An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. UCNPs Synthesis and Surface Modifications
2.4. Synthesis of Amine-Functionalized Fe3O4 Magnetic Nanoparticles
2.5. Synthesis of Aptamer and cDNA-Conjugated Nanoparticles
2.6. Analytical Procedure
2.7. Method Specificity
2.8. Preparation and Detection of Authentic Samples
3. Results and Discussion
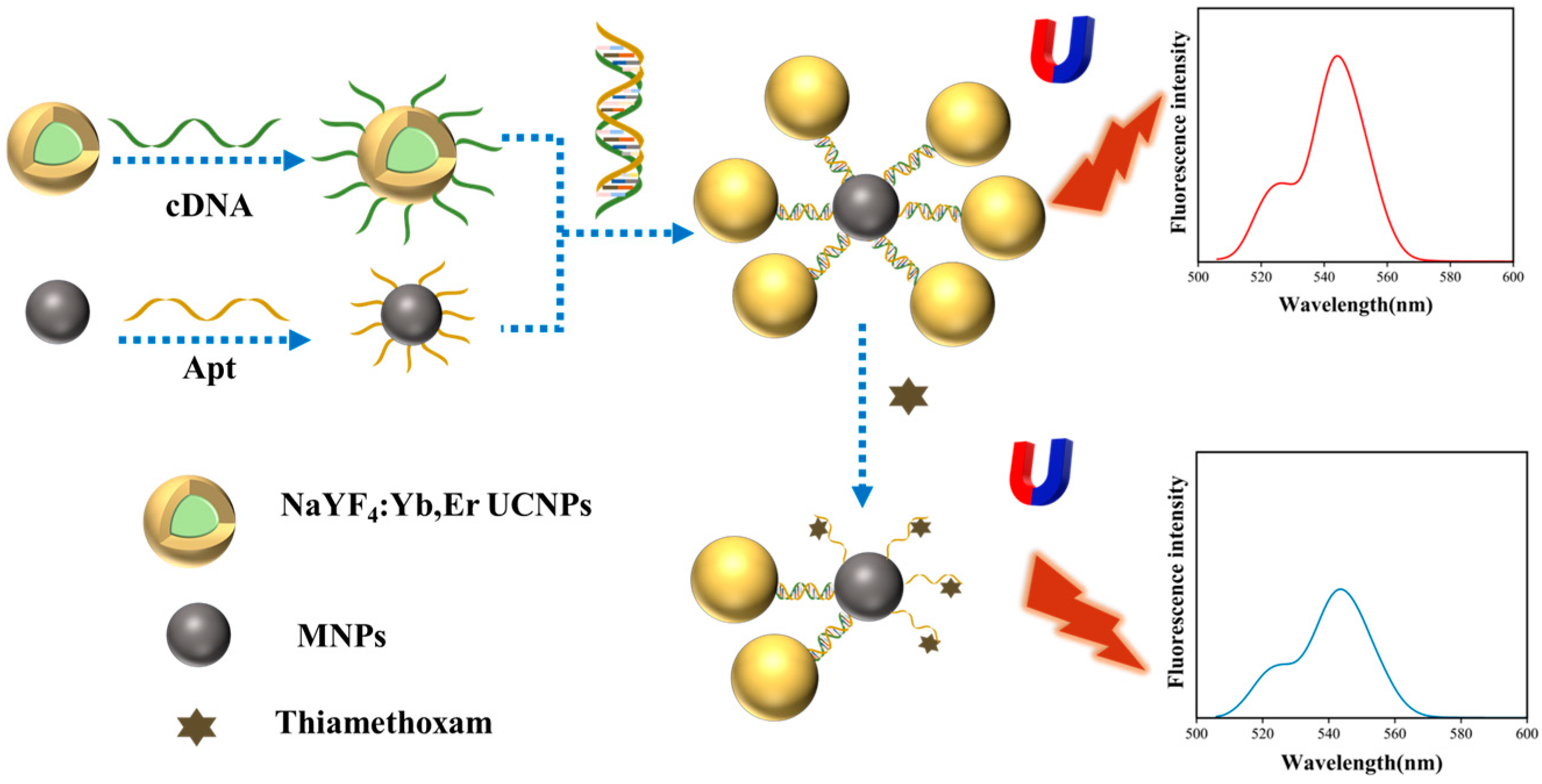
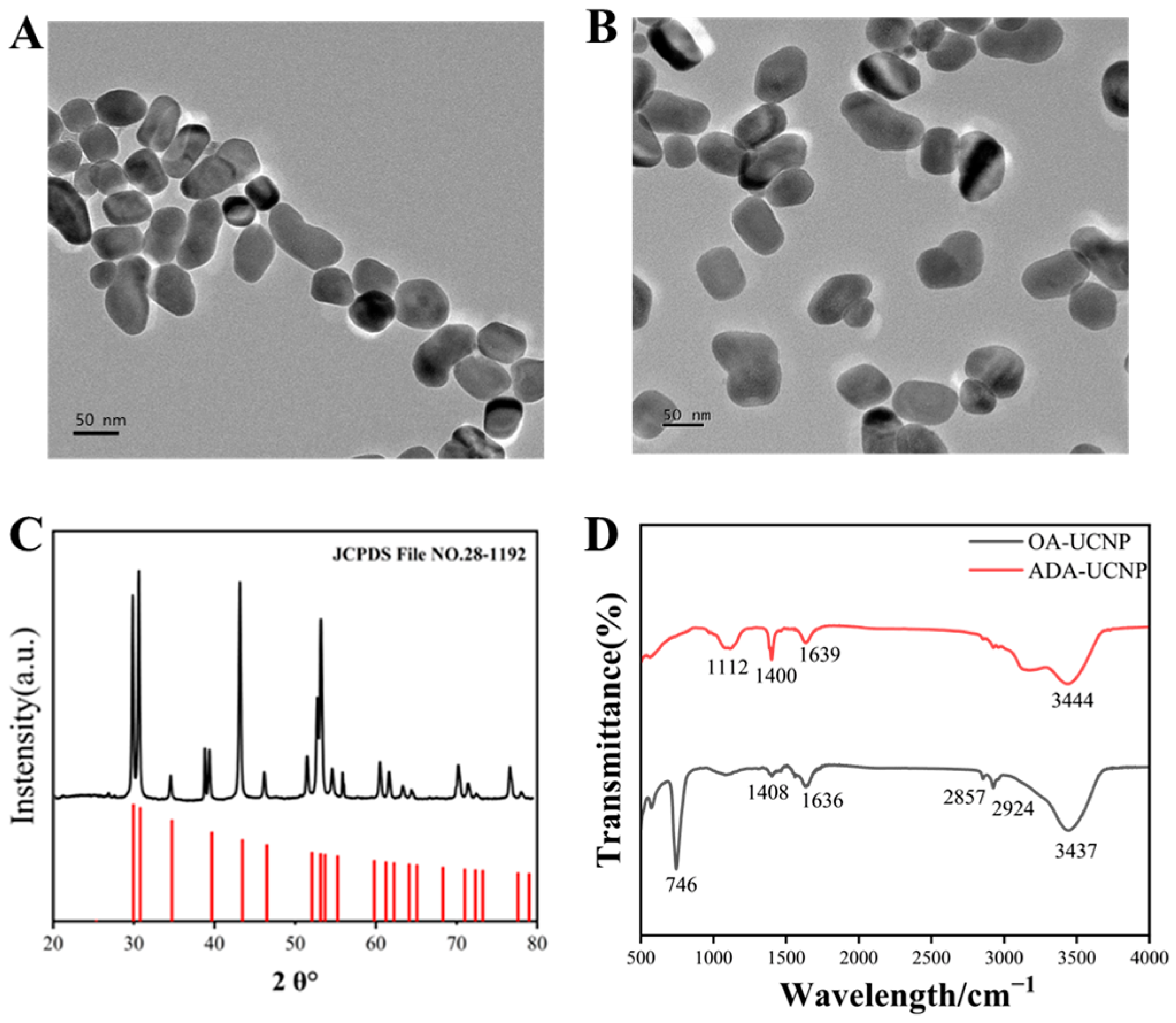
3.1. Characterization of MNPs@UCNPs Fluorescence Biosensor
3.2. Optimization of Detection Conditions
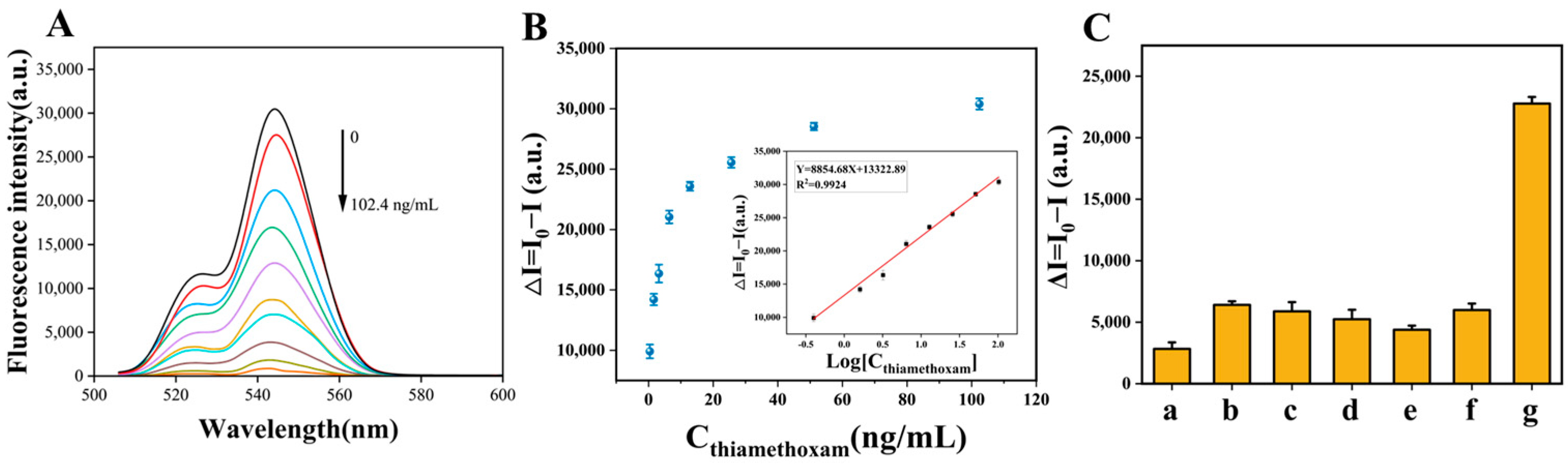
3.3. Analytical Performance of MNPs@UCNPs Fluorescence Biosensor
3.4. Specificity Analysis
3.5. Determination of Thiamethoxam in Authentic Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.H.; Ji, P.X.; Chen, Q.; Wu, M. Advances in adsorption and degradation mechanism on neonicotinoids mediated by biochar in soil. Huanjing Huaxue-Environ. Chem. 2023, 42, 4304–4315. [Google Scholar]
- Akter, S.; Hulugalle, N.R.; Jasonsmith, J.; Strong, C.L. Changes in soil microbial communities after exposure to neonicotinoids: A systematic review. Environ. Microbiol. Rep. 2023, 15, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Strolihova, A.; Velisek, J.; Stara, A. Selected neonicotinoids and associated risk for aquatic organisms. Vet. Med. 2023, 68, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Wang, X.H.; Yang, Y.Q.; Ares, I.; Martinez, M.; Lopez-Torres, B.; Martinez-Larranaga, M.-R.; Wang, X.; Anadon, A.; Martinez, M.-A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Neupane, S.; Gill, T.A.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Comparative transcriptome analysis of thiamethoxam susceptible and resistant Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), using RNA-sequencing. Insect Sci. 2021, 28, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yang, D.J.; Fang, H.J.; Han, M.H.; Tang, C.X.; Wu, J.G.; Chen, Y.; Jiang, Q.Y. Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: A biomonitoring-based study. Environ. Int. 2020, 143, 105918. [Google Scholar] [CrossRef]
- Tooker, J.F.; Pearsons, K.A. Newer characters, same story: Neonicotinoid insecticides disrupt food webs through direct and indirect effects. Curr. Opin. Insect Sci. 2021, 46, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Demortain, D. The science behind the ban: The outstanding impact of ecotoxicological research on the regulation of neonicotinoids. Curr. Opin. Insect Sci. 2021, 46, 78–82. [Google Scholar] [CrossRef]
- Ma, J.; Hao, Y.; Guo, L.; Li, K.; Zhang, J.; Xu, W.; Gong, S. Advances in the contamination and control of neonicotinoid insecticides in food. J. Food Saf. Qual. Insp. 2022, 13, 278–286. [Google Scholar]
- Ma, Y.; Mao, Y.P.; Liu, F.; Song, Y.Q.; Chai, L.Q.; Wang, X.; Yuan, N.; Ma, M.Y. Reproductive toxic effects of neonicotinoid insecticides on humans and mammals. J. Shenyang Med. Coll. 2022, 24, 191–195. [Google Scholar]
- Jameel, M.; Jamal, K.; Alam, M.F.; Ameen, F.; Younus, H.; Siddique, H.R. Interaction of thiamethoxam with DNA: Hazardous effect on biochemical and biological parameters of the exposed organism. Chemosphere 2020, 254, 126875. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Li, X.Y.; Jiang, S.X.; Han, J.J.; Wu, J.X.; Yan, M.L.; Yao, Z.L. Enantioselective Behaviors of Chiral Pesticides and Enantiomeric Signatures in Foods and the Environment. J. Agric. Food Chem. 2023, 71, 12372–12389. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Cabrera, J.F.; Montero, L.; Meckelmann, S.W.; Uteschil, F.; Schmitz, O.J. Review on atmospheric pressure ionization sources for gas chromatography-mass spectrometry. Part I: Current ion source developments and improvements in ionization strategies. Anal. Chim. Acta 2023, 1238, 340353. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.Z.; Zhang, Y.C.; Huang, B.J.; Han, L. Selective inhibition toward dual enzyme-like activities of iridium nanozymes for a specific colorimetric assay of malathion without enzymes. J. Agric. Food Chem. 2022, 70, 3898–3906. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Feng, H.W.; Liu, S.; Liu, C.; Zhao, P.Y.; Zhang, M.; Zhang, L.; Zhao, J.; Li, J.Z.; Yu, X.M.; et al. The preparation of polyclonal antibody against chlordimeform and establishment of detection by indirect competitive ELISA. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2022, 57, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yan, K.T.; Wang, L.L.; Chen, P.C.; Han, Y.F.; Lan, Y.B. Research progress of pesticide residue detection based on fluorescence spectrum analysis. Spectrosc. Spectr. Anal. 2021, 41, 2364–2371. [Google Scholar]
- Yang, J.; Chen, S.W.; Zhang, B.; Tu, Q.; Wang, J.; Yuan, M.S. Non-biological fluorescent chemosensors for pesticides detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef] [PubMed]
- Nesakumar, N.; Srinivasan, S.; Alwarappan, S. Graphene quantum dots: Synthesis, properties, and applications to the development of optical and electrochemical sensors for chemical sensing. Microchim Acta 2022, 189, 258–294. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. TrAC Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Jia, Z.X.; Shi, C.; Yang, X.T.; Zhang, J.R.; Sun, X.; Guo, Y.M.; Ying, X. QD-based fluorescent nanosensors: Production methods, optoelectronic properties, and recent food applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4644–4669. [Google Scholar] [CrossRef]
- Lin, X.F.; Yu, Q.R.; Yang, W.; He, C.X.; Zhou, Y.; Duan, N.; Wu, S.J. Double-enzymes-mediated fluorescent assay for sensitive determination of organophosphorus pesticides based on the quenching of upconversion nanoparticles by Fe3+. Food Chem. 2021, 345, 128809. [Google Scholar] [CrossRef]
- Feng, W.; Han, C.M.; Li, F.Y. Upconversion-nanophosphor-based functional Nanocomposites. Adv. Mater. 2013, 25, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Wang, A.C.; Hassan, M.M.; Ouyang, Q.; Li, H.H.; Chen, Q.S. A highly sensitive upconversion nanoparticles-WS2 nanosheet sensing platform for Escherichia coli detection. Sens. Actuators B-Chem. 2020, 320, 128434. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Q.Q.; Yan, L.; Zhang, Q.Y. Controlling upconversion through interfacial energy transfer (IET): Fundamentals and applications. J. Rare Earths 2020, 38, 474–482. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.W.; Li, H.H.; Hu, Y.Q.; Chen, Q.S. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yi, J.Q.; Li, X.S.; He, F.; Niu, N.; Chen, L.G. A comprehensive review on upconversion nanomaterials-based fluorescent sensor for environment, biology, food and medicine applications. Biosensors 2022, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, N.N.; Li, Z.H. Recent advances in chromophore-assembled upconversion nanoprobes for chemo/biosensing. Trac-Trends Anal. Chem. 2022, 151, 116602. [Google Scholar] [CrossRef]
- Liang, G.; Song, L.; Gao, Y.; Wu, K.; Guo, R.; Chen, R.; Zhen, J.; Pan, L. Aptamer Sensors for the Detection of Anti-biotic Residues— A Mini-Review. Toxics 2023, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tian, X.; Wang, H.Y.; Guo, X.Y.; Wen, Y.; Yang, H.F. Magnetically optimized surface enhanced Raman scattering detection strategy and its sensing applications. Coord. Chem. Rev. 2024, 510, 215848. [Google Scholar] [CrossRef]
- Zhao, Y.; Sang, J.W.; Fu, Y.S.; Guo, J.C.; Guo, J.H. Magnetic nanoprobe-enabled lateral flow assays: Recent advances. Analyst 2023, 148, 3418–3431. [Google Scholar] [CrossRef]
- Li, T.A.; Wang, K.; Zheng, C.J.; Zheng, W.; Cheng, Y.M.; Ning, Q.H.; Xu, H.; Cui, D.X. Magnetic frequency mixing technological advances for the practical improvement of point-of-care testing. Biotechnol. Bioeng. 2022, 119, 347–360. [Google Scholar] [CrossRef] [PubMed]
- He, C.A.; Liu, X.R.; Yu, M.M.; Qiu, Z.; Huang, T.; Xie, W.C.; Cheng, H.X.; Yang, Y.F.; Hao, X.; Wang, X.L. Smartphone conducted DNA portable quantitative detection platform based on photonic crystals chip and magnetic nanoparticles. Talanta 2023, 265, 124849. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Zhang, D.G.; Zu, Y.; Zhang, L.X. Procalcitonin Detection Using Immunomagnetic Beads-Mediated Surface-Enhanced Raman Spectroscopy. Biosensors 2024, 14, 164. [Google Scholar] [CrossRef]
- Li, S.H.; Deng, Y.; Pang, C.H.; Ma, X.H.; Wu, Y.W.; Wang, M.Y.; Li, J.P.; Xu, Z.; Zhang, L.M. An electrochemiluminescence sensor based on Fe/Zn-BTC@C-dots sensitisation for thiamethoxam detection. Sens. Actuators B-Chem. 2023, 394, 134415. [Google Scholar] [CrossRef]
- Feng, A.L.; Lin, M.; Tian, L.; Zhu, H.Y.; Guo, H.; Singamaneni, S.; Duan, Z.F.; Lu, T.J.; Xu, F. Selective enhancement of red emission from upconversion nanoparticles via surface plasmon-coupled emission. Rsc Adv. 2015, 5, 76825–76835. [Google Scholar] [CrossRef]
- You, M.L.; Zhong, J.J.; Hong, Y.; Duan, Z.F.; Lin, M.; Xu, F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale 2015, 7, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Duan, N.; Shen, M.F.; Wang, J.; Wang, Z.P. Surface-enhanced Raman spectroscopic single step detection of Vibrio parahaemolyticus using gold coated polydimethylsiloxane as the active substrate and aptamer modified gold nanoparticles. Microchim. Acta 2019, 186, 401–408. [Google Scholar] [CrossRef]
- Hua, X.D.; You, H.J.; Luo, P.W.; Tao, Z.X.; Chen, H.; Liu, F.Q.; Wang, M.H. Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal. Bioanal. Chem. 2017, 409, 6885–6892. [Google Scholar] [CrossRef]
- Chen, M.; Song, Y.Q.; Han, L.; Zhou, D.D.; Wang, Y.; Pan, L.Q.; Tu, K. An ultrasensitive upconversion fluorescence aptasensor based on graphene oxide release and magnetic separation for Staphylococcus aureus Detection. Food Anal. Methods 2022, 15, 2791–2800. [Google Scholar] [CrossRef]
- Gu, Y.; Qiao, X.; Zhang, J.; Sun, Y.Y.; Tao, Y.M.; Qiao, S.X. Effects of surface modification of upconversion nanoparticles on cellular uptake and cytotoxicity. Chem. Res. Chin. Univ. 2016, 32, 474–479. [Google Scholar] [CrossRef]
- Kong, Q.Q.; Yue, F.L.; Liu, M.Y.; Huang, J.C.; Yang, F.Z.; Liu, J.J.; Li, F.L.; Sun, X.; Guo, Y.M.; Zhu, Y.L. Non-immobilized GO-SELEX of aptamers for label-free detection of thiamethoxam in vegetables. Anal. Chim. Acta 2022, 1202, 339677. [Google Scholar] [CrossRef]
- Fang, X.Y.; Duan, D.; Ye, J.P.; Li, K. A sensitive visual detection of thiamethoxam based on fluorescence resonance energy transfer from NH2-SiO2@CsPbBr3 to merocyanine configuration of spiropyran. Anal. Chim. Acta 2021, 1183, 338938. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.C.; Zhang, H.; Zhang, M.; Yang, X.; Gu, C.; Zhou, G.P.; Zhao, H.T.; Wang, Z.Y.; Dong, A.J.; Wang, J. A rapid elec-trochemical monitoring platform for sensitive determination of thiamethoxam based on β-cyclodextrin-graphene composite. Environ. Toxicol. Chem. 2017, 36, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Sun, W.L.; Huang, L.R.; Sun, N.N.; Hua, X.D.; Wang, M.H.; Liu, F.Q. Development of a multicolor upconversion fluorescence immunoassay for the simultaneous detection of thiamethoxam and dextran by magnetic separation. Rsc Adv. 2021, 11, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Song, P.; Xia, L.X.; Yu, L. A simple and sensitive method for detecting thiamethoxam residues using β-CD-AgNP. Plasmonics 2024, 24, 11468. [Google Scholar] [CrossRef]


| Method | Material | LOD (ng·mL−1) | Detection Range (ng·mL−1) | Detection Time (min) | Reference |
|---|---|---|---|---|---|
| Colorimetric aptasensor | AuNPs | 0.49 | 1.46–43.76 | 40 | [41] |
| Fluorescence biosensor | PQD, β-CD-SP | 1.20 | 4.20–18.20 | >30 | [42] |
| Electrochemical detection | β-CD, GO | 78.8 | 145.9–4600 | 30 | [43] |
| UCFIA | UCNPs, MNPs | 0.09 | 0.09–2.34 | >60 | [44] |
| SERS | β-CD, AgNPs | 21.3 | 29.2–29,200 | >60 | [45] |
| UCNPs-MNPs biosensor | UCNPs, MNPs | 0.08 | 0.4–104.2 | 25 | This work |
| Samples | Additive Concentration (ng·mL−1) | Intra-Batch (n = 3) | Inter-Batch RSD (%) (n = 9) | |||||
|---|---|---|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | ||||||
| Average Recovery (%) | RSD (%) | Average Recovery (%) | RSD (%) | Average Recovery (%) | RSD (%) | |||
| Cucumber | 0.5 | 94.67 | 6.97 | 98.67 | 5.81 | 99.33 | 9.05 | 6.83 |
| 5 | 96.33 | 9.54 | 103.65 | 4.21 | 101.04 | 4.77 | 7.14 | |
| 10 | 101.91 | 3.73 | 102.17 | 5.01 | 99.97 | 2.29 | 3.98 | |
| Cabbage | 0.5 | 82.67 | 4.97 | 92.03 | 9.12 | 102.12 | 3.63 | 9.83 |
| 2 | 102.33 | 3.99 | 102.83 | 4.51 | 106.50 | 2.13 | 4.08 | |
| 10 | 98.40 | 6.24 | 98.33 | 3.48 | 95.07 | 3.70 | 4.93 | |
| Apple | 0.5 | 108.67 | 8.55 | 93.33 | 4.19 | 102.67 | 3.02 | 10.68 |
| 2 | 109.33 | 7.32 | 94.51 | 3.02 | 107.17 | 6.72 | 8.85 | |
| 10 | 95.97 | 5.65 | 99.03 | 5.43 | 103.07 | 4.69 | 6.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Han, L.; Ma, H.; Lan, W.; Tu, K.; Peng, J.; Su, J.; Pan, L. An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods 2025, 14, 182. https://doi.org/10.3390/foods14020182
Huang Q, Han L, Ma H, Lan W, Tu K, Peng J, Su J, Pan L. An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods. 2025; 14(2):182. https://doi.org/10.3390/foods14020182
Chicago/Turabian StyleHuang, Qian, Lu Han, Hui Ma, Weijie Lan, Kang Tu, Jing Peng, Jing Su, and Leiqing Pan. 2025. "An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam" Foods 14, no. 2: 182. https://doi.org/10.3390/foods14020182
APA StyleHuang, Q., Han, L., Ma, H., Lan, W., Tu, K., Peng, J., Su, J., & Pan, L. (2025). An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods, 14(2), 182. https://doi.org/10.3390/foods14020182







