | 37–85 | 58.33 ± 2.85 | 57.00 ± 0.08 |
| LDL-C (mg/dL) | 10–110 | 5.00 ± 2.00 | 5.33 ± 2.33 |
| Albumin (g/dL) | 3.8–5.4 | 4.50 ± 0.49 | 4.27 ± 0.19 |
| Total bilirubin (mg/dL) | 0.1–1.2 | 0.10 ± 0.00 | 0.10 ± 0.06 |
| Estradiol (pg/dL) | 30–400 | 24.19 ± 3.64 | 23.10 ± 5.49 |
| ALT (U/L) | 7–56 | 23.00 ± 3.51 | 39.00 ± 0.07 |
| AST (U/L) | 10–36 | 63.67 ± 2.61 | 69.00 ± 5.42 |
| LDH (U/L) | 140–280 | 684.67 ± 8.03 | 687.00 ± 2.58 |
| Uric acid (mg/dL) | 2.7–7.0 | 1.07 ± 0.12 | 10.3 ± 0.13 |
| Sodium (mmol/L) | 135–148 | 144.00 ± 0.85 | 142.67 ± 0.88 |
| Potassium (mmol/L) | 3.5–5.0 | 4.53 ± 0.07 | 4.47 ± 0.18 |
| Bicarbonate (mEq/L) | 22.0–26.0 | 22.63 ± 0.67 | 24.40 ± 0.06 |
| Chloride (mEq/L) | 99–111 | 97.67 ± 0.88 | 96.33 ± 0.88 |
| Cholesterol (mg/dL) | 127–262 | 78.67 ± 0.88 | 75.67 ± 2.32 |
| Protein (g/dL) | 6.0–8.3 | 6.10 ± 0.44 | 5.97 ± 0.22 |
| Alkaline phosphate (U/L) | 75–250 | 12.33 ± 2.85 | 11.00 ± 1.15 |
| Triglyceride (mg/dL) | 26–145 | 129.67 ± 4.26 | 125.67 ± 0.67 |
| T3 (ng/dL) | 80–200 | 86.43 ± 0.97 | 68.64 ± 2.61 |
| T4 (ng/dL) | 4.5–12.5 | 1.98 ± 0.12 | 1.73 ± 0.30 |
| Relative Organ Weight (%) | Female Wistar Rats | ||
|---|---|---|---|
| Control (Mean ± SEM) | RJ6601 (2000 mg/kg BW) (Mean ± SEM) | ||
| Brain | 2.15 ± 0.08 | 2.18 ± 0.02 | |
| Salivary gland | Rt. | 0.07 ± 0.00 | 0.06 ± 0.00 |
| Lt. | 0.08 ± 0.00 | 0.08 ± 0.00 | |
| Thymus gland | 0.30 ± 0.01 | 0.35 ± 0.07 | |
| Lung | 1.41 ± 0.02 | 1.43 ± 0.08 | |
| Heart | 0.86 ± 0.3 | 0.85 ± 0.01 | |
| Liver | 7.75 ± 0.09 | 7.89 ± 0.02 | |
| Spleen | 0.65 ± 0.01 | 0.68 ± 0.04 | |
| Pancreas | 1.02 ± 0.07 | 0.98 ± 0.06 | |
| Kidney | Rt. | 1.13 ± 0.02 | 1.13 ± 0.04 |
| Lt. | 1.11 ± 0.04 | 1.13 ± 0.05 | |
| Adrenal gland | Rt. | 0.08 ± 0.00 | 0.05 ± 0.00 |
| Lt. | 0.03 ± 0.00 | 0.04 ± 0.00 | |
| Stomach | 1.45 ± 0.13 | 1.45 ± 1.14 | |
| Intestine | 4.02 ± 0.15 | 4.00 ± 0.12 | |
| Bladder | 0.17 ± 0.00 | 0.15 ± 0.07 | |
| Ovary | Rt. | 0.10 ± 0.00 | 0.10 ± 0.00 |
| Lt. | 0.10 ± 0.00 | 0.11 ± 0.00 | |
| Relative Organ Weight (%) | Female Wistar Rats | |
|---|---|---|
| Control (Mean ± SEM) | RJ6601 (2000 mg/kg BW) (Mean ± SEM) | |
| Body weight (g) | 404.14 ± 3.02 | 399.18 ± 5.59 |
| Food intake (g) | 11.48 ± 0.23 | 15.10 ± 0.32 |
| Water intake (mL) | 23.63 ± 0.79 | 27.13 ± 0.21 |
| Hippocampus | ||||
|---|---|---|---|---|
| Groups | AChE Activity | MAO Activity | MAO-A Activity | MAO-B Activity |
| (nmol/mg. Protein) (Mean ± SEM) | (µmol/mg. Protein) (Mean ± SEM) | (µmol/mg. Protein) (Mean ± SEM) | (µmol/mg. Protein) (Mean ± SEM) | |
| Placebo | 1.41 ± 0.08 | 0.22 ± 0.01 | 0.23 ± 0.05 | 0.23 ± 0.04 |
| Positive control | 1.09 ± 0.06 ** | 0.14 ± 0.02 ** | 0.17 ± 0.01 | 0.26 ± 0.02 |
| RJ6601 200 | 1.08 ± 0.04 ** | 0.09 ± 0.03 *** | 0.07 ± 0.03 *** | 0.11 ± 0.02 ** |
| RJ6601 400 | 0.96 ± 0.07 *** | 0.06 ± 0.01 *** | 0.05 ± 0.02 *** | 0.10 ± 0.01 ** |
| Groups | Hippocampus | |||
|---|---|---|---|---|
| MDA Levels | SOD Activity | CAT Activity | GSH-Px Activity | |
| (ng/mg Protein) (Mean ± SEM) | (Units/mg Protein) (Mean ± SEM) | (Units/mg Protein) (Mean ± SEM) | (Units/mg Protein) (Mean ± SEM) | |
| Placebo | 1.30 ± 0.03 | 26.92 ± 0.21 | 3.40 ± 0.20 | 3.76 ± 0.20 |
| Positive control | 0.41 ± 0.05 *** | 41.75 ± 1.65 *** | 5.92 ± 0.02 *** | 3.85 ± 0.02 |
| RJ6601 200 | 0.61 ± 0.04 *** | 53.62 ± 3.38 *** | 6.22 ± 0.40 *** | 7.17 ± 0.40 *** |
| RJ6601 400 | 0.53 ± 0.03 *** | 59.32 ± 2.32 *** | 7.10 ± 0.44 *** | 8.27 ± 0.44 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaisanam, R.; Wattanathorn, J. Acute Toxicity and Neuroprotective Effect of “RJ6601”, a Newly Formulated Instant Soup, in Geriatric Rats. Foods 2025, 14, 277. https://doi.org/10.3390/foods14020277
Chaisanam R, Wattanathorn J. Acute Toxicity and Neuroprotective Effect of “RJ6601”, a Newly Formulated Instant Soup, in Geriatric Rats. Foods. 2025; 14(2):277. https://doi.org/10.3390/foods14020277
Chicago/Turabian StyleChaisanam, Rujikarn, and Jintanaporn Wattanathorn. 2025. "Acute Toxicity and Neuroprotective Effect of “RJ6601”, a Newly Formulated Instant Soup, in Geriatric Rats" Foods 14, no. 2: 277. https://doi.org/10.3390/foods14020277
APA StyleChaisanam, R., & Wattanathorn, J. (2025). Acute Toxicity and Neuroprotective Effect of “RJ6601”, a Newly Formulated Instant Soup, in Geriatric Rats. Foods, 14(2), 277. https://doi.org/10.3390/foods14020277







