Threat and Control of tet(X)-Mediated Tigecycline-Resistant Acinetobacter sp. Bacteria
Abstract
1. Introduction
2. Evolution of tet(X)-Mediated Tigecycline Resistance
2.1. Emergence of tet(X) Genes
2.2. Structural Insights into Tet(X) Proteins
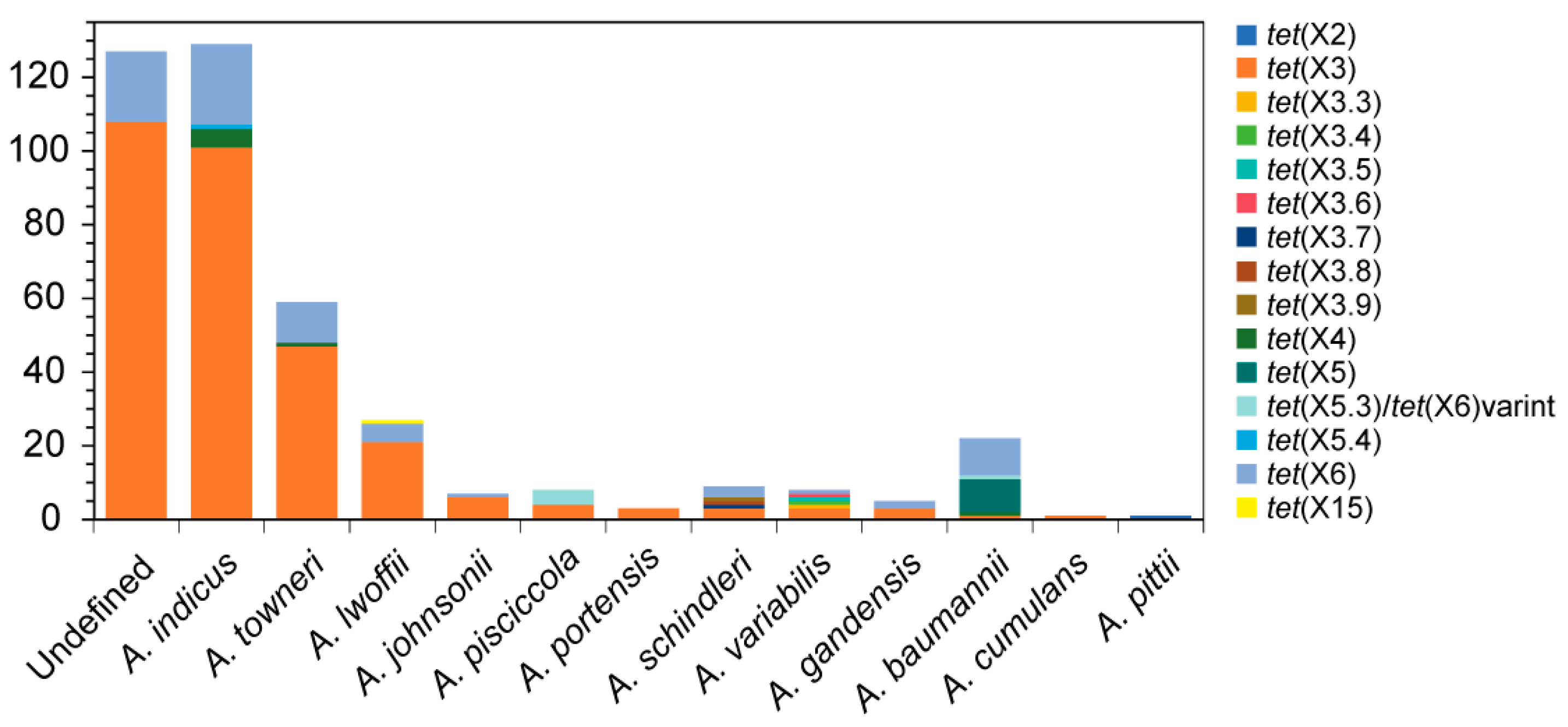
3. Global Epidemiology of tet(X)-Positive Acinetobacter sp. Strains
3.1. Classification of tet(X) Genes
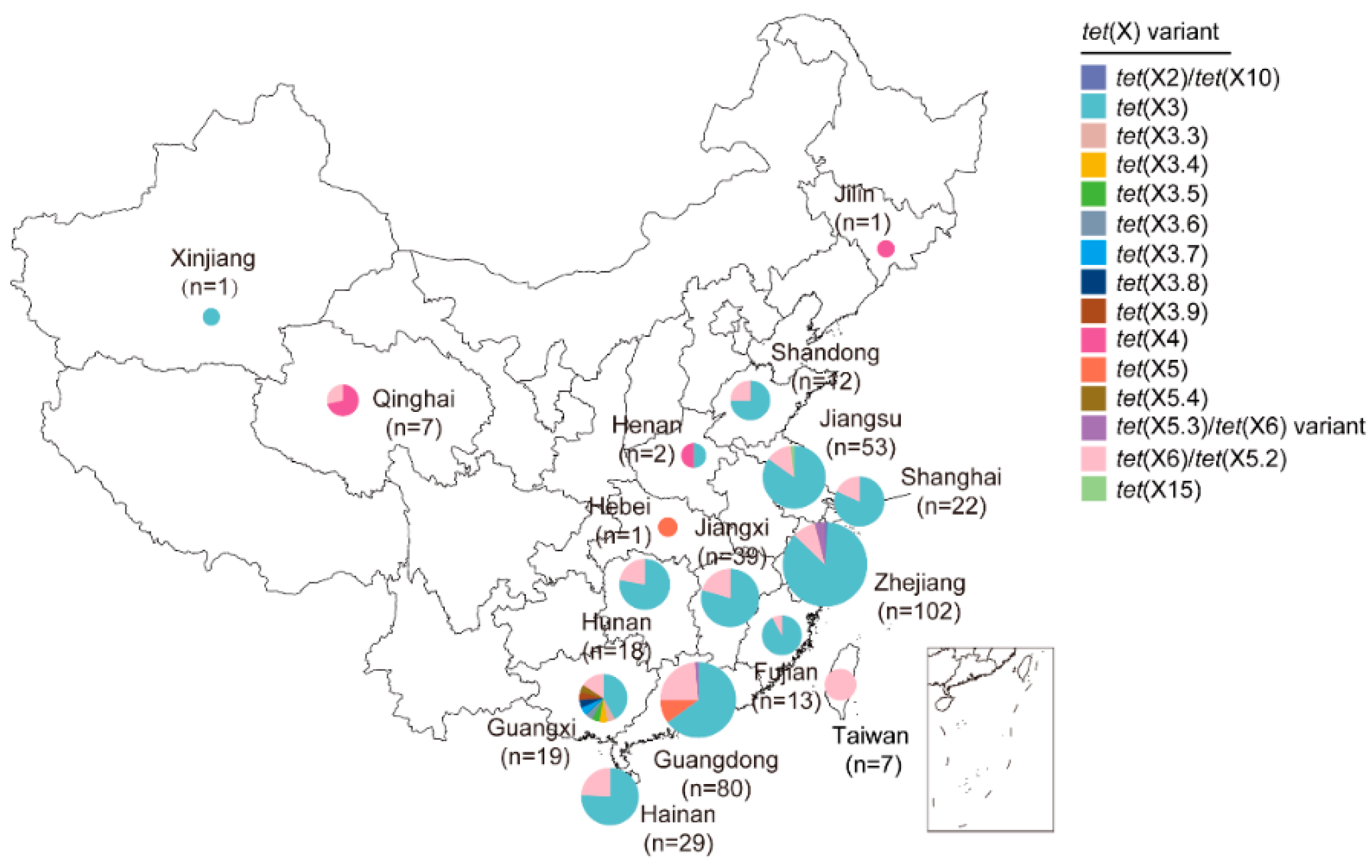
3.2. China
3.2.1. Predominate Groups of tet(X3) and tet(X6)
3.2.2. Sporadic Groups of tet(X2), tet(X4), tet(X5), and tet(X15)
3.3. Other Countries
4. Horizontal Transmission of tet(X) Genes
4.1. Mobilizable Plasmids
4.2. ISCR2-Mediated Transposons
5. Rapid Detection Methods of tet(X) Variants
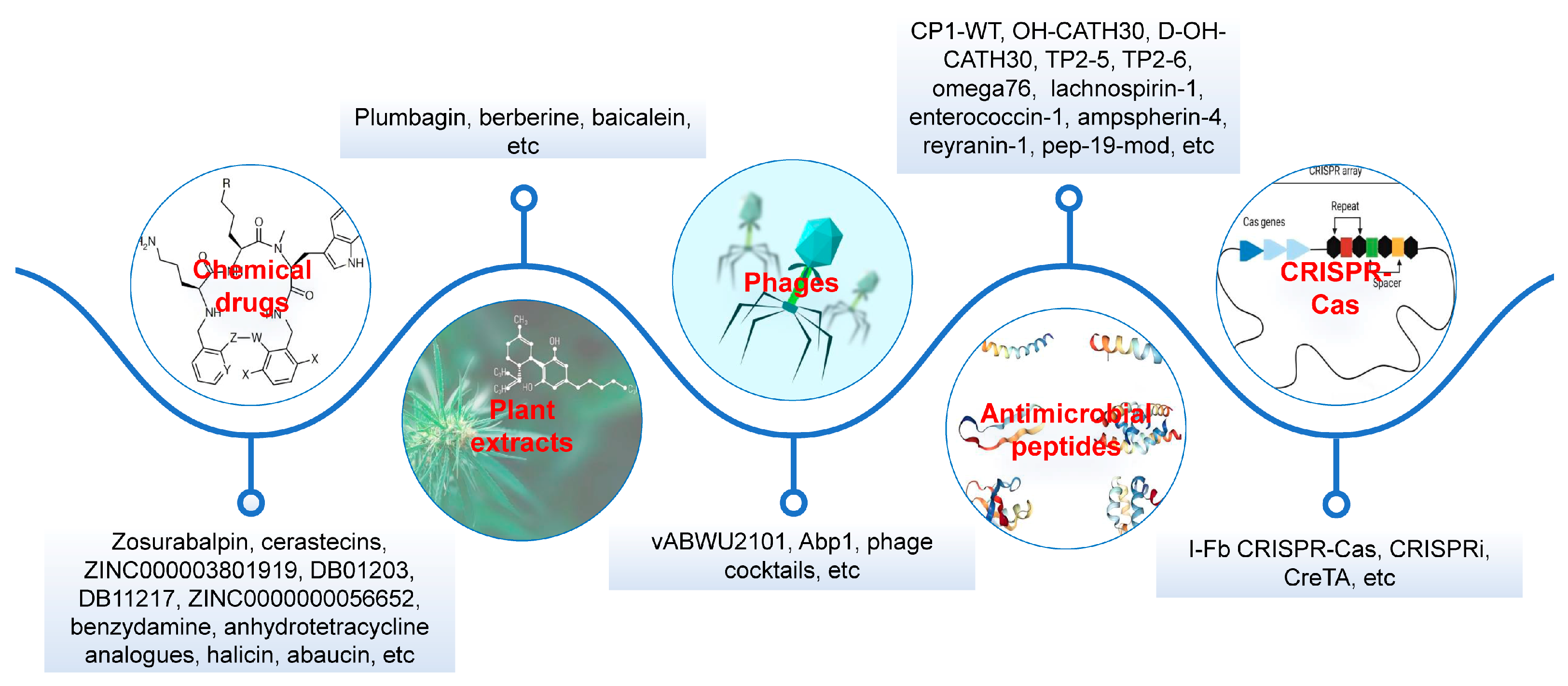
6. Treatment Options
6.1. Chemical Drugs
6.2. Plant Extracts
6.3. Phages
6.4. AMPs
6.5. CRISPR-Cas
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant |
| AMPs | Antimicrobial peptides |
| CRAb | Carbapenem-resistant A. baumannii |
| USFDA | United States Food and Drug Administration |
| MIC | Minimum inhibitory concentration |
| FAD | Flavin adenine dinucleotide |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| WGS | Whole genome sequencing |
| IS | Insertion sequence |
| OTG | Orange to green |
| MALDI-TOF MS | Matrix-assisted laser desorption ionization–time of flight mass spectrometry |
| MCP | Macrocyclic peptide |
| TP2 | Tilapia piscidin 2 |
References
- Qin, J.; Feng, Y.; Lu, X.; Zong, Z. Precise Species Identification for Acinetobacter: A Genome-Based Study with Description of Two Novel Acinetobacter Species. mSystems 2021, 6, e0023721. [Google Scholar] [CrossRef]
- Al Atrouni, A.; Hamze, M.; Rafei, R.; Eveillard, M.; Joly-Guillou, M.L.; Kempf, M. Diversity of Acinetobacter species isolated from different environments in Lebanon: A nationwide study. Future Microbiol. 2016, 11, 1147–1156. [Google Scholar] [CrossRef]
- Al Atrouni, A.; Joly-Guillou, M.L.; Hamze, M.; Kempf, M. Reservoirs of Non-baumannii Acinetobacter Species. Front. Microbiol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Carvalheira, A.; Casquete, R.; Silva, J.; Teixeira, P. Prevalence and antimicrobial susceptibility of Acinetobacter spp. isolated from meat. Int. J. Food Microbiol. 2017, 243, 58–63. [Google Scholar] [CrossRef]
- Carvalheira, A.; Silva, J.; Teixeira, P. Lettuce and fruits as a source of multidrug resistant Acinetobacter spp. Food Microbiol. 2017, 64, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Wang, Y.; Tang, K.; Wang, D.; Hong, J.; Wang, P.; Ye, S.; Yan, J.; Li, S.; et al. Genetic landscape and evolution of Acinetobacter pittii, an underestimated emerging nosocomial pathogen. Commun. Biol. 2025, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Vera, A.; Bello-López, E.; Pantoja-Nuñez, G.I.; Rodríguez-López, G.M.; Morales-Erasto, V.; Castillo-Ramírez, S. Acinetobacter junii: An emerging One Health pathogen. mSphere 2024, 9, e0016224. [Google Scholar] [CrossRef]
- Castanheira, M.; Mendes, R.E.; Gales, A.C. Global Epidemiology and Mechanisms of Resistance of Acinetobacter baumannii-calcoaceticus Complex. Clin. Infect. Dis. 2023, 76, S166–S178. [Google Scholar] [CrossRef]
- Luo, Q.; Chang, M.; Lu, P.; Guo, Q.; Jiang, X.; Xiao, T.; Zhang, H.; Ma, Y.; Zhang, Y.; Yu, W.; et al. Genomic epidemiology and phylodynamics of Acinetobacter baumannii bloodstream isolates in China. Nat. Commun. 2025, 16, 3536. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H. Epidemiology and antimicrobial resistance trends of Acinetobacter species in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2024, 11, 1245131. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, P.; Chen, Y.; Shen, P.; Zheng, B.; Ji, J.; Ying, C.; Liu, Z.; Xiao, Y. ESKAPE in China: Epidemiology and characteristics of antibiotic resistance. Emerg. Microbes Infect. 2024, 13, 2317915. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control. Hosp. Epidemiol. 2019, 41, 1–18. [Google Scholar] [CrossRef]
- Mathur, P.; Malpiedi, P.; Walia, K.; Srikantiah, P.; Gupta, S.; Lohiya, A.; Chakrabarti, A.; Ray, P.; Biswal, M.; Taneja, N.; et al. Health-care-associated bloodstream and urinary tract infections in a network of hospitals in India: A multicentre, hospital-based, prospective surveillance study. Lancet Glob. Health 2022, 10, e1317–e1325. [Google Scholar] [CrossRef]
- Khateb, A.M.; Barefah, A.S.; Radhwi, O.O.; Algiraigri, A.; Azhar, E.I. Beyond neutropenia: 14 years analysis of bloodstream infections in hematological malignancies. J. Infect. Public Health 2025, 18, 102948. [Google Scholar] [CrossRef]
- Meiring, S.; Quan, V.; Mashau, R.; Perovic, O.; Magobo, R.; Smith, M.; Mpembe, R.; von Gottberg, A.; de Gouveia, L.; Walaza, S.; et al. Pathogen aetiology and risk factors for death among neonates with bloodstream infections at lower-tier South African hospitals: A cross-sectional study. Lancet Microbe 2025, 6, 100989. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrod, T.; Reuter, S.; Gross, A.; Kocer, K.; Günther, F.; Zimmermann, S.; Heeg, K.; Mutters, N.T.; Nurjadi, D. Molecular characterization of carbapenem-resistant Acinetobacter baumannii using WGS revealed missed transmission events in Germany from 2012–15. J. Antimicrob. Chemother. 2019, 74, 3473–3480. [Google Scholar] [CrossRef] [PubMed]
- Novovic, K.; Jovcic, B. Colistin Resistance in Acinetobacter baumannii: Molecular Mechanisms and Epidemiology. Antibiotics 2023, 12, 516. [Google Scholar] [CrossRef]
- Xu, Q.; Mu, X.; He, J.; Liu, H.; Liu, X.; Wang, Y.; Hua, X.; Yu, Y.; Manning, S.D. Phenotypic and genotypic characterization of clinical carbapenem-resistant Acinetobacter species harboring the metallo-beta-lactamases IMP-8 or NDM-1 in China. Microbiol. Spectr. 2025, 13, e0115824. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 6 September 2025).
- Chen, C.; Cui, C.Y.; Wu, X.T.; Fang, L.X.; He, Q.; He, B.; Long, T.F.; Liao, X.P.; Chen, L.; Liu, Y.H.; et al. Spread of tet(X5) and tet(X6) genes in multidrug-resistant Acinetobacter baumannii strains of animal origin. Vet. Microbiol. 2021, 253, 108954. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Rybak, M.J. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacotherapy 2012, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Lai, C.-C.; Ko, W.-C.; Hsueh, P.-R. Geographical patterns of in vitro susceptibilities to tigecycline and colistin among worldwide isolates of Acinetobacter baumannii, Escherichia coli and Klebsiella pneumoniae: Data from the Antimicrobial Testing Leadership and Surveillance (ATLAS) programme, 2016–2021. Int. J. Antimicrob. Agents 2023, 62, 106930. [Google Scholar] [CrossRef]
- Deng, M.; Zhu, M.-H.; Li, J.-J.; Bi, S.; Sheng, Z.-K.; Hu, F.-S.; Zhang, J.-J.; Chen, W.; Xue, X.-W.; Sheng, J.-F.; et al. Molecular Epidemiology and Mechanisms of Tigecycline Resistance in Clinical Isolates of Acinetobacter baumannii from a Chinese University Hospital. Antimicrob. Agents Chemother. 2014, 58, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, Y.; Hua, X. Resistance mechanisms of tigecycline in Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2023, 13, 1141490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H. The tigecycline resistance mechanisms in Gram-negative bacilli. Front. Cell. Infect. Microbiol. 2024, 14, 1471469. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhang, J.; Wang, X.; Zhang, Y.; Wang, H. Identification of a novel plasmid-mediated tigecycline resistance-related gene, tet(Y), in Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 77, 58–68. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.J.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.B.; Ji, Q.J.; Wei, R.C.; Liu, Z.H.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Chen, C.; Cui, C.Y.; Yu, J.J.; He, Q.; Wu, X.T.; He, Y.Z.; Cui, Z.H.; Li, C.; Jia, Q.L.; Shen, X.G.; et al. Genetic diversity and characteristics of high-level tigecycline resistance Tet(X) in Acinetobacter species. Genome Med. 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, K.; Xiao, X.; Wang, Y.; Wang, Z. Characterization of novel ISAba1-bounded tet(X15)-bearing composite transposon Tn6866 in Acinetobacter variabilis. J. Antimicrob. Chemother. 2021, 76, 2481–2483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, Y.; Yu, Y.; Jia, H.; Kong, Y.; Zhang, J.; Xie, X.; Stehling, E.G.; Furlan, J.P.R.; Zhou, Z.; et al. Interplay of multiple carbapenemases and tigecycline resistance in Acinetobacter species: A serious combined threat. Clin. Microbiol. Infect. 2025, 31, 128–133. [Google Scholar] [CrossRef]
- Cui, C.Y.; Chen, C.; Liu, B.T.; He, Q.; Wu, X.T.; Sun, R.Y.; Zhang, Y.; Cui, Z.H.; Guo, W.Y.; Jia, Q.L.; et al. Co-occurrence of Plasmid-Mediated Tigecycline and Carbapenem Resistance in Acinetobacter spp. from Waterfowls and Their Neighboring Environment. Antimicrob. Agents Chemother. 2020, 64, e02502-19. [Google Scholar] [CrossRef]
- Mallonga, Z.; Tokuda, M.; Yamazaki, R.; Tsuruga, S.; Nogami, I.; Sato, Y.; Tarrayo, A.G.; Fuentes, R.; Parilla, R.; Kimbara, K.; et al. Emergence of Acinetobacter towneri harbouring a novel plasmid with blaNDM-1 and tet(X7) from hospital wastewater in the Philippines. J. Glob. Antimicrob. Resist. 2025, 41, 287–289. [Google Scholar] [CrossRef]
- Guiney, D.G., Jr.; Hasegawa, P.; Davis, C.E. Expression in Escherichia coli of cryptic tetracycline resistance genes from bacteroides R plasmids. Plasmid 1984, 11, 248–252. [Google Scholar] [CrossRef]
- Speer, B.S.; Bedzyk, L.; Salyers, A.A. Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J. Bacteriol. 1991, 173, 176–183. [Google Scholar] [CrossRef]
- Yang, W.R.; Moore, I.F.; Koteva, K.P.; Bareich, D.C.; Hughes, D.W.; Wright, G.D. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 2004, 279, 52346–52352. [Google Scholar] [CrossRef] [PubMed]
- Moore, I.F.; Hughes, D.W.; Wright, G.D. Tigecycline Is Modified by the Flavin-Dependent Monooxygenase TetX. Biochemistry 2005, 44, 11829–11835. [Google Scholar] [CrossRef] [PubMed]
- Whittle, G.; Hund, B.D.; Shoemaker, N.B.; Salyers, A.A. Characterization of the 13-kilobase ermF region of the Bacteroides conjugative transposon CTnDOT. Appl. Environ. Microb. 2001, 67, 3488–3495. [Google Scholar] [CrossRef]
- Cui, C.Y.; He, Q.; Jia, Q.L.; Li, C.; Chen, C.; Wu, X.T.; Zhang, X.J.; Lin, Z.Y.; Zheng, Z.J.; Liao, X.P.; et al. Evolutionary Trajectory of the Tet(X) Family: Critical Residue Changes towards High-Level Tigecycline Resistance. mSystems 2021, 6, e00050-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, N.; Shen, Z.; Zeng, Y.; Lu, J.; Liu, C.; Zhou, H.; Hu, Y.; Sun, Q.; Cheng, Q.; et al. Epidemiological and phylogenetic analysis reveals Flavobacteriaceae as potential ancestral source of tigecycline resistance gene tet(X). Nat. Commun. 2020, 11, 4648. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, Y.; Wu, T.; Liu, J.; Guo, Y.; Huang, J. Concurrence of Inactivation Enzyme-Encoding Genes tet(X), blaEBR, and estT in Empedobacter Species from Chickens and Surrounding Environments. Foods 2024, 13, 3201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Cheung, Y.; Liu, C.; Chan, E.W.C.; Wong, K.Y.; Zhang, R.; Chen, S. Functional and phylogenetic analysis of TetX variants to design a new classification system. Commun. Biol. 2022, 5, 522. [Google Scholar] [CrossRef]
- Blake, K.S.; Xue, Y.-P.; Gillespie, V.J.; Fishbein, S.R.S.; Tolia, N.H.; Wencewicz, T.A.; Dantas, G. The tetracycline resistome is shaped by selection for specific resistance mechanisms by each antibiotic generation. Nat. Commun. 2025, 16, 1452. [Google Scholar] [CrossRef]
- Zhang, R.-M.; Sun, J.; Sun, R.-Y.; Wang, M.-G.; Cui, C.-Y.; Fang, L.-X.; Liao, M.-N.; Lu, X.-Q.; Liu, Y.-X.; Liao, X.-P.; et al. Source Tracking and Global Distribution of the Tigecycline Non-Susceptible tet(X). Microbiol. Spectr. 2021, 9, e0116421. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zeng, Y.; Cai, C.; Sun, C.; Lu, J.; Liu, C.; Zhou, H.; Sun, Q.; Shu, L.; Wang, H.; et al. Prevalence, transmission, and molecular epidemiology of tet(X)-positive bacteria among humans, animals, and environmental niches in China: An epidemiological, and genomic-based study. Sci. Total Environ. 2021, 818, 151767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.M.; Li, Y.K.; Yu, R.H.; Ma, M.X.; Yang, M.; Si, H.B. Identification of Novel tet(X3) Variants Resistant To Tigecycline in Acinetobacter Species. Microbiol. Spectr. 2022, 10, e0133322. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, G.; Liu, J.; Liu, L.; Zhao, S.; Wang, L.; He, D. A novel tigecycline resistance gene, tet(X6), on an SXT/R391 integrative and conjugative element in a Proteus genomospecies 6 isolate of retail meat origin. J. Antimicrob. Chemother. 2020, 75, 1159–1164. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Li, Y.K.; Yang, M.; He, Y.; Shi, X.R.; Zhang, Z.D.; Zhong, Y.S.; Zhang, Y.; Si, H.B. Emergence of novel tigecycline resistance gene tet(X5) variant in multidrug-resistant Acinetobacter indicus of swine farming environments. Vet. Microbiol. 2023, 284, 109837. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Wu, J.-W.; Chen, Y.-Y.; Quyen, T.L.T.; Liao, W.-C.; Li, S.-W.; Chen, Y.-C.; Pan, Y.-J. An Outbreak of tet(X6)-Carrying Tigecycline-Resistant Acinetobacter baumannii Isolates with a New Capsular Type at a Hospital in Taiwan. Antibiotics 2021, 10, 1239. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Liu, Y.; Song, J.; Chen, Y.; Shan, T.; Xiao, Y.; Zhou, K. Detection of a new tet(X6)-encoding plasmid in Acinetobacter towneri. J. Glob. Antimicrob. Resist. 2021, 25, 132–136. [Google Scholar] [CrossRef]
- Zheng, X.-R.; Zhu, J.-H.; Zhang, J.; Cai, P.; Sun, Y.-H.; Chang, M.-X.; Fang, L.-X.; Sun, J.; Jiang, H.-X. A novel plasmid-borne tet(X6) variant co-existing with blaNDM-1 and blaOXA-58 in a chicken Acinetobacter baumannii isolate. J. Antimicrob. Chemother. 2020, 75, 3397–3399. [Google Scholar] [CrossRef]
- Qian, C.R.; Ma, Z.X.; Feng, L.Z.; Guo, W.H.; Han, Y.J.; Zhang, Y.; Xu, C.Q.; Cao, J.M.; Zhou, T.L. Emergence of tet(X2) in Acinetobacter pittii confers clinical resistance to tigecycline. J. Antimicrob. Chemother. 2023, 78, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Yu, R.H.; Zhao, W.B.; Schwarz, S.; Li, C.L.; Yao, H.; Du, X.D. Characterization of a genomic Island carrying the tet(X4) gene in porcine Acinetobacter towneri co-harboring plasmid-borne blaNDM-1 and blaOXA-58 genes. Front. Vet. Sci. 2022, 9, 1002149. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Lv, Y.; Cui, L.; Li, Y.; Li, T.; Song, H.; Hao, Y.; Shen, J.; Wang, Y.; et al. Novel Plasmid-Mediated tet(X5) Gene Conferring Resistance to Tigecycline, Eravacycline, and Omadacycline in a Clinical Acinetobacter baumannii Isolate. Antimicrob. Agents Chemother. 2019, 64, e01326-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, P.-Y.; Cui, C.-Y.; He, Q.; Sun, J.; Liu, Y.-H.; Huang, J.-L. Classification and molecular characteristics of tet(X)-carrying plasmids in Acinetobacter species. Front. Microbiol. 2022, 13, 974432. [Google Scholar] [CrossRef]
- Deng, Y.; Zeng, Z.; Liu, J.; Chen, Z. Insertion sequence common region element: A novel gene-capturing system in bacteria--a review. Acta Microbiol. Sin. 2009, 49, 987–993. [Google Scholar]
- Ji, K.; Xu, Y.; Sun, J.; Huang, M.; Jia, X.; Jiang, C.; Feng, Y. Harnessing efficient multiplex PCR methods to detect the expanding Tet(X) family of tigecycline resistance genes. Virulence 2019, 11, 49–56. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, D.; Song, H.; Liu, Z.; Jiang, H.; Wang, Y. Development of a Multiplex Real-Time PCR Assay for Rapid Detection of Tigecycline Resistance Gene tet(X) Variants from Bacterial, Fecal, and Environmental Samples. Antimicrob. Agents Chemother. 2020, 64, e02292-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, L.; Lin, S.; Yang, C.; Liang, H.; Huang, K.; Guo, Z.; Lv, F. Sensitive and rapid detection of tet(X2) ~ tet(X5) by loop-mediated isothermal amplification based on visual OTG dye. BMC Microbiol. 2023, 23, 329. [Google Scholar] [CrossRef]
- Cui, Z.H.; Ni, W.N.; Tang, T.; He, B.; Zhong, Z.X.; Fang, L.X.; Chen, L.; Chen, C.; Cui, C.Y.; Liu, Y.H.; et al. Rapid detection of plasmid-mediated high-level tigecycline resistance in Escherichia coli and Acinetobacter spp. J. Antimicrob. Chemother. 2020, 75, 1479–1483. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Pan, Q.; Wang, J.; Jiao, X.a.; Zhang, Y. Development and evaluation of rapid and accurate one-tube RPA-CRISPR-Cas12b-based detection of mcr-1 and tet(X4). Appl. Microbiol. Biotechnol. 2024, 108, 345. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Zheng, Z.-J.; Tang, T.; Zhong, Z.-X.; Cui, C.-Y.; Lian, X.-L.; Fang, L.-X.; He, Q.; Wang, X.-R.; Chen, C.; et al. Rapid Detection of High-Level Tigecycline Resistance in Tet(X)-Producing Escherichia coli and Acinetobacter spp. Based on MALDI-TOF MS. Front. Cell. Infect. Microbiol. 2020, 10, 583341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.J.; Cui, Z.H.; Diao, Q.Y.; Ye, X.Q.; Zhong, Z.X.; Tang, T.; Wu, S.B.; He, H.L.; Lian, X.L.; Fang, L.X.; et al. MALDI-TOF MS for rapid detection and differentiation between Tet(X)-producers and non-Tet(X)-producing tetracycline-resistant Gram-negative bacteria. Virulence 2022, 13, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zampaloni, C.; Mattei, P.; Bleicher, K.; Winther, L.; Thäte, C.; Bucher, C.; Adam, J.-M.; Alanine, A.; Amrein, K.E.; Baidin, V.; et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 2024, 625, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Pahil, K.S.; Gilman, M.S.A.; Baidin, V.; Clairfeuille, T.; Mattei, P.; Bieniossek, C.; Dey, F.; Muri, D.; Baettig, R.; Lobritz, M.; et al. A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature 2024, 625, 572–577. [Google Scholar] [CrossRef]
- Wang, H.; Ishchenko, A.; Skudlarek, J.; Shen, P.; Dzhekieva, L.; Painter, R.E.; Chen, Y.-T.; Bukhtiyarova, M.; Leithead, A.; Tracy, R.; et al. Cerastecins inhibit membrane lipooligosaccharide transport in drug-resistant Acinetobacter baumannii. Nat. Microbiol. 2024, 9, 1244–1255. [Google Scholar] [CrossRef]
- Alagesan, K.; Nagarajan, H.; Jeyakanthan, J. Repurposing FDA-approved drugs for combating tigecycline resistance in Acinetobacter baumannii: In silico screening against BaeR protein. Mol. Divers. 2024, 29, 2243–2264. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Deng, T.; Wang, Z. Reversion of antibiotic resistance in multidrug-resistant pathogens using non-antibiotic pharmaceutical benzydamine. Commun. Biol. 2021, 4, 1328. [Google Scholar] [CrossRef]
- Park, J.; Gasparrini, A.J.; Reck, M.R.; Symister, C.T.; Elliott, J.L.; Vogel, J.P.; Wencewicz, T.A.; Dantas, G.; Tolia, N.H. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat. Chem. Biol. 2017, 13, 730–736. [Google Scholar] [CrossRef]
- Markley, J.L.; Fang, L.; Gasparrini, A.J.; Symister, C.T.; Kumar, H.; Tolia, N.H.; Dantas, G.; Wencewicz, T.A. Semisynthetic Analogues of Anhydrotetracycline as Inhibitors of Tetracycline Destructase Enzymes. ACS Infect. Dis. 2019, 5, 618–633. [Google Scholar] [CrossRef]
- Kumar, H.; Williford, E.E.; Blake, K.S.; Virgin-Downey, B.; Dantas, G.; Wencewicz, T.A.; Tolia, N.H. Structure of anhydrotetracycline-bound Tet(X6) reveals the mechanism for inhibition of type 1 tetracycline destructases. Commun. Biol. 2023, 6, 423. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e613. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Niu, S.; Liu, Z.; Zou, Y.; Yang, Y.; Feng, H.; Liu, D.; Niu, X.; Deng, X.; et al. A novel inhibitor of monooxygenase reversed the activity of tetracyclines against tet(X3)/tet(X4)-positive bacteria. EBioMedicine 2022, 78, 103943. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, L.; Kang, G.; Wang, P.; Yin, H.; Huang, H. A Potential Combination Therapy of Berberine Hydrochloride with Antibiotics Against Multidrug-Resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2021, 11, 660431. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 7313–7326. [Google Scholar] [CrossRef]
- Güran, M.; Çakıral, K.; Teralı, K.; Kandemir, T.; Şanlıtürk, G.; Öcal, M.M.; Nagiyev, T.; Köksal, F. Meropenem in combination with baicalein exhibits synergism against extensively drug resistant and pan-drug-resistant Acinetobacter baumannii clinical isolates in vitro. Pathog. Dis. 2023, 81, ftad007. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Zhou, Z.; Yang, J.; Liu, W.; Zhang, Y.; Zhang, P.; Guo, T.; Li, G. Baicalein Resensitizes Multidrug-Resistant Gram-Negative Pathogens to Doxycycline. Microbiol. Spectr. 2023, 11, e0470222. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Galgano, S.; Houdijk, J.; Xie, W.; Jin, Y.; Lin, J.; Song, W.; Fu, Y.; Li, X.; et al. Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry. Antibiotics 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Surachat, K.; Singkhamanan, K. Isolation and Characterization of a Novel Autographiviridae Phage and Its Combined Effect with Tigecycline in Controlling Multidrug-Resistant Acinetobacter baumannii-Associated Skin and Soft Tissue Infections. Viruses 2022, 14, 194. [Google Scholar] [CrossRef]
- Yin, S.; Huang, G.; Zhang, Y.; Jiang, B.; Yang, Z.; Dong, Z.; You, B.; Yuan, Z.; Hu, F.; Zhao, Y.; et al. Phage Abp1 Rescues Human Cells and Mice from Infection by Pan-Drug Resistant Acinetobacter baumannii. Cell. Physiol. Biochem. 2017, 44, 2337–2345. [Google Scholar] [CrossRef]
- Koncz, M.; Stirling, T.; Hadj Mehdi, H.; Méhi, O.; Eszenyi, B.; Asbóth, A.; Apjok, G.; Tóth, Á.; Orosz, L.; Vásárhelyi, B.M.; et al. Genomic surveillance as a scalable framework for precision phage therapy against antibiotic-resistant pathogens. Cell 2024, 187, 5901–5918.e28. [Google Scholar] [CrossRef]
- Cha, K.; Oh, H.K.; Jang, J.Y.; Jo, Y.; Kim, W.K.; Ha, G.U.; Ko, K.S.; Myung, H. Characterization of Two Novel Bacteriophages Infecting Multidrug-Resistant (MDR) Acinetobacter baumannii and Evaluation of Their Therapeutic Efficacy in Vivo. Front. Microbiol. 2018, 9, 696. [Google Scholar] [CrossRef]
- Hussain, F.A.; Dubert, J.; Elsherbini, J.; Murphy, M.; VanInsberghe, D.; Arevalo, P.; Kauffman, K.; Rodino-Janeiro, B.K.; Gavin, H.; Gomez, A.; et al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science 2021, 374, 488–492. [Google Scholar] [CrossRef]
- Lucidi, M.; Imperi, F.; Artuso, I.; Capecchi, G.; Spagnoli, C.; Visaggio, D.; Rampioni, G.; Leoni, L.; Visca, P. Phage-mediated colistin resistance in Acinetobacter baumannii. Drug Resist. Updates 2024, 73, 101061. [Google Scholar] [CrossRef]
- Krahn, T.; Wibberg, D.; Maus, I.; Winkler, A.; Bontron, S.; Sczyrba, A.; Nordmann, P.; Puhler, A.; Poirel, L.; Schluter, A. Intraspecies Transfer of the Chromosomal Acinetobacter baumannii blaNDM-1 Carbapenemase Gene. Antimicrob. Agents Chemother. 2016, 60, 3032–3040. [Google Scholar] [CrossRef]
- MacNair, C.R.; Rutherford, S.T.; Tan, M.-W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 2023, 22, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Júnior, N.G.; Souza, C.M.; Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial peptides: Structure, functions and translational applications. Nat. Rev. Microbiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, J.; Nag, M.; Ghosh, P.; Rajmani, R.S.; Chatterjee, R.; Karmakar, K.; Chandra, K.; Chatterjee, J.; Chakravortty, D.; Varadarajan, R. A CcdB toxin-derived peptide acts as a broad-spectrum antibacterial therapeutic in infected mice. EMBO Rep. 2023, 24, e55338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lan, X.-Q.; Du, Y.; Chen, P.-Y.; Zhao, J.; Zhao, F.; Lee, W.-H.; Zhang, Y. King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates. Zool. Res. 2018, 39, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hazam, P.K.; Cheng, C.-C.; Hsieh, C.-Y.; Lin, W.-C.; Hsu, P.-H.; Chen, T.-L.; Lee, Y.-T.; Chen, J.-Y. Development of Bactericidal Peptides against Multidrug-Resistant Acinetobacter baumannii with Enhanced Stability and Low Toxicity. Int. J. Mol. Sci. 2022, 23, 2191. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Roy, N.; Kulkarni, O.; Nanajkar, N.; Datey, A.; Ravichandran, S.; Thakur, C.; Thirunavukkarasu, S.; Aprameya, I.V.; Sarma, S.P.; et al. Ω76: A designed antimicrobial peptide to combat carbapenem- and tigecycline-resistant Acinetobacter baumannii. Sci. Adv. 2019, 5, eaax1946. [Google Scholar] [CrossRef]
- Santos-Júnior, C.D.; Torres, M.D.T.; Duan, Y.; Rodríguez del Río, Á.; Schmidt, T.S.B.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef]
- Wang, B.; Lin, P.; Zhong, Y.; Tan, X.; Shen, Y.; Huang, Y.; Jin, K.; Zhang, Y.; Zhan, Y.; Shen, D.; et al. Explainable deep learning and virtual evolution identifies antimicrobial peptides with activity against multidrug-resistant human pathogens. Nat. Microbiol. 2025, 10, 332–347. [Google Scholar] [CrossRef]
- Dance, A. Five ways science is tackling the antibiotic resistance crisis. Nature 2024, 632, 494–496. [Google Scholar] [CrossRef]
- Rossetti, P.; Trollmann, M.F.W.; Wichmann, C.; Gutsmann, T.; Eggeling, C.; Böckmann, R.A. From Membrane Composition to Antimicrobial Strategies: Experimental and Computational Approaches to AMP Design and Selectivity. Small 2025, e2411476. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e0082922. [Google Scholar] [CrossRef]
- de Dios, R.; Gadar, K.; McCarthy, R.R. A high-efficiency scar-free genome-editing toolkit for Acinetobacter baumannii. J. Antimicrob. Chemother. 2022, 77, 3390–3398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Q.; Huang, S.; Zhang, N.; Sun, D. Advancements in CRISPR-Cas-based strategies for combating antimicrobial resistance. Microbiol. Res. 2025, 298, 128232. [Google Scholar] [CrossRef]
- Bai, J.; Dai, Y.; Farinha, A.; Tang, A.Y.; Syal, S.; Vargas-Cuebas, G.; van Opijnen, T.; Isberg, R.R.; Geisinger, E.; O’Toole, G. Essential Gene Analysis in Acinetobacter baumannii by High-Density Transposon Mutagenesis and CRISPR Interference. J. Bacteriol. 2021, 203, e0056520. [Google Scholar] [CrossRef]
- Wang, R.; Shu, X.; Zhao, H.; Xue, Q.; Liu, C.; Wu, A.; Cheng, F.; Wang, L.; Zhang, Y.; Feng, J.; et al. Associate toxin-antitoxin with CRISPR-Cas to kill multidrug-resistant pathogens. Nat. Commun. 2023, 14, 2078. [Google Scholar] [CrossRef]
- Raza, A.; Fatima, P.; Yasmeen, B.; Rana, Z.A.; Ellakwa, D.E. From resistance to remedy: The role of clustered regularly interspaced short palindromic repeats system in combating antimicrobial resistance-a review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2259–2273. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef] [PubMed]
- Ali Agha, A.S.A.; Al-Samydai, A.; Aburjai, T. New frontiers in CRISPR: Addressing antimicrobial resistance with Cas9, Cas12, Cas13, and Cas14. Heliyon 2025, 11, e42013. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Harjai, K.; Chhibber, S. Repurposing prokaryotic clustered regularly interspaced short palindromic repeats-Cas adaptive immune system to combat antimicrobial resistance. Future Microbiol. 2023, 18, 443–459. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wu, T.; Liu, J.; Gao, J. Threat and Control of tet(X)-Mediated Tigecycline-Resistant Acinetobacter sp. Bacteria. Foods 2025, 14, 3374. https://doi.org/10.3390/foods14193374
Chen C, Wu T, Liu J, Gao J. Threat and Control of tet(X)-Mediated Tigecycline-Resistant Acinetobacter sp. Bacteria. Foods. 2025; 14(19):3374. https://doi.org/10.3390/foods14193374
Chicago/Turabian StyleChen, Chong, Taotao Wu, Jing Liu, and Jie Gao. 2025. "Threat and Control of tet(X)-Mediated Tigecycline-Resistant Acinetobacter sp. Bacteria" Foods 14, no. 19: 3374. https://doi.org/10.3390/foods14193374
APA StyleChen, C., Wu, T., Liu, J., & Gao, J. (2025). Threat and Control of tet(X)-Mediated Tigecycline-Resistant Acinetobacter sp. Bacteria. Foods, 14(19), 3374. https://doi.org/10.3390/foods14193374





