Nutritional Characterization of Annual and Perennial Glassworts from the Apulia Region (Italy)
Abstract
1. Introduction
2. Materials and Methods
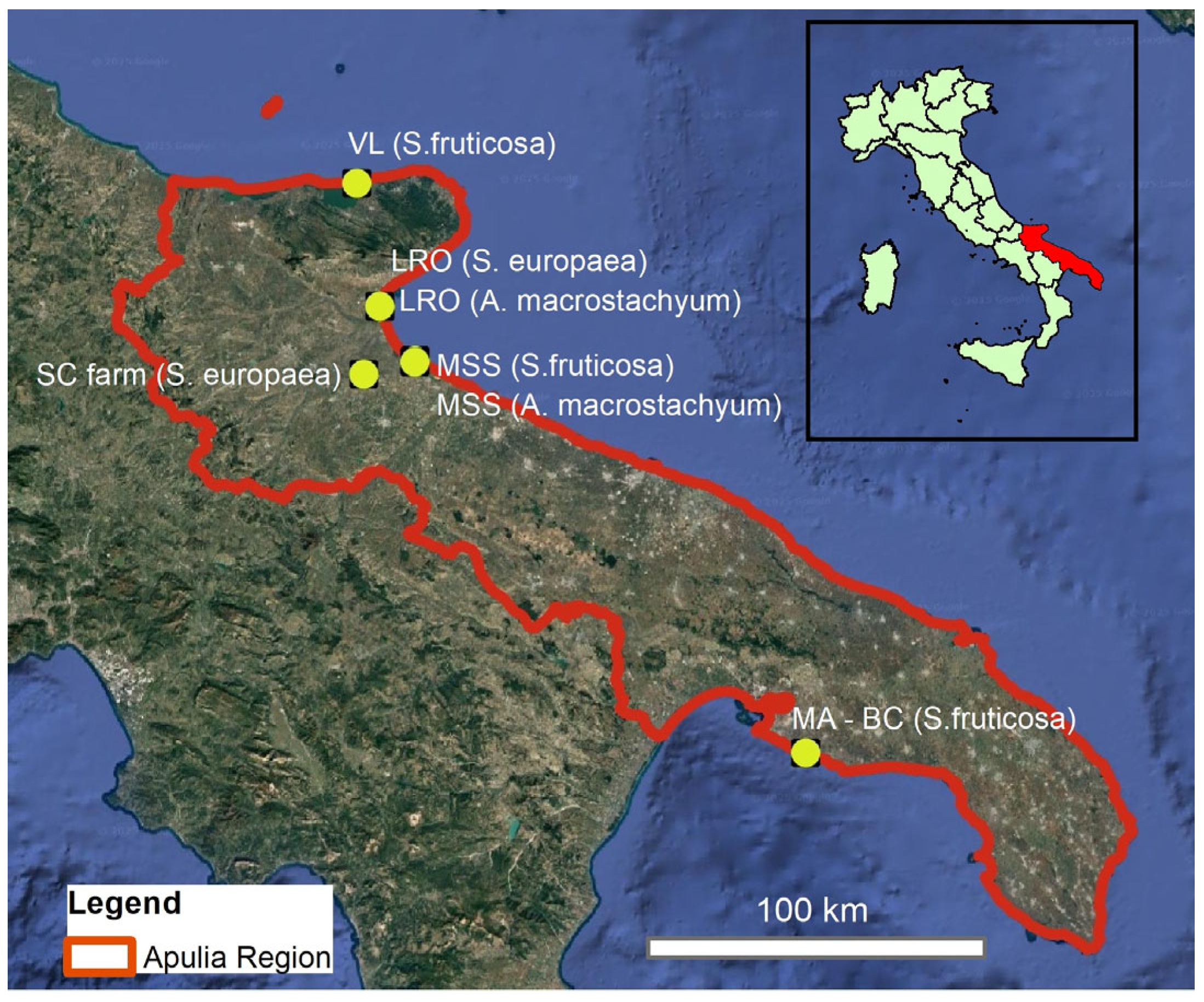
2.1. Sampling Area and Glasswort Description
- (1)
- Margherita di Savoia Saltworks (MSS), [Margherita di Savoia Barletta–Andria-Trani province (BT)] (site a: 41°25′54.39″ N; 16°0′31.395″ E; sito b: 41°25′39.512″ N; 16°0′28.46″ E);
- (2)
- Laguna del Re Oasis (LRO) [Manfredonia, Foggia province (FG)] (site a: 41°34′40.8″ N; 15°53′5.8″ E; site b: 41°34′47.86″ N; 15°53′0.12″ E);
- (3)
- Varano lake (VL) (Cagnano Varano, FG) (41°54′51.5″ N; 15°48′17.6″ E);
- (4)
- Monti d’Arena-Bosco Caggione (MA-BC) [Taranto province (TA)] (40°20′54.4″ N; 17°22′31.9″ E);
- (5)
- ‘Spirito Contadino’ (SC) commercial farm (Borgo Tressanti, FG) (41°23′44.8″ N; 15°49′25.2″ E).
2.2. Inorganic Ions and Iodine Determination
2.2.1. Anions and Cations
2.2.2. Iodine
2.3. Pigments and Antioxidant Compounds
2.3.1. Chlorophylls, Carotenoids and Anthocyanins
2.3.2. Phenols and Flavonoids
2.3.3. Phytosterols
2.4. Antioxidant Capacity
2.5. Statistical Analysis
3. Results
3.1. Dry Mass and Mineral Concentrations
3.2. Bioactive Compound Concentrations and Antioxidant Capacity
4. Discussion
4.1. Mineral Nutrients and Antinutrients
4.2. Visual Quality, Nutritional and Antioxidative Traits
4.3. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agudelo, A.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture 2020; Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020; ISBN 978-92-5-133441-6. [Google Scholar]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable Agricultural Management of Saline Soils in Arid and Semi-Arid Mediterranean Regions through Halophytes, Microbial and Soil-Based Technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Chaturvedi, T.; Thomsen, M.H. Extraction and Quantification of Chlorophylls, Carotenoids, Phenolic Compounds, and Vitamins from Halophyte Biomasses. Appl. Sci. 2022, 12, 840. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural Products from Extreme Marine Environments: Searching for Potential Industrial Uses within Extremophile Plants. Ind. Crops Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A Second Update to the Checklist of the Vascular Flora Native to Italy. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole, Ed.; Edagricole di New Business Media: Milan, Italy, 2017; ISBN 978-88-506-5242-6. [Google Scholar]
- POWO. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 24 September 2025).
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Custódio, L.; Rodrigues, M.J.; Pereira, C.G.; Castañeda-Loaiza, V.; Fernandes, E.; Standing, D.; Neori, A.; Shpigel, M.; Sagi, M. A Review on Sarcocornia Species: Ethnopharmacology, Nutritional Properties, Phytochemistry, Biological Activities and Propagation. Foods 2021, 10, 2778. [Google Scholar] [CrossRef]
- Ramírez, E.; Rodríguez, N.; de la Fuente, V. Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition. Plants 2024, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Sousa, J.; Bento-Silva, A.; Duarte, B.; Caçador, I.; Salazar, M.; Mecha, E.; Serra, A.T.; Bronze, M.R. Soilless Cultivated Halophyte Plants: Volatile, Nutritional, Phytochemical, and Biological Differences. Antioxidants 2023, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, S.; Egodawatta, C.; Attanayake, R.N.; Perera, D. From Salt Pan to Saucepan: Salicornia, a Halophytic Vegetable with an Array of Potential Health Benefits. Food Front. 2023, 4, 641–676. [Google Scholar] [CrossRef]
- Haldimann, M.; Alt, A.; Blanc, A.; Blondeau, K. Iodine Content of Food Groups. J. Food Compos. Anal. 2005, 18, 461–471. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A Survey of Nitrate and Oxalate Content in Fresh Vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- Park, J.; Kwock, C.; Yang, Y. The Effect of the Sodium to Potassium Ratio on Hypertension Prevalence: A Propensity Score Matching Approach. Nutrients 2016, 8, 482. [Google Scholar] [CrossRef]
- Sánchez Gavilán, I.; Velázquez Ybarzabal, D.; de la Fuente, V.; Cámara, R.M.; Sánchez-Mata, M.C.; Cámara, M. Valorization of Salicornia Patula Duval-Jouve Young Shoots in Healthy and Sustainable Diets. Nutrients 2024, 16, 358. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as Source of Bioactive Phenolic Compounds and Their Potential Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1078–1101. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramirez Chueca, E.; de la Fuente García, V. Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants 2021, 10, 2218. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of Seawater Concentration on the Productivity and Nutritional Value of Annual Salicornia and Perennial Sarcocornia halophytes as Leafy Vegetable Crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, F.; Veríssimo, A.C.; Maciel, E.; Domingues, M.R.; Calado, R. Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization. Plants 2024, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Mateos-Naranjo, E.; Carreiras, J.; Feijão, E.; Duarte, B.; Matos, A.R.; Betti, M.; Del Rio, C.; Romero-Bernal, M.; Montaner, J.; et al. Interactive Temperature and CO2 Rise, Salinity, Drought, and Bacterial Inoculation Alter the Content of Fatty Acids, Total Phenols, and Oxalates in the Edible Halophyte Salicornia ramosissima. Plants 2023, 12, 1395. [Google Scholar] [CrossRef]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.d.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Micke, G.A.; Tavares, L.B.B.; Fett, R. Nutrient Composition and, Identification/Quantification of Major Phenolic Compounds in Sarcocornia Ambigua (Amaranthaceae) Using HPLC–ESI-MS/MS. Food Res. Int. 2014, 55, 404–411. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Cifarelli, S.; Losavio, F.; Sonnante, G.; Elia, A. Exploring On-Farm Agro-Biodiversity: A Study Case of Vegetable Landraces from Puglia Region (Italy). Biodivers. Conserv. 2020, 29, 747–770. [Google Scholar] [CrossRef]
- Cammerino, A.R.B.; Ingaramo, M.; Rizzi, V.; Gioiosa, M.; Monteleone, M. Glasswort as a Strategic Crop in Coastal Wetlands: Intercropping Results with Swiss Chard. Agronomy 2025, 15, 158. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morpho-Biometrical, Nutritional and Phytochemical Characterization of Carrot Landraces from Puglia Region (Southern Italy). Sustainability 2021, 13, 3940. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Natrella, G.; Lazzizera, C.; Elia, A. Peeling Affects the Nutritional Properties of Carrot Genotypes. Foods 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Somma, A.; Palmitessa, O.D.; Conversa, G.; Serio, F.; Santamaria, P. Localized Foliar Application of Iodine on Tomato: An Effective Approach for Targeted Agronomic Biofortification. Sci. Hortic. 2024, 327, 112807. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Pre-Harvest Strategy for Improving Harvest and Post-Harvest Performance of Kale and Chicory Baby Leaves. Plants 2025, 14, 863. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol Reductase Activity in Relation to Differential Anthocyanin Accumulation in Juvenile and Mature Phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evidence-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, Y.; Li, H.; Lin, J. Simultaneous Extraction and Determination of Free and Conjugated Phytosterols in Tobacco. J. Sep. Sci. 2016, 39, 2466–2473. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols Extraction from Hickory (Carya Cathayensis Sarg.) Husk with a Green Direct Citric Acid Hydrolysis Extraction Method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lisanti, A.; Formica, V.; Ianni, F.; Albertini, B.; Marinozzi, M.; Sardella, R.; Natalini, B. Antioxidant Activity of Phenolic Extracts from Different Cultivars of Italian Onion (Allium Cepa) and Relative Human Immune Cell Proliferative Induction. Pharm. Biol. 2016, 54, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Conversa, G.; Botticella, L.; Lazzizera, C.; Bonasia, A.; Elia, A. Ecophysiological and Nutritional Characterisation of Two Morphotypes of Cakile Maritima Subsp. Maritima Scop. from Puglia Region, Southern Italy. Front. Plant Sci. 2024, 15, 1397852. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as New Model Plant Species for Salt Tolerance Strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Mendis, C.L.; Padmathilake, R.E.; Attanayake, R.N.; Perera, D. Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance. Int. J. Mol. Sci. 2025, 26, 5936. [Google Scholar] [CrossRef]
- Homayouni, H.; Razi, H.; Izadi, M.; Alemzadeh, A.; Kazemeini, S.A.; Niazi, A.; Vicente, O. Temporal Changes in Biochemical Responses to Salt Stress in Three Salicornia Species. Plants 2024, 13, 979. [Google Scholar] [CrossRef]
- Puccinelli, M.; Marchioni, I.; Botrini, L.; Carmassi, G.; Pardossi, A.; Pistelli, L. Growing Salicornia europaea L. with Saline Hydroponic or Aquaculture Wastewater. Horticulturae 2024, 10, 196. [Google Scholar] [CrossRef]
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Magné, C.; Foggi, G.; Conte, G.; Santin, M.; et al. Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae 2022, 8, 828. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar. Plants 2022, 11, 1497. [Google Scholar] [CrossRef]
- Rahmani, R.; El Arbi, K.; Aydi, S.S.; Hzami, A.; Tlahig, S.; Najar, R.; Aydi, S.; Debouba, M. Biochemical Composition and Biological Activities of Salicornia europaea L. from Southern Tunisia. J. Food Meas. Charact. 2022, 16, 4833–4846. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima. J. Woods Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150483-6. [Google Scholar]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary Reference Values for Sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Raheem, D.; Ahmed, F.; Alhodieb, F.S.; Alsharari, Z.D.; Alhaji, J.H.; BinMowyna, M.N.; Saraiva, A.; Raposo, A. Salicornia Bigelovii, S. Brachiata and S. Herbacea: Their Nutritional Characteristics and an Evaluation of Their Potential as Salt Substitutes. Foods 2022, 11, 3402. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or Foe? Chloride Patterning in Halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef]
- Ralph, Y.; Manley, S.L. Spatial and Temporal Variation in Tissue Halide Levels of Salicornia Virginica. Wetlands 2006, 26, 97–106. [Google Scholar] [CrossRef]
- Lv, S.; Nie, L.; Fan, P.; Wang, X.; Jiang, D.; Chen, X.; Li, Y. Sodium Plays a More Important Role than Potassium and Chloride in Growth of Salicornia Europaea. Acta Physiol. Plant. 2012, 34, 503–513. [Google Scholar] [CrossRef]
- Zahran, M.A.; El-Amier, Y.A. Non-Traditional Fodders from the Halophytic Vegetation of the Deltaic Mediterranean Coastal Desert, Egypt. J. Biol. Sci. 2013, 13, 226–233. [Google Scholar] [CrossRef]
- Lopes, M.; Silva, A.S.; Séndon, R.; Barbosa-Pereira, L.; Cavaleiro, C.; Ramos, F. Towards the Sustainable Exploitation of Salt-Tolerant Plants: Nutritional Characterisation, Phenolics Composition, and Potential Contaminants Analysis of Salicornia ramosissima and Sarcocornia perennis Alpini. Molecules 2023, 28, 2726. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; La Rotonda, P.; Elia, A. Reduction of Nitrate Content in Baby-Leaf Lettuce and Cichorium Endivia through the Soilless Cultivation System, Electrical Conductivity and Management of Nutrient Solution. Front. Plant Sci. 2021, 12, 645671. [Google Scholar] [CrossRef]
- Shi, R.; Liang, L.; Liu, W.; Zeb, A. Kochia scoparia L., a Newfound Candidate Halophyte, for Phytoremediation of Cadmium-Contaminated Saline Soils. Environ. Sci. Pollut. Res. 2022, 29, 44759–44768. [Google Scholar] [CrossRef]
- Duarte, B.; Feijão, E.; Pinto, M.V.; Matos, A.R.; Silva, A.; Figueiredo, A.; Fonseca, V.F.; Reis-Santos, P.; Caçador, I. Nutritional Valuation and Food Safety of Endemic Mediterranean Halophytes Species Cultivated in Abandoned Salt Pans under a Natural Irrigation Scheme. Estuar. Coast. Shelf Sci. 2022, 265, 107733. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Panel on Dietetic Products Nutrition and Allergies) Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [CrossRef]
- Backer, H.; Hollowell, J. Use of Iodine for Water Disinfection: Iodine Toxicity and Maximum Recommended Dose. Environ. Health Perspect. 2000, 108, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Conversa, G.; Miedico, O.; Chiaravalle, A.E.; Elia, A. Heavy Metal Contents in Green Spears of Asparagus (Asparagus officinalis L.) Grown in Southern Italy: Variability among Farms, Genotypes and Effect of Soil Mycorrhizal Inoculation. Sci. Hortic. 2019, 256, 108559. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes Have Potential as Heavy Metal Phytoremediators: A Comprehensive Review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Ashikhmin, A.; Bolshakov, M.; Pashkovskiy, P.; Vereshchagin, M.; Khudyakova, A.; Shirshikova, G.; Kozhevnikova, A.; Kosobryukhov, A.; Kreslavski, V.; Kuznetsov, V.; et al. The Adaptive Role of Carotenoids and Anthocyanins in Solanum Lycopersicum Pigment Mutants under High Irradiance. Cells 2023, 12, 2569. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll Cycle Regulates the Construction and Destruction of the Light-Harvesting Complexes. Biochim. Biophys. Acta-Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- D’ambrosio, C.; Stigliani, A.L.; Giorio, G. Food from Genetically Engineered Plants: Tomato with Increased β-Carotene, Lutein, and Xanthophylls Contents. In Genetically Modified Organisms in Food: Production, Safety, Regulation and Public Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 361–380. ISBN 978-012802530-7. [Google Scholar]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pentieva, K.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Preformed Vitamin A and Β-carotene. EFSA J. 2024, 22, e8814. [Google Scholar] [CrossRef]
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Gargouri, M.; Magné, C.; Dauvergne, X.; Ksouri, R.; El Feki, A.; Metges, M.-A.G.; Talarmin, H. Cytoprotective and Antioxidant Effects of the Edible Halophyte Sarcocornia perennis L. (Swampfire) against Lead-Induced Toxicity in Renal Cells. Ecotoxicol. Environ. Saf. 2013, 95, 44–51. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Zhao, C.-X. Water-Deficit Stress-Induced Anatomical Changes in Higher Plants. Comptes Rendus. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In Vitro and in Silico Perspectives on Biological and Phytochemical Profile of Three Halophyte Species—A Source of Innovative Phytopharmaceuticals from Nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; Canini, A.; El Haci, I.A.; Atik Bekkara, F. Phytochemical Analysis and Antioxidant Activity of Tamarix Africana, Arthrocnemum Macrostachyum and Suaeda Fruticosa, Three Halophyte Species from Algeria. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2019, 153, 843–852. [Google Scholar] [CrossRef]
- Rodrigues, M.; Gangadhar, K.; Vizetto-Duarte, C.; Wubshet, S.; Nyberg, N.; Barreira, L.; Varela, J.; Custódio, L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Stojanović-Radić, Z.; Jakovljević, D.; Zlatić, N.; Luković, M.; Dajić-Stevanović, Z. Coastal Halophytes: Potent Source of Bioactive Molecules from Saline Environment. Plants 2023, 12, 1857. [Google Scholar] [CrossRef]
- Normén, L.; Johnsson, M.; Andersson, H.; van Gameren, Y.; Dutta, P. Plant Sterols in Vegetables and Fruits Commonly Consumed in Sweden. Eur. J. Nutr. 1999, 38, 84–89. [Google Scholar] [CrossRef]
- Laitinen, K.; Gylling, H. Dose-Dependent LDL-Cholesterol Lowering Effect by Plant Stanol Ester Consumption: Clinical Evidence. Lipids Health Dis. 2012, 11, 140. [Google Scholar] [CrossRef]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Vear, F.; Merah, O. Sterol Content in Sunflower Seeds (Helianthus annuus L.) as Affected by Genotypes and Environmental Conditions. Food Chem. 2010, 121, 990–995. [Google Scholar] [CrossRef]
- Pavlík, M.; Pavlíková, D.; Balík, J.; Neuberg, M. The Contents of Amino Acids and Sterols in Maize Plants Growing under Different Nitrogen Conditions. Plant Soil Environ. 2010, 56, 125–132. [Google Scholar] [CrossRef]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Effect of Sowing Dates on Fatty Acids and Phytosterols Patterns of Carthamus tinctorius L. Appl. Sci. 2019, 9, 2839. [Google Scholar] [CrossRef]
- Magni, N.N.; Veríssimo, A.C.S.; Silva, H.; Pinto, D.C.G.A. Metabolomic Profile of Salicornia Perennis Plant’s Organs under Diverse in Situ Stress: The Ria de Aveiro Salt Marshes Case. Metabolites 2023, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.A.; Nesterov, V.N.; Bogdanova, E.S. Lipids of Halophyte Species Growing in Lake Elton Region (South East of the European Part of Russia). In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 2013–2037. ISBN 978-3-030-57634-9. [Google Scholar]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, H.; Silva, A.M.S. Lipophilic Profile of the Edible Halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-H.; Yang, Y.-X.; Feng, M.-Y. Contents of Phytosterols in Vegetables and Fruits Commonly Consumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Hupel, M.; Lecointre, C.; Meudec, A.; Poupart, N.; Gall, E.A. Comparison of Photoprotective Responses to UV Radiation in the Brown Seaweed Pelvetia Canaliculata and the Marine Angiosperm Salicornia ramosissima. J. Exp. Mar. Bio. Ecol. 2011, 401, 36–47. [Google Scholar] [CrossRef]
- Kumari, N.; Rani, B.; Manne, H.; Jattan, M.; Jattan, M.S.; Avtar, R.; Kumari, A.; Duhan, J.; Kodidhala, V. Antioxidative Response Mechanisms in Halophytes: Their Role in Stress Defence. In Halophytes vis-à-vis Saline Agriculture; Springer: Singapore, 2024; pp. 329–350. ISBN 978-981-97-3156-5. [Google Scholar]


| Sampling Area | Taxonomic Classification | Sample Identification |
|---|---|---|
| Margherita di Savoia Saltworks (MSS), site a | Sarcocornia fruticosa L. | S. fruticosa-MSS |
| Margherita di Savoia Saltworks (MSS), site b | Arthrocaulon macrostachyum Moric. | A. macrostachyum-MSS |
| Laguna del Re Oasis (LRO), site a | Arthrocaulon macrostachyum Moric. | A. macrostachyum-LRO |
| Laguna del Re Oasis (LRO), site b | Salicornia europaea Auct. | S. europaea-LRO |
| Varano lake (VL) | Sarcocornia fruticosa L. | S. fruticosa-VL |
| Monti d’Arena-Bosco Caggione (MA-BC) | Arthrocaulon macrostachyum Moric. | A. macrostachyum-MA-BC |
| Spirito Contadino farm (SC) | Salicornia europaea Auct. | S. europaea-SC |
| Glasswort Sample | Dry Mass | Na | K | Mg | Ca | Cl | NO3 | C2O4 | Na/K | I |
|---|---|---|---|---|---|---|---|---|---|---|
| g·kg−1 FW | µg 100 g−1 FW | |||||||||
| S. europaea-LRO | 178.3 b (1) (±3.9) | 20.9 a (±0.8) | 8.7 ab (±0.6) | 2.2 ab (±0.1) | 6.1 ab (±0.4) | 21.9 b (±1.0) | 0.08 b (±0.00) | 0.15 d (±0.01) | 2.9 bc (±0.2) | 349.4 a (±21.0) |
| S. europaea-SC | 194.2 b (±9.8) | 14.8 abc (±1.0) | 9.0 a (±0.6) | 2.8 a (±0.3) | 6.9 a (±0.8) | 15.4 c (±1.0) | 0.39 a (±0.06) | 0.78 cd (±0.09) | 1.6 c (±0.1) | 96.5 c (±8.8) |
| S. fruticosa-MSS | 187.0 b (±6.8) | 13.4bcd (±1.0) | 4.0 bc (±0.4) | 1.4 bc (±0.1) | 4.2 abc (±1.0) | 1.9 e (±1.8) | 0.07 b (±0.04) | 0.01 d (±0.00) | 3.8 ab (±0.7) | 104.8 c (±9.3) |
| S. fruticosa-VL | 189.0 b (±5.5) | 14.9 abc (±0.2) | 4.1 abc (±0.3) | 1.3 c (±0.1) | 2.5 bc (±1.0) | 12.9 cd (±0.7) | 0.09 b (±0.01) | 6.57 a (±0.47) | 3.7 ab (±0.2) | 212.0 abc (±13.2) |
| A. macrostachyum-MA-BC | 187.0 b (±5.7) | 17.1 ab (±0.4) | 3.4 c (±0.3) | 1.2 c (±0.04) | 3.6 abc (±0.4) | 21.7 b (±0.7) | 0.40 a (±0.01) | 1.47 c (±0.06) | 5.2 a (±0.4) | 223.7 abc (±16.1) |
| A. macrostachyum-LRO | 104.6 c (±5.3) | 6.9 d (±0.3) | 1.9 c (±0.2) | 0.8 c (±0.04) | 1.8 c (±0.3) | 8.9 d (±0.5) | n.d. (3) | 0.79 cd (±0.12) | 4.0 ab (±0.3) | 277.4 ab (±9.8) |
| A. macrostachyum-MSS | 242.2 a (±5.1) | 9.8 cd (±0.4) | 5.4 abc (±0.7) | 1.5 bc (±0.01) | 5.7 ab (±0.5) | 37.5 a (±0.2) | 0.11 b (±0.00) | 2.96 b (±0.27) | 1.9 c (±0.3) | 112.1 bc (±2.9) |
| Significance (2) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Glasswort Sample | CHLa | CHLb | CHLt | Car | TP | FL | Anth | Sterols |
|---|---|---|---|---|---|---|---|---|
| µg g−1 FW | mg ßc.e. (2) 100 g−1 FW | mg g.a.e. (2) 100 g−1 FW | mg q.e. (2) 100 g−1 FW | mg c.g.e (2) 100 g−1 FW | mg s.e. (2) 100 g−1 FW | |||
| S. europaea-LRO | 134.4 a (1) (±4.7) | 49.5 a (±2.0) | 180.8 a (±6.4) | 4.1 b (±0.20) | 258.3 a (±7.7) | 31.7 bc (±0.00) | 0.7 bc (±0.10) | 7.4 c (±0.5) |
| S. europaea-SC | 127.0 a (±21.3) | 43.0 a (±8.8) | 167.7 a (±29.3) | 6.2 a (±0.30) | 192.9 a (±22.3) | 39.3 b (±2.20) | 1.0 abc (±0.20) | 12.2 bc (±1.4) |
| S. fruticosa-MSS | 11.5 b (±0.6) | 8.0 b (±1.2) | 18.7 b (±1.6) | 3.5 b (±0.03) | 19.3 b (±2.0) | 0.3 e (±0.02) | 1.6 a (±0.03) | n.a. (3) |
| S. fruticosa-VL | 7.8 b (±0.2) | 6.3 b (±0.1) | 13.4 b (±0.3) | 1.7 c (±0.02) | 26.6 b (±1.0) | 26.2 cd (±0.60) | 1.1 abc (±0.02) | 17.0 b (±0.9) |
| A. macrostachyum-MA-BC | 12.3 b (±0.2) | 10.8 b (±0.2) | 21.8 b (±0.3) | 1.3 c (±0.05) | 78.4 b (±3.5) | 22.0 cd (±1.70) | 1.4 ab (±0.03) | 15.3 bc (±1.0) |
| A. macrostachyum-LRO | 6.9 b (±1.2) | 4.7 b (±1.0) | 11.0 b (±2.1) | 1.2 c (±0.10) | 83.7 b (±6.6) | 18.0 d (±0.90) | 0.6 bc (±0.20) | 7.3 c (±0.9) |
| A. macrostachyum-MSS | 119.7 a (±3.3) | 41.5 a (±0.7) | 159.0 a (±3.8) | 2.8 bc (±0.10) | 256.2 a (±7.3) | 73.9 a (±2.00) | 0.4 c (±0.10) | 53.3 a (±10.0) |
| Significance (4) | *** | *** | *** | *** | *** | *** | *** | *** |
| Glasswort Sample | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|
| HA (2) | LA (2) | Total | HA (2) | LA (2) | Total | ||
| µmol T.E. g−1 FW (3) | |||||||
| S. europaea-LRO | 8.2 b (1) (±0.2) | 12.4 c (±0.4) | 0.60 a (±0.02) | 13.0 c (±0.4) | 19.5 b (±0.7) | n.d. | 19.5 b (±0.7) |
| S. europaea-SC | 13.0 a (±0.5) | 22.9 a (±0.9) | 0.73 a (±0.05) | 23.7 a (±1.0) | 71.6 a (±1.2) | n.d. | 71.6 a (±1.2) |
| S. fruticosa-MSS | 3.6 d (±0.1) | 6.9 d (±0.2) | n.d. (4) | 6.9 d (±0.2) | 5.8 d (±0.2) | 0.26 b (±0.03) | 6.2 d (±0.2) |
| S. fruticosa-VL | 5.8 c (±0.2) | 7.3 d (±0.3) | n.d. | 7.3 d (±0.3) | 8.4 cd (±0.4) | 0.43 a (±0.03) | 8.8 cd (±0.4) |
| A. macrostachyum-MA-BC | 2.6 d (±0.1) | 5.4 d (±0.4) | n.d. | 5.4 d (±0.4) | 5.0 d (±0.2) | 0.33 ab (±0.02) | 5.3 d (±0.1) |
| A. macrostachyum-LRO | 4.1 d (±0.2) | 4.4 d (±0.2) | n.d. | 4.4 d (±0.2) | 7.8 d (±0.4) | 0.40 ab (±0.04) | 7.5 d (±0.4) |
| A. macrostachyum-MSS | 9.4 b (±0.2) | 17.8 b (±1.2) | 0.27 b (±0.02) | 18.1 b (±1.2) | 14.3 bc (±0.6) | 0.10 c (±0.00) | 14.4 bc (±0.6) |
| Significance (5) | *** | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duri, L.G.; Botticella, L.; Lazzizera, C.; Perrino, E.V.; Giancaspro, A.; Cammerino, A.R.B.; Bonasia, A.; Elia, A.; Conversa, G. Nutritional Characterization of Annual and Perennial Glassworts from the Apulia Region (Italy). Foods 2025, 14, 3433. https://doi.org/10.3390/foods14193433
Duri LG, Botticella L, Lazzizera C, Perrino EV, Giancaspro A, Cammerino ARB, Bonasia A, Elia A, Conversa G. Nutritional Characterization of Annual and Perennial Glassworts from the Apulia Region (Italy). Foods. 2025; 14(19):3433. https://doi.org/10.3390/foods14193433
Chicago/Turabian StyleDuri, Luigi Giuseppe, Lucia Botticella, Corrado Lazzizera, Enrico Vito Perrino, Angelica Giancaspro, Anna Rita Bernadette Cammerino, Anna Bonasia, Antonio Elia, and Giulia Conversa. 2025. "Nutritional Characterization of Annual and Perennial Glassworts from the Apulia Region (Italy)" Foods 14, no. 19: 3433. https://doi.org/10.3390/foods14193433
APA StyleDuri, L. G., Botticella, L., Lazzizera, C., Perrino, E. V., Giancaspro, A., Cammerino, A. R. B., Bonasia, A., Elia, A., & Conversa, G. (2025). Nutritional Characterization of Annual and Perennial Glassworts from the Apulia Region (Italy). Foods, 14(19), 3433. https://doi.org/10.3390/foods14193433











