Elucidating Sugar–Acid Metabolic Diversity and Screening Breeding Materials in Xinjiang Pear (Pyrus) Germplasm Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sensory Evaluation
2.3. Determination of Appearance Quality and Soluble Solid Content
2.4. Analysis of Sugar Composition and Content
2.5. Analysis of Organic Acid Composition and Content
2.6. Data Analysis
3. Results
3.1. Phenotypic Trait Analysis and Sensory Evaluation of Pear Germplasm Resources
3.2. Analysis of Sugar Composition and Content Variation
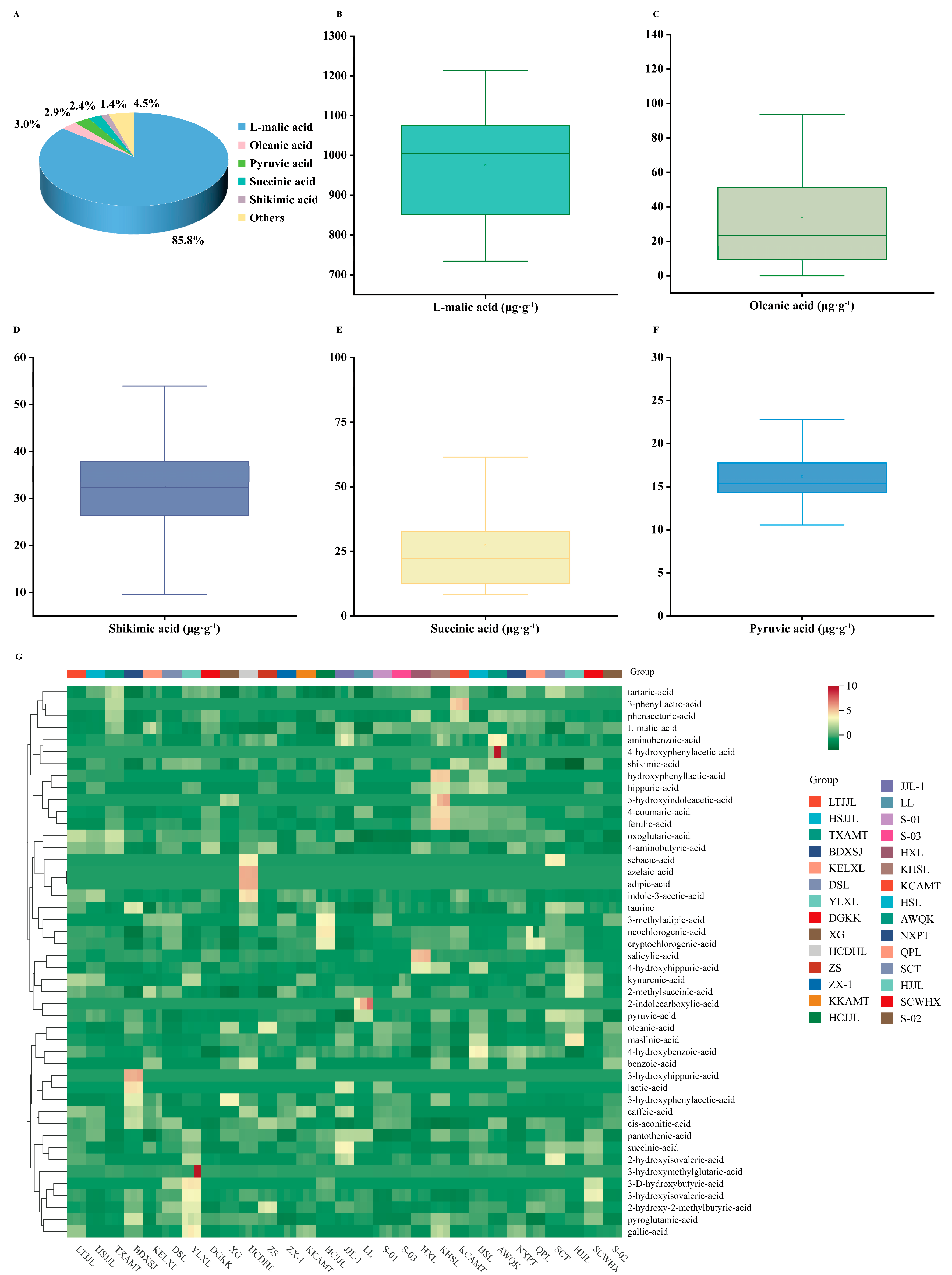
3.3. Analysis of the Organic Acids Composition and Content
3.4. Variation in Sugar and Organic Acid Contents at Different Maturation Stages
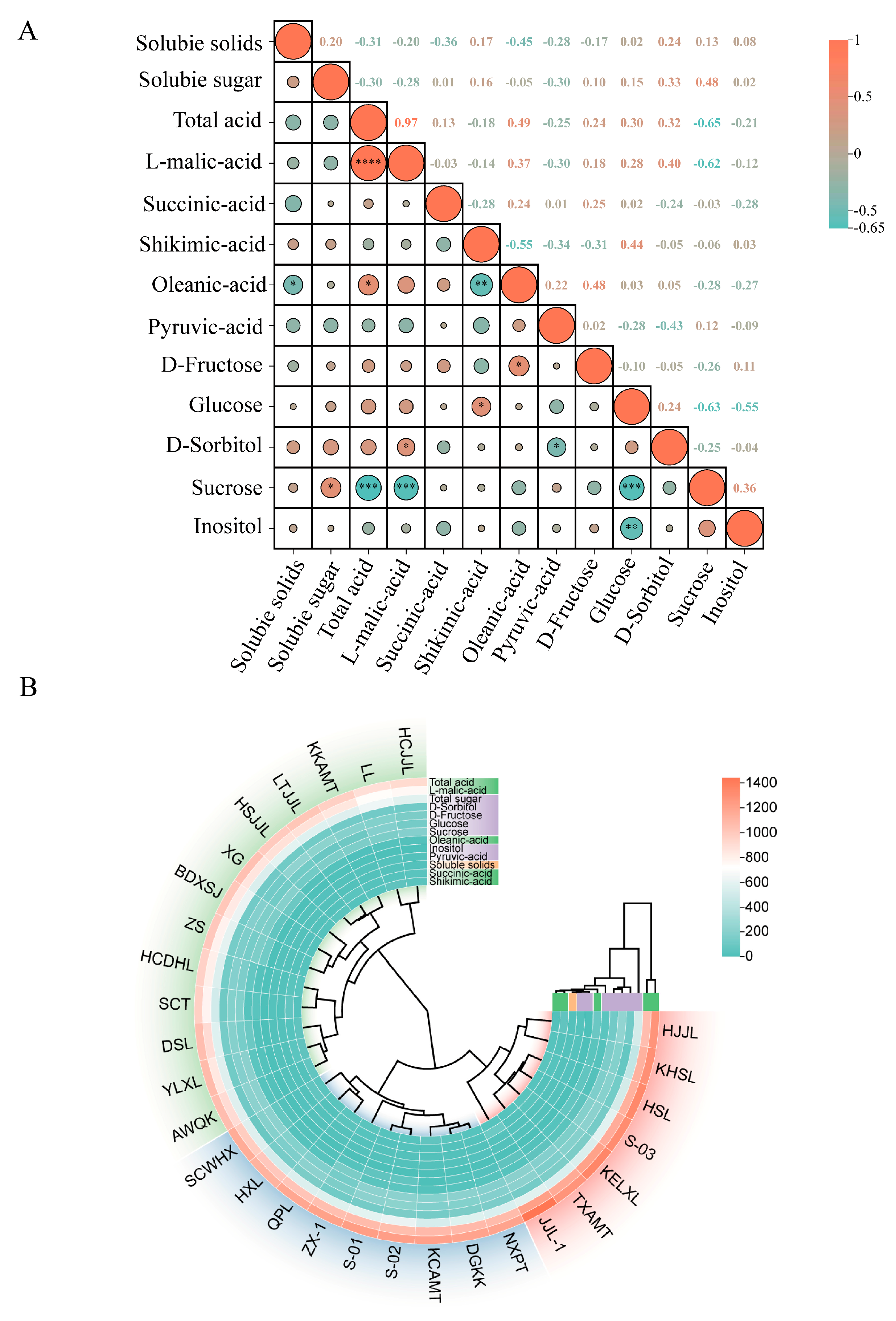
3.5. Correlation Analysis of Fruit Quality Traits in Pear Germplasm Resources
3.6. Cluster Analysis
3.7. Correlation Between Sensory Evaluations and Sugar and Organic Acid Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Zhou, W.Q.; Chen, X.Y.; Fan, G.Q.; Zhang, S.K.; Liao, K. Genome size and chromosome ploidy identification in pear germplasm represented by Asian pears-Local pear varieties. Sci. Hortic. 2020, 265, 109202. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.; Yang, X.P.; Wu, C.Y.; Ming, J.Q.; Zhang, H.Y.; Chen, J.J.; Wang, J.B.; Xu, J. Metabolome and transcriptome profiling revealed the enhanced synthesis of volatile esters in Korla pear. BMC Plant Biol. 2023, 23, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Yin, H.; Wu, X.; Shi, X.J.; Qi, K.J.; Zhang, S.L. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, C.K.; Zhao, Y.W.; Sun, C.H.; Hu, D.G. Mechanisms and regulation of organic acid accumulation in plant vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Li, Z.Y.; Liao, G.L.; Chen, L.; Zhong, M.; Huang, C.H.; Jia, D.F.; Xu, X.B. Variation in fruit quality within wild Actinidia eriantha germplasm. N. Z. J. Crop Hortic. Sci. 2020, 48, 153–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Ma, Y.; Guan, J.; Zhang, H. Regulation of pear fruit quality: A review based on chinese pear varieties. Agronomy 2025, 15, 30. [Google Scholar] [CrossRef]
- Cao, J.P.; Jiang, Q.; Lin, J.Y.; Li, X.; Sun, C.D.; Chen, K.S. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.Z.; Zheng, W.L.; Tan, Q.L.; Xie, Z.Z.; Zheng, C.S.; Hu, C.X. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds—A review. Front. Plant Sci. 2020, 11, 572601. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, T.; Zhou, J.T.; Huang, F.; Wang, Y.Y.K.; Liu, Y.X.; Liu, Z.S.; He, W.; Li, M.Y.; Lin, Y.X.; et al. Fruit sugar and organic acid composition and inheritance analysis in an intraspecific cross of Chinese cherry. LWT—Food Sci. Technol. 2024, 198, 116101. [Google Scholar] [CrossRef]
- Xi, W.P.; Zheng, H.W.; Zhang, Q.Y.; Li, W.H. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef]
- Zanon, L.; Falchi, R.; Santi, S.; Vizzotto, G. Sucrose transport and phloem unloading in peach fruit: Potential role of two transporters localized in different cell types. Physiol. Plant. 2015, 154, 179–193. [Google Scholar] [CrossRef]
- Zhou, J.T.; Yang, S.W.; Ma, Y.; Liu, Z.S.; Tu, H.X.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Li, M.Y.; et al. Soluble sugar and organic acid composition and flavor evaluation of Chinese cherry fruits. Food Chem. X 2023, 20, 100953. [Google Scholar] [CrossRef]
- Li, Y.; Yan, L.; Zhang, B.; Yang, S.; Zhao, Z. A study on sugar and organic acid components in different apple cultivars. J. Fruit Sci. 2021, 38, 1877–1889. (In Chinese) [Google Scholar]
- Wang, L.; Huang, Y.; Liu, Z.A.; He, J.X.; Jiang, X.L.; He, F.; Lu, Z.H.; Yang, S.Z.; Chen, P.; Yu, H.W.; et al. Somatic variations led to the selection of acidic and acidless orange cultivars. Nat. Plants 2021, 7, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Battistelli, A.; Bonghi, C.; Drincovich, M.F.; Falchi, R.; Lara, M.V.; Moscatello, S.; Vizzotto, G.; Famiani, F. Non-structural carbohydrate metabolism in the flesh of stone fruits of the genus Prunus (Rosaceae)—A review. Front. Plant Sci. 2020, 11, 549921. [Google Scholar] [CrossRef]
- Yao, G.F.; Yang, Z.J.; Zhang, S.L.; Cao, Y.F.; Liu, J.; Wu, J. Characteristics of components and contents of organic acid in pear fruits from different cultivated species. Acta Hortic. Sinica. 2014, 41, 755–764. (In Chinese) [Google Scholar]
- Akagić, A.; Oras, A.; Gaši, F.; Meland, M.; Drkenda, P.; Memić, S.; Spaho, N.; Žuljević, S.O.; Jerković, I.; Musić, O.; et al. A comparative study of ten pear (Pyrus communis L.) cultivars in relation to the content of sugars, organic acids, and polyphenol compounds. Foods 2022, 11, 3031. [Google Scholar] [CrossRef]
- Wang, L.B.; Ma, M.; Zhang, Y.R.; Wu, Z.F.; Guo, L.; Luo, W.Q.; Wang, L.; Zhang, Z.; Zhang, S.L. Characterization of the genes involved in malic acid metabolism from pear fruit and their expression profile after postharvest 1-MCP/ethrel treatment. J. Agric. Food Chem. 2018, 66, 8772–8782. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, Z.; Bai, X.; Yu, S.; An, S.; Zheng, Q.; Tang, Z.; Zhi, J. Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region. Open Life Sci. 2024, 19, 20220911. [Google Scholar] [CrossRef]
- Li, W.B.; Wu, Z.Q.; Xu, Y.; Long, H.P.; Deng, Y.H.; Li, S.W.; Xi, Y.; Li, W.Q.; Cai, H.L.; Zhang, B.K.; et al. Emerging LC-MS/MS-based molecular networking strategy facilitates foodomics to assess the function, safety, and quality of foods: Recent trends and future perspectives. Trends Food Sci. Technol. 2023, 139, 104114. [Google Scholar] [CrossRef]
- Lozano, L.; Iglesias, I.; Puy, J.; Echeverria, G. Performance of an expert sensory panel and instrumental measures for assessing eating fruit quality attributes in a pear breeding programme. Foods 2023, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, S.M.; Zhang, H.P.; Zhang, H.Y.; Wu, C.Y.; Tang, Z.H.; Wang, J.B.; Xu, J. Sensory quality evaluation of korla pear from different orchards and analysis of their primary and volatile metabolites. Molecules 2020, 25, 5567. [Google Scholar] [CrossRef]
- Yin, H.; Wu, J.Y.; Fan, J.B.; Xu, L.L.; Zhang, W.W.; Li, Q.H.; Jia, L.T.; Wu, X.; Wang, Z.W.; Li, H.X.; et al. Profiling of soluble sugar compositions in mature fruits of a diverse pear (Pyrus spp.) germplasm by UPLC. J. Food Compos. Anal. 2024, 132, 106281. [Google Scholar] [CrossRef]
- Wu, J.Y.; Fan, J.B.; Li, Q.H.; Jia, L.T.; Xu, L.L.; Wu, X.; Wang, Z.W.; Li, H.X.; Qi, K.J.; Qiao, X.; et al. Variation of organic acids in mature fruits of 193 pear (Pyrus spp.) cultivars. J. Food Compos. Anal. 2022, 109, 104483. [Google Scholar] [CrossRef]
- Ma, W.F.; Li, B.Y.; Zheng, L.T.; Peng, Y.J.; Tian, R.; Yuan, Y.Y.; Zhu, L.C.; Su, J.; Ma, F.W.; Li, M.J.; et al. Combined profiling of transcriptome and DNA methylome reveal genes involved in accumulation of soluble sugars and organic acid in apple fruits. Foods 2021, 10, 2198. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wu, J.Y.; Qin, G.H.; Yao, G.F.; Qi, K.J.; Wang, L.F.; Zhang, S.L. The role of sucrose-metabolizing enzymes in pear fruit that differ in sucrose accumulation. Acta Physiol. Plant. 2014, 36, 71–77. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, B.; Lu, J.Y.; Xiang, M.J.; Lin, Y.X.; Zhang, Y.T.; Wang, Y.; He, W.; Li, M.Y.; Chen, Q. Comprehensive analysis and evaluation of the intrinsic fruit quality of Sichuan pear germplasm resources in China. Sci. Hortic. 2025, 343, 114084. [Google Scholar] [CrossRef]
- Ma, B.Q.; Chen, J.; Zheng, H.Y.; Fang, T.; Ogutu, C.; Li, S.H.; Han, Y.P.; Wu, B.H. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Basson, C.E.; Groenewald, J.H.; Kossmann, J.; Cronjé, C.; Bauer, R. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme. Food Chem. 2010, 121, 1156–1162. [Google Scholar] [CrossRef]
- Wang, X.H.; Chen, Y.Y.; Zhang, J.J.; Wang, Z.W.; Qi, K.J.; Li, H.X.; Yin, H. Comparative analysis of volatile aromatic compounds from a wide range of pear (Pyrus L.) germplasm resources based on HS-SPME with GC–MS. Food Chem. 2023, 418, 135963. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, X.; Li, Z.G.; Zhang, X.S.; Jemric, T.; Wang, X. Quality monitoring and analysis of Xinjiang ‘Korla’ fragrant pear in cold chain logistics and home storage with multi-sensor technology. Appl. Sci. 2019, 9, 3895. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.X.; Jiang, J.B.; Yang, H.H.; Li, J.F. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Alcaraz-Marmol, F.; Nuncio-Jauregui, N.; Garcia-Sanchez, F.; Martinez-Nicolas, J.J.; Hernandez, F. Characterization of twenty pomegranate (Punica granatum L.) cultivars grown in Spain: Aptitudes for fresh consumption and processing. Sci. Hortic. 2017, 219, 152–160. [Google Scholar] [CrossRef]
- Wu, J.H.; Gao, H.Y.; Zhao, L.; Liao, X.J.; Chen, F.; Wang, Z.F.; Hu, X.S. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Lee, J. Sorbitol, Rubus fruit, and misconception. Food Chem. 2015, 166, 616–622. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects—An updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 119–135. [Google Scholar] [CrossRef]
- Yin, C.; Tian, L.M.; Li, J.; Cao, Y.F.; Dong, X.G.; Huo, H.L.; Xu, J.Y.; Liu, C. Evaluation of pear fruit quality in different ripening stages based on internal quality characteristics. J. Food Compos. Anal. 2025, 140, 107282. [Google Scholar] [CrossRef]
- Sokol-Letowska, A.; Kucharska, A.Z.; Hodun, G.; Golba, M. Chemical composition of 21 cultivars of sour cherry (Prunus cerasus) fruit cultivated in Poland. Molecules 2020, 25, 4581. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]


| Number | Number | Cultivar | Harvest Date | Single-Fruit Weight (g) | Transverse Diameter (mm) | Longitudinal Diameter (mm) | Fruit Shape Index (%) | Fruit Firmness (kg/cm2) | Soluble Solids (%) | Sensory Flavor | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rating | Score | ||||||||||
| 1 | LTJJL | Luntai Jujuli | 3 September | 102.7 ± 0.3 ij | 55.3 ± 1.0 kl | 57.8 ± 1.3 k | 1.0 ± 0.0 cdef | 7.6 ± 0.4 ghi | 13.4 ± 0.3 cde | sour–sweet | 6.1 |
| 2 | HSJJL | Hese Jujuli | 19 September | 109.2 ± 0.7 hij | 60.0 ± 0.4 ijk | 65.4 ± 0.4 ghi | 0.9 ± 0.0 defg | 6.9 ± 0.7 ij | 12.0 ± 0.4 fghi | sweet–sour | 4.8 |
| 3 | TXAMT | Taxamut | 1 October | 157.0 ± 0.6 defg | 64.7 ± 1.9 fghi | 73.0 ± 4.5 efgh | 0.9 ± 0.1 efgh | 14.6 ± 0.3 a | 15.5 ± 0.2 ab | sour | 1.6 |
| 4 | BDXSJ | Baodaoxinshiji | 4 September | 355.0 ± 7.0 a | 85.3 ± 0.8 bc | 77.0 ± 2.9 cde | 1.1 ± 0.0 b | 8.7 ± 0.7 g | 12.1 ± 0.6 fgh | sour–sweet | 7.1 |
| 5 | KELXL | Korla Fragrant Pear | 23 September | 140.7 ± 2.3 efghij | 61.0 ± 0.6 hij | 73.7 ± 3.8 def | 0.8 ± 0.1 gh | 5.9 ± 0.3 jkl | 12.4 ± 0.4 efgh | sweet | 7.1 |
| 6 | DSL | Dangshanli | 18 September | 303.2 ± 47.9 b | 88.2 ± 0.2 b | 90.8 ± 2.8 b | 1.0 ± 0.0 cde | 12.1 ± 0.4 cde | 16.3 ± 0.7 a | sour–sweet | 7.0 |
| 7 | YLXL | Ili Fragrant Pear | 14 August | 127.5 ± 11.0 ghij | 56.7 ± 0.3 jkl | 57.5 ± 1.7 k | 1.0 ± 0.0 c | 12.7 ± 0.6 bcd | 13.2 ± 0.6 def | sweet–sour | 4.8 |
| 8 | DGKK | Daguokuikeamut | 1 October | 151.3 ± 0.8 defgh | 45.6 ± 1.1 m | 44.8 ± 0.8 l | 1.2 ± 0.0 b | 12.6 ± 0.4 bcd | 14.5 ± 0.2 bcd | sour | 2.4 |
| 9 | XG | Xingao | 4 September | 189.0 ± 0.6 d | 74.0 ± 0.6 e | 62.0 ± 0.6 ijk | 0.7 ± 0.0 i | 8.9 ± 0.1 g | 11.9 ± 0.1 fghi | sweet–sour | 3.0 |
| 10 | HCDHL | Huocheng Donghuangli | 2 September | 191.4 ± 30.2 d | 63.3 ± 4.2 ghi | 89.3 ± 1.5 b | 0.9 ± 0.0 efgh | 13.1 ± 0.2 bcd | 12.5 ± 0.1 efgh | sweet | 8.1 |
| 11 | ZS | Zaosu | 20 September | 142.7 ± 3.1 efghij | 63.0 ± 1.1 ghi | 71.4 ± 2.7 defg | 0.9 ± 0.0 cdef | 5.1 ± 0.4 lm | 11.8 ± 0.2 ghi | sweet–sour | 4.8 |
| 12 | ZX-1 | Zhuxuan 1 | 23 September | 190.7 ± 11.7 d | 69.7 ± 1.7 ef | 74.3 ± 1.5 def | 1.0 ± 0.0 c | 4.4 ± 0.7 m | 14.7 ± 0.7 b | sweet | 7.2 |
| 13 | KKAMT | Kuikeamut | 27 July | 47.2 ± 0.5 k | 45.6 ± 1.1 m | 44.8 ± 0.8 l | 1.0 ± 0.1 c | 12.6 ± 0.4 bcd | 14.5 ± 0.2 bcd | sour–sweet | 5.8 |
| 14 | HCJJL | Huocheng Jujuli | 2 August | 117.7 ± 3.3 ghij | 59.3 ± 1.0 ijk | 58.7 ± 3.4 jk | 1.1 ± 0.1 b | 9.0 ± 0.2 g | 12.3 ± 0.4 efgh | sweet | 7.2 |
| 15 | JJL-1 | Jujuli 1 | 19 August | 101.2 ± 8.9 ij | 54.2 ± 0.4 l | 49.1 ± 1.7 l | 1.1 ± 0.0 b | 10.6 ± 0.6 f | 8.8 ± 0.1 j | sweet | 7.0 |
| 16 | LL | Lüli | 19 August | 184.0 ± 17.0 de | 73.8 ± 3.5 e | 65.1 ± 2.5 ghij | 0.9 ± 0.0 fgh | 12.1 ± 0.4 cdef | 10.7 ± 0.3 i | sweet | 6.9 |
| 17 | S-03 | Sha 03 | 23 September | 130.3 ± 2.4 fghij | 60.3 ± 0.9 hijk | 69.3 ± 0.3 fgh | 0.9 ± 0.0 efgh | 5.4 ± 0.7 klm | 12.2 ± 0.2 fghi | sweet | 7.1 |
| 18 | S-01 | Sha 01 | 23 September | 142.7 ± 3.1 efghij | 63.0 ± 1.1 ghi | 71.4 ± 2.7 defg | 0.9 ± 0.0 efgh | 5.1 ± 0.6 lm | 11.8 ± 0.2 ghi | sweet | 8.3 |
| 19 | HXL | Hongxiangli | 7 October | 158.7 ± 12.4 defg | 62.7 ± 0.9 ghi | 70.3 ± 1.5 def | 0.9 ± 0.0 fgh | 13.4 ± 0.7 abc | 14.6 ± 1.1 bc | sour | 2.1 |
| 20 | KHSL | Korla Huangsuanli | 23 September | 123.0 ± 8.1 ghij | 60.3 ± 2.4 hijk | 69.7 ± 2.2 fgh | 1.0 ± 0.0 cdef | 13.9 ± 0.5 ab | 9.4 ± 0.6 j | sour | 1.9 |
| 21 | KCAMT | Kuqa Amut | 23 September | 139.1 ± 4.1 efghij | 65.6 ± 1.8 fgh | 69.5 ± 2.0 fgh | 0.8 ± 0.0 gh | 13.4 ± 0.7 abc | 11.4 ± 0.3 hi | sour | 2.1 |
| 22 | HSL | Heisuanli | 3 September | 147.3 ± 3.5 defghi | 63.9 ± 1.2 ghi | 77.0 ± 0.8 cde | 0.8 ± 0.0 h | 8.4 ± 0.3 gh | 8.8 ± 0.5 j | sour | 2.2 |
| 23 | AWQK | Aiwenqieke | 1 October | 253.3 ± 27.6 c | 78.7 ± 1.2 d | 98.0 ± 2.0 a | 0.9 ± 0.0 defg | 10.8 ± 0.6 ef | 16.5 ± 0.2 a | sour–sweet | 6.8 |
| 24 | NXPT | Naxput | 1 October | 181.7 ± 3.3 de | 69.7 ± 2.7 ef | 77.0 ± 1.5 cde | 1.0 ± 0.0 cd | 11.0 ± 0.3 ef | 12.8 ± 0.7 efgh | sour | 1.9 |
| 25 | QPL | Qipanli | 23 September | 157.2 ± 0.8 defg | 81.0 ± 0.6 cd | 82.0 ± 1.0 c | 1.0 ± 0.0 cde | 6.8 ± 0.1 ijk | 14.3 ± 0.4 bcd | sour–sweet | 7.1 |
| 26 | SCT | Suanchengtuo | 20 September | 175.0 ± 21.0 def | 64.6 ± 2.8 fghi | 66.0 ± 2.7 ghi | 1.5 ± 0.0 a | 10.9 ± 0.9 ef | 12.5 ± 0.2 efgh | sour–sweet | 6.8 |
| 27 | HJJL | Huang Jujuli | 3 September | 97.3 ± 0.7 j | 98.3 ± 0.1 a | 64.0 ± 1.2 hijk | 0.9 ± 0.0 efgh | 11.8 ± 0.1 def | 11.9 ± 0.1 fghi | sour–sweet | 7.0 |
| 28 | SCWHX | Shache Wanhongxiang | 3 October | 124.7 ± 0.9 ghij | 60.2 ± 0.1 hijk | 67.7 ± 0.9 fghi | 0.9 ± 0.0 fgh | 8.8 ± 0.1 g | 12.8 ± 0.1 efg | sour–sweet | 7.2 |
| 29 | S-02 | Sha 02 | 20 September | 159.1 ± 3.8 def | 67.0 ± 0.9 fg | 77.4 ± 0.4 cd | 1.0 ± 0.0 cdef | 7.2 ± 0.5 hij | 13.2 ± 0.2 def | sweet | 8.2 |
| Cultivar | Total Sugar Content (mg·g−1) | D-Fructose (mg·g−1) | Proportion (%) | Glucose (mg·g−1) | Proportion(%) | D-Sorbitol (mg·g−1) | Proportion (%) | Sucrose (mg·g−1) | Proportion (%) | Inositol (mg·g−1) | Proportion (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LTJJL | 576.2 ± 1.3 e | 214.3 ± 1.2 c | 37.2 | 81.4 ± 1.0 t | 14.1 | 120.4 ± 0.5 j | 20.9 | 153.7 ± 1.3 d | 26.7 | 3.7 ± 0.0 a | 0.6 |
| HSJJL | 588.9 ± 4.4 c | 225.9 ± 2.2 b | 38.4 | 119.2 ± 0.7 q | 20.3 | 118.8 ± 1.0 j | 20.2 | 119.1 ± 0.9 f | 20.2 | 3.6 ± 0.0 a | 0.6 |
| TXAMT | 549.4 ± 2.2 k | 164.8 ± 1.0 n | 30.0 | 184.2 ± 0.8 h | 33.5 | 154.0 ± 1.3 a | 28.0 | 42.2 ± 1.6 i | 7.7 | 2.0 ± 0.0 l | 0.4 |
| BDXSJ | 617.1 ± 2.8 b | 181.2 ± 0.8 k | 29.4 | 162.2 ±1.7 l | 26.3 | 116.1 ± 0.9 k | 18.8 | 153.5 ± 0.9 d | 24.9 | 2.2 ± 0.0 g | 0.4 |
| KELXL | 541.0 ± 3.4 l | 196.3 ± 1.3 fg | 36.3 | 161.8 ±0.3 l | 29.9 | 144.0 ± 1.3 c | 26.6 | 35.6 ± 0.8 k | 6.6 | 1.2 ± 0.0 t | 0.2 |
| DSL | 576.7 ± 2.8 e | 197.3 ± 1.1 f | 34.2 | 145.5 ± 0.8 m | 25.2 | 137.2 ± 0.9 e | 23.8 | 92.8 ± 1.6 f | 16.1 | 2.1 ± 0.0 ij | 0.4 |
| YLXL | 557.7 ± 0.8 ij | 185.4 ± 0.9 j | 33.2 | 203.1 ± 1.6 bc | 36.4 | 119.1 ± 0.5 j | 21.4 | 46.0 ± 1.0 h | 8.3 | 1.7 ± 0.1 o | 0.3 |
| DGKK | 569.7 ± 2.1 fg | 189.1 ± 0.8 i | 33.2 | 205.4 ± 0.9 b | 36.1 | 143.0 ± 1.7 cd | 25.1 | 26.8 ± 0.7 mn | 4.7 | 2.6 ± 0.0 d | 0.5 |
| XG | 542.0 ± 3.0 l | 116.0 ± 0.8 q | 21.4 | 122.1 ± 1.6 p | 22.5 | 105.0 ± 0.8 n | 19.4 | 195.2 ± 0.8 a | 36.0 | 1.9 ± 0.0 m | 0.4 |
| HCDHL | 553.5 ± 1.8 jk | 185.6 ± 0.9 j | 33.5 | 160.7 ± 0.9 l | 29.0 | 124.9 ± 0.5 i | 22.6 | 76.1 ± 0.8 g | 13.8 | 3.6 ± 0.0 b | 0.6 |
| ZS | 593.5 ± 0.9 c | 159.8 ± 0.8 o | 26.9 | 135.4 ± 1.2 n | 22.8 | 136.0 ± 0.8 ef | 22.9 | 156.6 ± 0.8 c | 26.4 | 2.1 ± 0.0 jk | 0.4 |
| ZX-1 | 562.3 ± 0.5 hi | 194.9 ± 0.8 g | 34.7 | 191.2 ± 0.8 f | 34.0 | 134.6 ± 1.7 f | 23.9 | 38.4 ± 1.6 j | 6.8 | 1.2 ± 0.0 t | 0.2 |
| KKAMT | 566.4 ± 3.5 gh | 195.4 ± 1.2 fg | 34.5 | 95.0 ± 0.8 s | 16.8 | 115.3 ± 1.2 kl | 20.4 | 154.4 ± 0.8 cd | 27.3 | 3.4 ± 0.0 c | 0.6 |
| HCJJL | 574.2 ± 3.5 ef | 145.1 ± 1.5 p | 25.3 | 129.8 ± 1.8 o | 22.6 | 113.0 ± 1.8 l | 19.7 | 183.2 ± 2.1 b | 31.9 | 1.0 ± 0.0 u | 0.2 |
| JJL-1 | 573.7 ± 1.5 ef | 211.5 ± 0.8 d | 36.9 | 198.9 ± 0.4 d | 34.7 | 130.9 ± 1.1 g | 22.8 | 26.7 ± 0.7 no | 4.7 | 1.9 ± 0.0 m | 0.3 |
| LL | 633.6 ± 0.1 a | 174.9 ± 0.9 l | 27.6 | 184.9 ± 0.9 h | 29.2 | 114.4 ± 0.6 kl | 18.1 | 154.7 ± 1.2 cd | 24.4 | 1.8 ± 0.0 n | 0.3 |
| S-03 | 583.0 ± 2.1 d | 209.5 ± 0.8 d | 35.9 | 195.7 ± 0.8 e | 33.6 | 132.4 ± 1.7 g | 22.7 | 42.2 ± 0.0 i | 7.2 | 1.4 ± 0.0 r | 0.2 |
| S-01 | 589.9 ± 2.6 c | 211.7 ± 1.2 d | 35.9 | 204.8 ± 0.8 b | 34.7 | 131.6 ± 0.8 g | 22.3 | 38.4 ± 1.3 j | 6.5 | 1.5 ± 0.0 p | 0.3 |
| HXL | 518.5 ± 0.8 n | 164.8 ± 1.2 n | 31.8 | 187.8 ± 0.9 g | 36.2 | 136.9 ± 0.9 ef | 26.4 | 24.9 ± 0.7 o | 4.8 | 2.0 ± 0.0 k | 0.4 |
| KHSL | 434.9 ± 0.9 p | 187.4 ± 0.8 ij | 43.1 | 146.7 ± 0.9 m | 33.7 | 75.8 ± 0.8 p | 17.4 | 19.9 ± 0.8 p | 4.6 | 2.5 ± 0.0 e | 0.6 |
| KCAMT | 529.7 ± 2.7 m | 172.5 ± 2.1 m | 32.6 | 201.1 ± 0.8 cd | 38.0 | 131.9 ± 0.9 g | 24.9 | 20.0 ± 0.0 p | 3.8 | 2.3 ± 0.0 f | 0.4 |
| HSL | 514.0 ± 1.8 no | 200.4 ± 0.8 e | 39.0 | 164.7 ± 0.8 k | 32.0 | 128.4 ± 1.2 h | 25.0 | 15.2 ± 0.7 q | 3.0 | 1.9 ± 0.0 n | 0.4 |
| AWQK | 557.7 ± 4.5 ij | 211.9 ± 0.8 d | 38.0 | 208.5 ± 1.7 a | 37.4 | 105.1 ± 1.2 n | 18.9 | 27.4 ± 0.9 mn | 4.9 | 1.7 ± 0.0 o | 0.3 |
| NXPT | 548.4 ± 0.5 k | 181.8 ± 0.7 k | 33.2 | 184.8 ± 0.8 h | 33.7 | 147.1 ± 0.9 b | 26.8 | 30.5 ± 0.8 l | 5.6 | 2.1 ± 0.0 i | 0.4 |
| QPL | 555.6 ± 1.2 j | 211.1 ± 1.2 d | 38.0 | 170.6 ± 1.3 j | 30.7 | 141.2 ± 0.8 d | 25.4 | 28.8 ± 1.3 lmn | 5.2 | 1.4 ± 0.0 q | 0.3 |
| SCT | 509.8 ± 3.3 o | 191.4 ± 0.5 g | 37.5 | 178.2 ± 1.6 i | 35.0 | 108.1 ± 1.2 m | 21.2 | 29.2 ± 0.8 lm | 5.7 | 0.3 ± 0.0 v | 0.1 |
| HJJL | 513.0 ± 2.3 o | 236.3 ± 1.0 a | 46.1 | 112.1 ± 1.7 r | 21.9 | 114.9 ± 1.2 kl | 22.4 | 45.1 ± 0.8 h | 8.8 | 2.2 ± 0.0 h | 0.4 |
| SCWHX | 568.2 ± 3.3 g | 195.6 ± 0.8 fg | 34.4 | 208.3 ± 1.2 a | 36.7 | 85.8 ± 0.8 o | 15.1 | 74.5 ± 0.8 g | 13.1 | 1.5 ± 0.0 p | 0.3 |
| S-02 | 617.8 ± 2.4 b | 214.9 ± 0.8 c | 34.8 | 209.5 ± 0.8 a | 33.9 | 143.2 ± 1.6 cd | 23.2 | 47.0 ± 0.8 h | 7.6 | 1.3 ± 0.0 s | 0.2 |
| Mean | 559.2 | 190.6 | 34.2 | 167.4 | 30.0 | 124.5 | 22.3 | 75.9 | 12.7 | 2.0 | 0.4 |
| SD | 39.0 | 25.0 | 5.1 | 36.7 | 6.6 | 17.9 | 3.1 | 57.5 | 9.8 | 0.8 | 0.1 |
| CV (%) | 7.0 | 13.1 | 14.9 | 21.9 | 22.0 | 14.4 | 13.9 | 75.8 | 77.2 | 40.0 | 25 |
| Cultivar | Total Acid Content (μg·g−1) | L-malic-Acid (μg·g−1) | Proportion (%) | Succinic-Acid (μg·g−1) | Proportion (%) | Shikimic-Acid (μg·g−1) | Proportion (%) | Oleanic-Acid (μg·g−1) | Proportion (%) | Pyruvic-Acid (μg·g−1) | Proportion (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LTJJL | 1017.8 ± 7.4 jkl | 878.3 ± 7.2 gh | 86.3 | 31.8 ± 1.0 fq | 3.1 | 24.4 ± 0.2 o | 2.4 | 23.2 ± 0.0 n | 2.3 | 15.8 ± 0.1 i | 1.6 |
| HSJJL | 995.9 ± 1.3 kl | 878.0 ± 0.3 gh | 88.2 | 11.8 ± 0.1 opq | 1.2 | 34.8 ± 0.1 gh | 3.5 | 7.7 ± 0.0 rs | 0.8 | 17.8 ± 0.6 g | 1.8 |
| TXAMT | 1280.7 ± 36.3 cde | 1166.1 ± 36.2 bc | 91.1 | 9.6 ± 0.1 opq | 0.8 | 31.3 ± 0.2 jk | 2.4 | 11.5 ± 0.0 p | 0.9 | 15.5 ± 0.2 ij | 1.2 |
| BDXSJ | 1011.5 ± 5.5 jkl | 836.9 ± 6.1 hi | 82.7 | 18.4 ± 0.1 kl | 1.8 | 20.5 ± 0.4 p | 2.0 | 61.3 ± 0.0 f | 6.1 | 17.8 ± 0.0 fg | 1.8 |
| KELXL | 1358.0 ± 133.7 b | 1204.1 ± 127.2 ab | 88.7 | 33.7 ± 11.4 ef | 2.5 | 32.4 ± 3.7 ij | 2.4 | 29.3 ± 4.1 l | 2.2 | 15.3 ± 0.8 jk | 1.1 |
| DSL | 1054.8 ± 5.5 jk | 907.9 ± 5.4 g | 86.1 | 26.0 ± 0.0 hi | 2.5 | 41.5 ± 0.0 d | 3.9 | 9.6 ± 0.0 q | 0.9 | 12.8 ± 0.0 q | 1.2 |
| YLXL | 1068.9 ± 94.9 ij | 843.6 ± 11.1 hi | 78.9 | 46.3 ± 0.5 d | 4.3 | 44.0 ± 0.4 c | 4.1 | 23.3 ± 1.2 n | 2.2 | 14.3 ± 0.1 mn | 1.3 |
| DGKK | 1197.3 ± 9.2 fg | 1074.4 ± 8.9 d | 89.7 | 8.2 ± 0.1 q | 0.7 | 33.6 ± 0.0 hi | 2.8 | 22.0 ± 0.3 n | 1.8 | 14.4 ± 0.1 mn | 1.2 |
| XG | 1041.8 ± 5.6 jk | 857.8 ± 5.0 h | 82.3 | 9.0 ± 0.0 pq | 0.9 | 25.1 ± 0.6 no | 2.4 | 93.7 ± 0.0 b | 9.0 | 14.0 ± 0.0 no | 1.4 |
| HCDHL | 957.9 ± 1.3 lm | 801.3 ± 0.1 ij | 83.7 | 32.7 ± 0.4 efg | 3.4 | 37.9 ± 1.1 e | 4.0 | 22.9 ± 0.4 n | 2.4 | 14.5 ± 0.1 m | 1.5 |
| ZS | 1006.4 ± 0.3 kl | 784.5 ± 0.1 j | 78.0 | 12.6 ± 0.2 nop | 1.3 | 28.2 ± 0.1 lm | 2.8 | 122.0 ± 0.4 a | 12.1 | 15.4 ± 0.0 ijk | 1.5 |
| ZX-1 | 1204.1 ± 6.3 fg | 1051.4 ± 6.0 de | 87.3 | 25.9 ± 0.0 hi | 2.2 | 33.9 ± 0.4 gh | 2.8 | 30.9 ± 0.0 k | 2.6 | 16.4 ± 0.1 h | 1.4 |
| KKAMT | 962.7 ± 1.8 lm | 851.5 ± 1.1 h | 88.5 | 23.4 ± 0.1 ij | 2.4 | 26.1 ± 0.7 n | 2.7 | 6.0 ± 0.0 t | 0.6 | 16.4 ± 0.1 h | 1.7 |
| HCJJL | 915.5 ± 5.7 mn | 756.4 ± 4.9 jk | 82.6 | 32.1 ± 0.2 fg | 3.5 | 33.4 ± 0.8 hi | 3.7 | 1.0 ± 0.0 qr | 1.0 | 14.2 ± 0.1 mn | 1.6 |
| JJL-1 | 1441.3 ± 19.5 a | 1213.2 ± 21.0 a | 84.2 | 98.0 ± 0.1 a | 6.8 | 28.0 ± 1.0 m | 1.9 | 43.8 ± 0.5 h | 3.0 | 10.6 ± 0.1 s | 0.7 |
| LL | 893.2 ± 0.1 n | 734.5 ± 0.0 k | 82.2 | 22.2 ± 0.1 ij | 2.5 | 42.7 ± 0.0 d | 4.8 | 25.4 ± 0.2 m | 2.8 | 23.7 ± 0.1 b | 2.7 |
| S-03 | 1322.1 ± 4.2 bcd | 1149.1 ± 4.0 c | 86.9 | 15.6 ± 0.3 lmn | 1.2 | 28.5 ± 0.0 lm | 2.2 | 66.6 ± 0.0 d | 5.0 | 15.1 ± 0.0 kl | 1.1 |
| S-01 | 1226.4 ± 1.0 ef | 1068.5 ± 0.7 d | 87.1 | 17.9 ± 0.1 kl | 1.5 | 30.8 ± 0.0 k | 2.5 | 51.1 ± 0.1 g | 4.2 | 15.7 ± 0.1 i | 1.3 |
| HXL | 1120.3 ± 0.0 hi | 1005.6 ± 0.1 f | 89.8 | 8.7 ± 0.0 pq | 0.8 | 35.1 ± 0.1 fg | 3.1 | 8.1 ± 0.0 rs | 0.7 | 14.9 ± 0.0 l | 1.3 |
| KHSL | 1296.9 ± 15.6 cd | 1127.9 ± 15.4 c | 87.0 | 11.4 ± 0.0 opq | 0.9 | 29.4 ± 0.2 l | 2.3 | 34.4 ± 0.0 j | 2.7 | 19.8 ± 0.0 d | 1.5 |
| KCAMT | 1212.2 ± 2.8 f | 1078.3 ± 1.6 d | 89.0 | 9.9 ± 0.0 opq | 0.8 | 54.0 ± 0.8 b | 4.5 | 0.0 ± 0.0 u | 0.0 | 14.9 ± 0.4 l | 1.2 |
| HSL | 1336.6 ± 1.1 bc | 1131.5 ± 1.0 c | 84.7 | 29.5 ± 0.0 gh | 2.2 | 36.4 ± 0.1 e | 2.7 | 64.6 ± 0.1 e | 4.8 | 19.0 ± 0.0 e | 1.4 |
| AWQK | 1054.0 ± 0.8 jk | 908.2 ± 0.1 g | 86.2 | 16.5 ± 0.1 lm | 1.6 | 55.5 ± 0.2 a | 5.3 | 12.3 ± 0.2 p | 1.2 | 13.7 ± 0.1 p | 1.3 |
| NXPT | 1198.8 ± 26.1 fg | 1071.2 ± 25.5 d | 89.4 | 13.4 ± 0.2 mno | 1.1 | 26.3 ± 0.6 n | 2.2 | 17.7 ± 0.2 o | 1.5 | 18.1 ± 0.7 f | 1.5 |
| QPL | 1179.1 ± 4.2 fg | 1020.9 ± 1.3 ef | 86.6 | 36.0 ± 0.0 e | 3.1 | 38.1 ± 0.3 e | 3.2 | 8.6 ± 0.0 qr | 0.7 | 13.7 ± 0.0 op | 1.2 |
| SCT | 967.8 ± 0.3 lm | 772.8 ± 0.4 jk | 79.9 | 60.7 ± 0.0 b | 6.3 | 17.6 ± 0.0 q | 1.8 | 38.6 ± 0.1 i | 4.0 | 24.3 ± 0.1 a | 2.5 |
| HJJL | 1276.4 ± 1.2 de | 1058.9 ± 1.0 de | 83.0 | 50.9 ± 0.1 c | 4.0 | 9.6 ± 0.0 r | 0.8 | 80.6 ± 0.1 c | 6.3 | 22.8 ± 0.1 c | 1.8 |
| SCWHX | 1147.4 ± 0.4 gh | 979.1 ± 0.2 f | 85.3 | 61.5 ± 0.4 b | 5.4 | 38.3 ± 0.0 e | 3.3 | 7.1 ± 0.0 st | 0.6 | 17.7 ± 0.0 g | 1.5 |
| S-02 | 1220.7 ± 0.6 f | 1060.2 ± 0.5 de | 86.9 | 20.5 ± 0.0 jk | 1.7 | 26.1 ± 0.1 n | 2.1 | 61.9 ± 0.0 f | 5.1 | 12.0 ± 0.0 r | 1.0 |
| Mean | 1136.8 | 974.9 | 85.6 | 27.4 | 2.4 | 32.5 | 2.9 | 34.2 | 3.0 | 16.2 | 1.5 |
| SD | 145.0 | 142.8 | 3.3 | 19.9 | 1.6 | 9.5 | 1.0 | 29.4 | 2.7 | 3.2 | 0.4 |
| CV (%) | 12.8 | 14.6 | 3.9 | 72.6 | 66.7 | 29.2 | 34.5 | 86.0 | 90.0 | 19.8 | 26.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, S.; Wang, S.; Xie, J.; Wusiman, A.; Zhou, W. Elucidating Sugar–Acid Metabolic Diversity and Screening Breeding Materials in Xinjiang Pear (Pyrus) Germplasm Resources. Foods 2025, 14, 3354. https://doi.org/10.3390/foods14193354
Zhang S, Wang S, Wang S, Xie J, Wusiman A, Zhou W. Elucidating Sugar–Acid Metabolic Diversity and Screening Breeding Materials in Xinjiang Pear (Pyrus) Germplasm Resources. Foods. 2025; 14(19):3354. https://doi.org/10.3390/foods14193354
Chicago/Turabian StyleZhang, Shikui, Shaopeng Wang, Shangdong Wang, Jinchao Xie, Amanguli Wusiman, and Weiquan Zhou. 2025. "Elucidating Sugar–Acid Metabolic Diversity and Screening Breeding Materials in Xinjiang Pear (Pyrus) Germplasm Resources" Foods 14, no. 19: 3354. https://doi.org/10.3390/foods14193354
APA StyleZhang, S., Wang, S., Wang, S., Xie, J., Wusiman, A., & Zhou, W. (2025). Elucidating Sugar–Acid Metabolic Diversity and Screening Breeding Materials in Xinjiang Pear (Pyrus) Germplasm Resources. Foods, 14(19), 3354. https://doi.org/10.3390/foods14193354






