Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
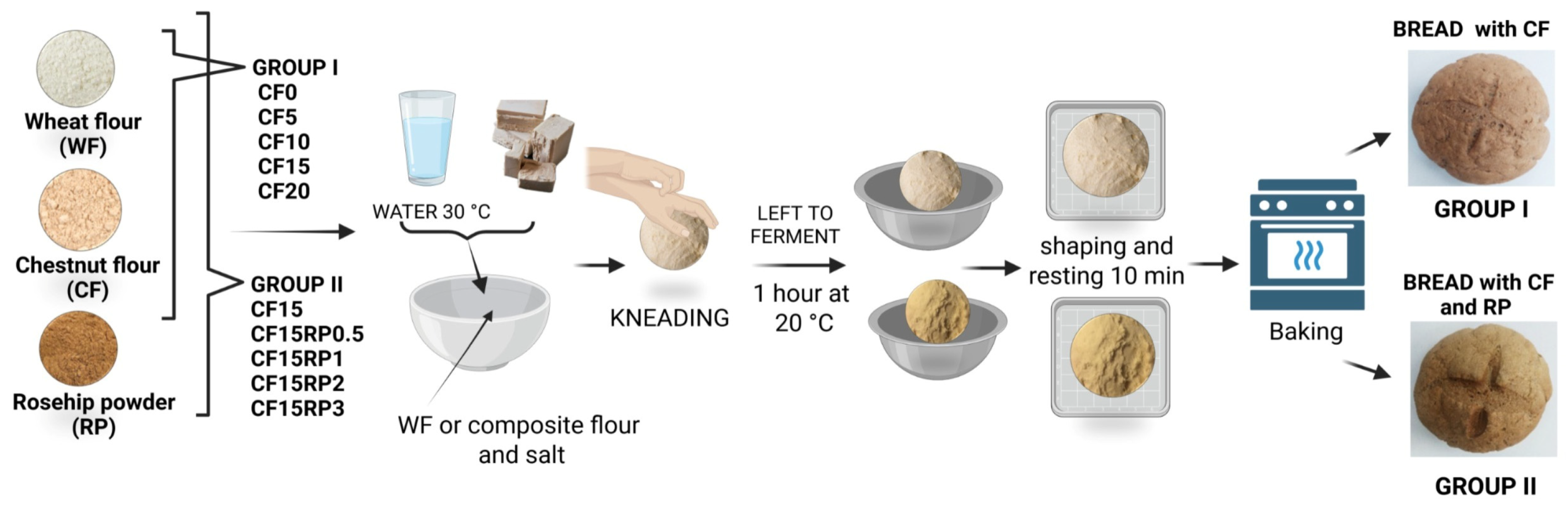
2.2. Bread Ingredients and Manufacturing Process
2.3. Proximate Composition and Energy Value Assessment of Wheat Flour, Chestnut Flour, Rosehip Powder, and Enriched Bread Samples
2.4. Rheological Determination of Composite Flours Using MIXOLAB System
2.5. Phytochemical Content and Antioxidant Activity of Wheat Flour, Chestnut Flour, Rosehip Powder, Dough, and Enriched Bread
2.5.1. Preparation of Alcoholic Extracts from Samples
2.5.2. Determination of Total Flavonoid Content
2.5.3. Determination of Total Phenolic Content
2.5.4. Evaluation of Antioxidant Activity via 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
2.5.5. Evaluation of Antioxidant Activity via Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6. Physical Characteristics Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Wheat Flour, Chestnut Flour, Rosehip Powder, and Enriched Bread Formulations
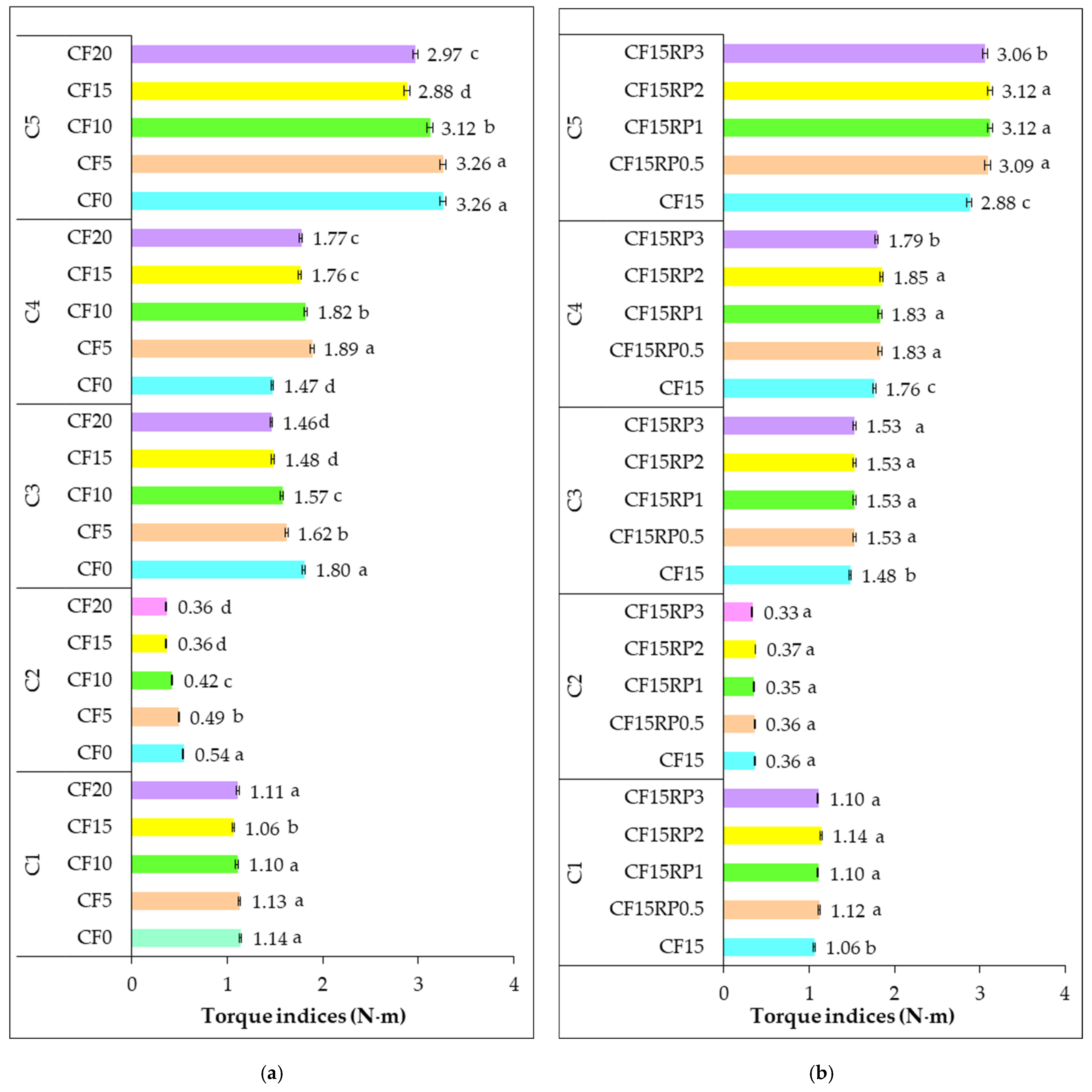
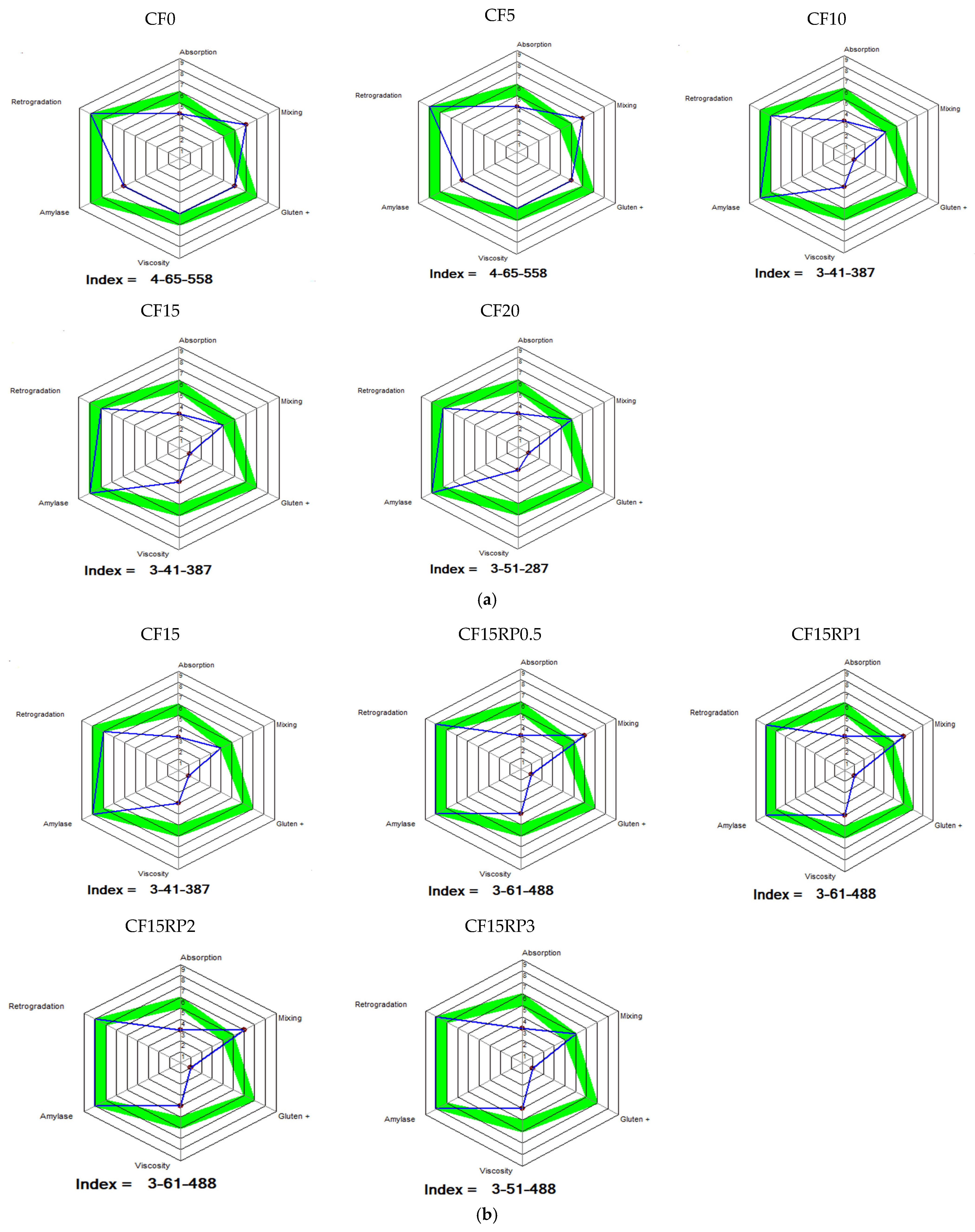
3.2. Rheological Properties of Composite Flours
3.3. Phytochemical Content and Antioxidant Activity of WF, CF, and RP
3.4. Phytochemical Content and Antioxidant Activity of Dough and Bread Formulations
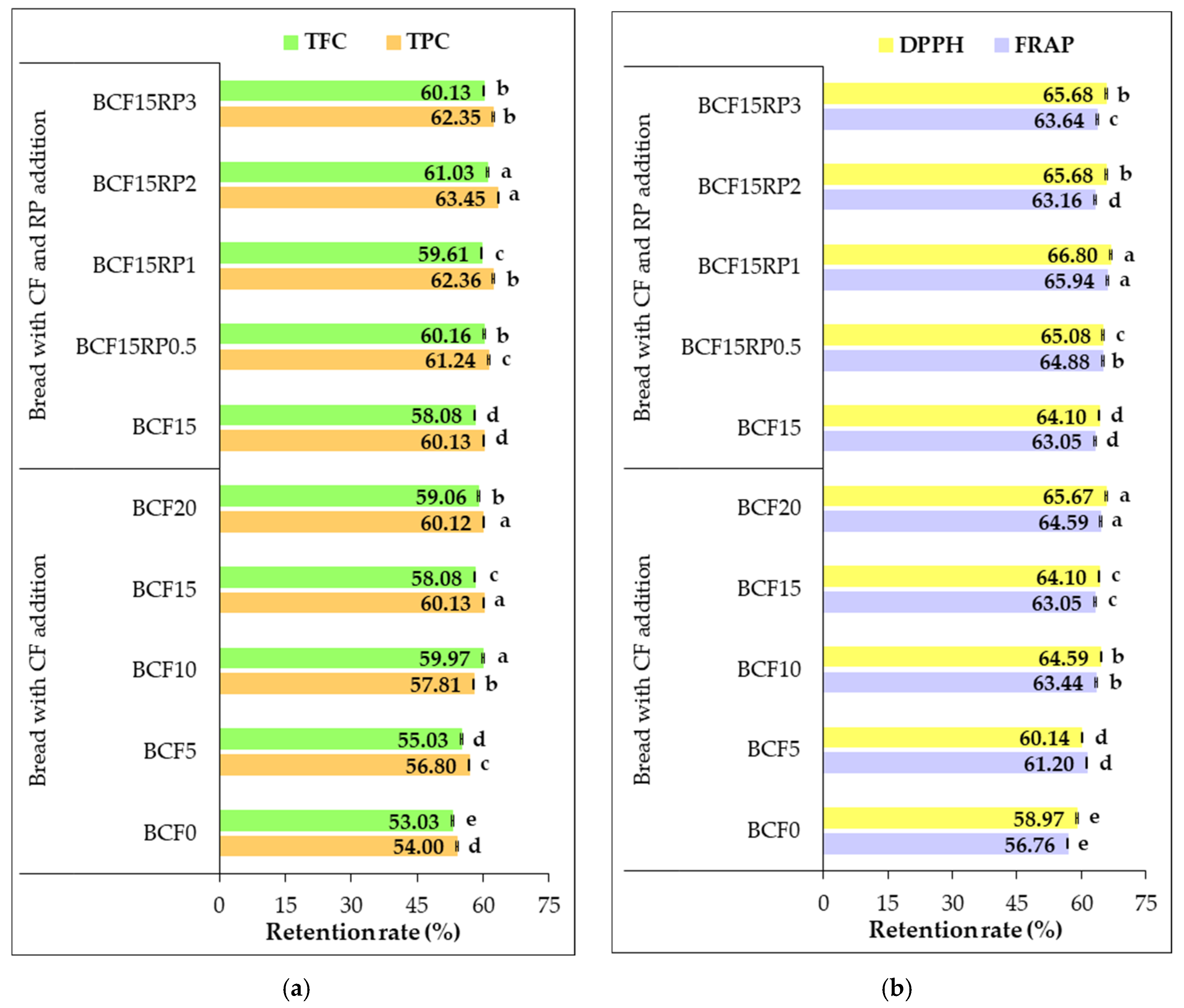
3.5. Retention Rate of Phytochemical Content and Antioxidant Activity in Bread Formulations After Baking
3.6. Physical Characteristics of Bread Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demiate, I.M.; Oetterer, M.; Wosiacki, G. Characterization of chestnut (Castanea sativa, Mill) starch for industrial utilization. Brazil. Arch. Biol. Technol. 2001, 44, 69–78. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Hrušková, M.; Švec, I.; Kadlčíková, I. Effect of chestnut and acorn flour on wheat/wheat–barley flour properties and bread quality. Int. J. Food Stud. 2019, 8, 41–57. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Şahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010, 101, 329–336. [Google Scholar] [CrossRef]
- Marciniak-Łukasiak, K.; Leśniewska, P.; Zielińska, D.; Sowiński, M.; Zbikowska, K.; Łukasiak, P.; Zbikowska, A. The Influence of Chestnut Flour on the Quality of Gluten-Free Bread. Appl. Sci. 2022, 12, 8340. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cirlini, M.; Scazzina, F.; Chiavaro, E. Chestnut flour addition in commercial gluten-free bread: A shelf-life study. LWT 2016, 70, 88–95. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Dall’Asta, C.; Cirlini, M.; Chiavaro, E. Short term storage evaluation of quality and antioxidant capacity in chestnut wheat bread. J. Sci. Food Agric. 2015, 95, 59–65. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ahn, H.-D.; Hwang, J.-M.; Kim, Y.-J.; Kim, S.-B.; Kim, I.-B.; Choi, H.-Y. Effects of chestnut powder content on the quality characteristics and antioxidant activity of rice muffins. Food Sci. Preserv. 2024, 31, 256. [Google Scholar] [CrossRef]
- Mert, S. Effect of Different Flours on Quality of Gluten-Free Wafer Sheet. Master’s Thesis, Middle East Technical University, Ankara, Türkiye, 2014. Available online: https://etd.lib.metu.edu.tr/upload/12617653/index.pdf (accessed on 14 March 2025).
- Shafi, M.; Baba, W.N.; Masoodi, F.A.; Bazaz, R. Wheat–water chestnut flour blends: Effect of baking on antioxidant properties of cookies. J. Food Sci. Technol. 2016, 53, 4278–4288. [Google Scholar] [CrossRef]
- Demirkesen, I. Formulation of chestnut cookies and their rheological and quality characteristics. J. Food Qual. 2016, 39, 264–273. [Google Scholar] [CrossRef]
- Šoronja-Simović, D.; Pajin, B.; Šubarić, D.; Dokić, L.; Šereš, Z.; Nikolić, I. Quality, sensory and nutritional characteristics of cookies fortified with chestnut flour. J. Food Process. Preserv. 2017, 41, e12887. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Kosović, I.; Jukić, M.; Jozinović, A.; Ačkar, Đ.; Komlenić, D. Influence of chestnut flour addition on quality characteristics of pasta made on extruder and minipress. Czech J. Food Sci. 2016, 34, 166–172. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Rheological properties of commercial chestnut flour doughs with different gums. Int. J. Food Sci. Technol. 2011, 46, 2085–2092. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Şahin, S. Rheological properties of gluten-free bread formulations. J. Food Eng. 2010, 96, 295–303. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Rinaldi, M.; Ganino, T.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread making. LWT 2013, 53, 233–239. [Google Scholar] [CrossRef]
- Li, R.; Sharma, A.K.; Zhu, J.; Zheng, B.; Xiao, G.; Chen, L. Nutritional biology of chestnuts: A perspective review. Food Chem. 2022, 395, 133575. [Google Scholar] [CrossRef] [PubMed]
- Littardi, P.; Paciulli, M.; Carini, E.; Rinaldi, M.; Rodolfi, M.; Chiavaro, E. Quality evaluation of chestnut flour addition on fresh pasta. LWT 2020, 126, 109303. [Google Scholar] [CrossRef]
- Kayahan, S.; Ozdemir, Y.; Gulbag, F. Functional compounds and antioxidant activity of Rosa species grown in Turkey. Erwerbs-Obstbau 2022, 64, 1079–1086. [Google Scholar] [CrossRef]
- Negrean, O.-R.; Farcaş, A.C.; Nemes, S.A.; Cic, D.-E.; Socaci, S.A. Recent advances and insights into the bioactive properties and applications of Rosa canina L. and its by-products. Heliyon 2024, 10, e30816. [Google Scholar] [CrossRef]
- Çürük, S.G.; Njjar, M.; Köseoğlu, D.; Akdoğan, A. Mineral and Bioactive Component Contents of Rosehip (Rosa canina L.) Seed Powder. Akad. Gıda 2023, 21, 323–332. [Google Scholar] [CrossRef]
- Fan, C.; Pacier, C.; Martirosyan, D. Rosehip (Rosa canina L): A functional food perspective. Funct. Foods Health Dis. 2014, 4, 493–509. [Google Scholar] [CrossRef]
- Vartolomei, N.; Turtoi, M. The Influence of the Addition of Rosehip Powder to Wheat Flour on the Dough Farinographic Properties and Bread Physico-Chemical Characteristics. Appl. Sci. 2021, 11, 12035. [Google Scholar] [CrossRef]
- Gül, H.; Şen, H. The Influence of Rosehip Seed Flour on Bread Quality. Sci. Bull. Ser. F Biotechnol. 2017, 21, 336–342. [Google Scholar]
- Antarkar, S.; Sharma, A.; Bhargava, A.; Gupta, H.; Tomar, R.; Srivastava, S. Physico-chemical and Nutritional Evaluation of Cookies with Different Levels of Rosehip and Hibiscus Powder Substitution. Arch. Curr. Res. Int. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Chochkov, R.; Zlateva, D.; Ivanova, P.; Stefanova, D. Effect of rosehip flour on the properties of wheat dough and bread. Ukr. Food J. 2023, 11, 558–572. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Canale, M.; Fascella, G.; Scarangella, M.; Strano, M.C.; Mangione, G.; Natalello, A.; Mammano, M.M.; Spina, A. Nutritional and technological characterization of rosehip seed flours as a functional ingredient in common wheat bread. Eur. Food Res. Technol. 2025, 251, 1033–1045. [Google Scholar] [CrossRef]
- Borşa, A.R.; Păucean, A.; Fogarasi, M.; Ranga, F.; Borşa, A.; Tanislav, A.E.; Mureșan, V.; Semeniuc, C.A. Utilisation of Rosehip Waste Powder as a Functional Ingredient to Enrich Waffle Cones with Fibres, Polyphenols, and Carotenoids. Foods 2025, 14, 90. [Google Scholar] [CrossRef]
- Peña, F.; Valencia, S.; Tereucán, G.; Nahuelcura, J.; Jiménez-Aspee, F.; Cornejo, P.; Ruiz, A. Bioactive Compounds and Antioxidant Activity in the Fruit of Rosehip (Rosa canina L. and Rosa rubiginosa L.). Molecules 2023, 28, 3544. [Google Scholar] [CrossRef]
- Ivanova, P.; Chochkov, R.; Zlateva, D.; Stefanova, D. TOTAL PHENOLIC AND FLAVONOID CONTENT, AND ANTIOXIDANT ACTIVITY OF WHEAT BREAD ENRICHED WITH PUMPKIN, CHESTNUT AND ROSEHIP FLOUR. Carpath. J. Food Sci. Technol. 2023, 15, 33–41. [Google Scholar] [CrossRef]
- Chochkov, R.; Zlateva, D.; Stefanova, D.; Ivanova, P. Effect of pumpkin seed flour, chestnut flour, and rosehip flour on wheat bread staling rate. Ukr. Food J. 2024, 13, 76–90. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Michałowska, D. Effect of coconut and chestnut flour supplementations on texture, nutritional and sensory properties of baked wheat based bread. Molecules 2021, 26, 4641. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Das, P.C.; Khan, M.J.; Rahman, M.S.; Majumder, S.; Islam, M.N. Comparison of the physico-chemical and functional properties of mango kernel flour with wheat flour and development of mango kernel flour based composite cakes. NFS J. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G. Lupin (Lupinus spp.) fortified bread: A sustainable, nutritionally, functionally, and technologically valuable solution for bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Chopin Application Laboratory (M.A. Handbook). Rheological and Enzymatic Analysis; Chopin Applications Laboratory: Villeneuve-la-Garenne, France, 2009; Available online: https://www.eqec.pt/doc_ficheiros/091023_mixolab_appli_en.pdf (accessed on 12 June 2025).
- Litwinek, D.; Gumul, D.; Łukasiewicz, M.; Zięba, T.; Kowalski, S. The Effect of Red Potato Pulp Preparation and Stage of Its Incorporation into Sourdough or Dough on the Quality and Health-Promoting Value of Bread. Appl. Sci. 2023, 13, 7670. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef]
- Blanch, G.P.; Ruiz del Castillo, M.L. Effect of Baking Temperature on the Phenolic Content and Antioxidant Activity of Black Corn (Zea mays L.) Bread. Foods 2021, 10, 1202. [Google Scholar] [CrossRef]
- Dimov, I.; Petkova, N.; Nakov, G.; Taneva, I.; Ivanov, I.; Stamatovska, V. Improvement of antioxidant potential of wheat flours and breads by addition of medicinal plants. Ukr. Food J. 2018, 7, 671–681. [Google Scholar] [CrossRef]
- Mekky, H.; El Sohafy, S.; Abu El-Khair, R.A.; El Hawiet, A.E. Total polyphenolic content and antioxidant activity of Silybum marianum cultures grown on different growth regulators. Int. J. Pharm. Pharm. Sci. 2017, 9, 44–47. [Google Scholar] [CrossRef]
- Metzner Ungureanu, C.-R.; Poiana, M.-A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to Improve the Thermo-Oxidative Stability of Sunflower Oil by Exploiting the Antioxidant Potential of Blueberries Processing Byproducts. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef] [PubMed]
- SR 91:2007; Romanian Standard for Bread, Confectionery and Bakery Specialties—Methods of Analysis. ASRO—Romanian Standards Association: Bucharest, Romania, 2007.
- Hegazy, N.A.; Kamil, M.M.; Hussein, A.M.S.; Bareh, G.F. Chemical and technological properties of improved biscuit by chestnut flour. Int. J. Food Sci. Nutr. 2014, 3, 7–15. [Google Scholar]
- Wang, J.; Rosell, C.M.; Benedito de Barber, C. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Sujka, K.; Ceglińska, A.; Romankiewicz, D.; Kacprzyk, E. The influence of dietary fiber on moisture and texture changes in wheat bread during storage. Acta Agrophys. 2018, 25, 73–84. [Google Scholar] [CrossRef]
- Vartolomei, N.P.; Arus, V.A.; Moroi, A.M.; Zaharia, D.; Turtoi, M. Influence of rosehip powder addition on quality indicators of mixtures obtained with different types of wheat flour. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 379–393. [Google Scholar] [CrossRef]
- Zlateva, D.; Stefanova, D.; Chochkov, R.; Ivanova, P. Mineral content of wheat bread enriched with chestnut and rosehip flour. Bulg. J. Agric. Sci. 2024, 30, 333–340. [Google Scholar]
- Collar, C.; Bollain, C.; Rosell, C.M. Rheological behaviour of formulated bread doughs during mixing and heating. Food Sci. Technol. Int. 2007, 13, 99–107. [Google Scholar] [CrossRef]
- Mariotti, M.; Lucisano, M.; Pagani, M.A.; Ng, P.K. The role of corn starch, amaranth flour, pea isolate, and Psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Wang, L.; Shi, D.; Chen, J.; Dong, H.; Chen, L. Effects of Chinese chestnut powder on starch digestion, texture properties, and staling characteristics of bread. Grain Oil Sci. Technol. 2023, 6, 82–90. [Google Scholar] [CrossRef]
- Demirkesen Mert, I.; Campanella, O.; Sumnu, G.; Sahin, S.; Hamaker, B. A study on staling characteristics of gluten free breads prepared with chestnut and rice flours. Food Bioprocess Technol. 2013, 7, 806–820. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Li, X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Mironeasa, S. Effects of plants’ ingredients on dough and final product. In Foods; MDPI: Basel, Switzerland, 2021; Available online: https://mdpi-res.com/bookfiles/book/5397/Effects_of_Plants_Ingredients_on_Dough_and_Final_Product.pdf (accessed on 17 July 2025).
- Rohn, S.; Rawel, H.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Jia, Y.; Zhang, H.; Ren, F. Formation and application of starch–polyphenol complexes: Influencing factors and rapid screening based on chemometrics. Foods 2024, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Rawel, H.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; de Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Ngo, T.V.; Kusumawardani, S.; Kunyanee, K.; Luangsakul, N. Polyphenol modified starches and their applications in the food industry: Recent updates and future directions. Foods 2022, 11, 3384. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Ye, X.; Kong, X.; Tian, J. Regulating the physicochemical, structural characteristics and digestibility of potato starch by complexing with different phenolic acids. Int. J. Biol. Macromol. 2023, 253, 127474. [Google Scholar] [CrossRef] [PubMed]
- Banu, I.; Stoenescu, G.; Ionescu, V.; Aprodu, I. Estimation of the baking quality of wheat flours based on rheological parameters of the Mixolab curve. Czech J. Food Sci. 2011, 29, 35–44. [Google Scholar] [CrossRef]
- Dabčević, T.; Hadnađev, M.; Pojić, M. Evaluation of the possibility to replace conventional rheological wheat flour quality control instruments with the new measurement tool–Mixolab. Agric. Conspec. Sci. 2009, 74, 169–174. [Google Scholar]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Prieto, D. Technological assessment of chestnut flour doughs regarding to doughs from other commercial flours and formulations. Food Bioprocess Technol. 2012, 5, 2301–2310. [Google Scholar] [CrossRef]
- Stoenescu, G.; Ionescu, V.; Banu, I. Rheological properties of the wheat flour supplemented with different additives. Ann. Univ. Dunarea De Jos Galati Fascicle VI-Food Technol. 2011, 35, 54–62. [Google Scholar]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Prieto, D. Influence of the particle size on the rheological behaviour of chestnut flour doughs. J. Food Eng. 2010, 100, 270–277. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Urbštaitė, R.; Liaudanskas, M.; Obelevičius, K.; Janulis, V. Phenolic content and antioxidant activity in fruit of the genus Rosa L. Antioxidants 2022, 11, 912. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and bioaccessibility of phenolic compounds in rosehip extracts during in vitro digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Soare, R.; Babeanu, C.; Bonea, D.; Păniță, O. The content of total phenols, flavonoids and antioxidant activity in rosehip from the spontaneous flora from south Romania. Sci. Pap. Ser. A Agron. 2015, 58, 307–317. [Google Scholar]
- Oniszczuk, A.; Widelska, G.; Wójtowicz, A.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Dib, A.; Matwijczuk, A. Content of phenolic compounds and antioxidant activity of new gluten-free pasta with the addition of chestnut flour. Molecules 2019, 24, 2623. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Su, Y.; Wang, M. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 338–344. [Google Scholar] [CrossRef]
- Suchowilska, E.; Bieńkowska, T.; Stuper-Szablewska, K.; Wiwart, M. Concentrations of phenolic acids, flavonoids and carotenoids and the antioxidant activity of the grain, flour and bran of Triticum polonicum as compared with three cultivated wheat species. Agriculture 2020, 10, 591. [Google Scholar] [CrossRef]
- Santetti, G.S.; Dacoreggio, M.V.; Silva, A.C.M.; Biduski, B.; Bressiani, J.; Oro, T.; de Francisco, A.; Gutkoski, L.C.; Amboni, R.D.M.C. Effect of yerba mate (Ilex paraguariensis) leaves on dough properties, antioxidant activity, and bread quality using whole wheat flour. J. Food Sci. 2021, 86, 4354–4364. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Changes of antioxidant potential of pasta fortified with parsley (Petroselinum Crispum mill.) leaves in the light of protein-phenolics interactions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 29–36. [Google Scholar] [CrossRef]
- Fedorková, S.; Musilová, J.; Ňorbová, M.; Vollmannová, A.; Čéryová, N.; Lidíková, J. IMPACT OF HEAT TREATMENTS ON THE ANTIOXIDANT ACTIVITY AND TOTAL PHENOLIC CONTENT OF SWEET CHESTNUTS (Castanea sativa MILL.). Sci. Bull. Ser. F Biotechnol. 2024, 28, 31–36. [Google Scholar]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative Study on Phytochemical Profiles and Antioxidant Capacities of Chestnuts Produced in Different Geographic Area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhieva, S.S.; Georgieva, S.S. Rosehip extraction: Process optimization and antioxidant capacity of extracts. Cent. Eur. J. Chem. 2014, 12, 502–508. [Google Scholar] [CrossRef]
- Dolek, U.; Gunes, M.; Genc, N.; Elmastas, M. Total Phenolic Compound and Antioxidant Activity Changes in Rosehip (Rosa sp.) during Ripening. J. Agric. Sci. Technol. 2018, 20, 817–828. [Google Scholar]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, Ü.; Güneş, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.; Baba, W.N.; Masoodi, F.A. Composite flour blends: Influence of particle size of water chestnut flour on nutraceutical potential and quality of Indian flat breads. Food Meas. 2017, 11, 1094–1105. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Radulov, I.; Raba, D.-N.; Cocan, I.; Negrea, M.; Misca, C.D.; Dragomir, C.; Dossa, S.; Suster, G. Strategies to Formulate Value-Added Pastry Products from Composite Flours Based on Spelt Flour and Grape Pomace Powder. Foods 2023, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of baking conditions and dough formulations on phenolic com-pound stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Han, H.-M.; Koh, B.-K. Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J. Sci. Food Agric. 2011, 91, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luthria, D.; Fuerst, E.P.; Kiszonas, A.M.; Yu, L.; Morris, C.F. Effect of Processing on Phenolic Composition of Dough and Bread Fractions Made from Refined and Whole Wheat Flour of Three Wheat Varieties. J. Agric. Food Chem. 2014, 62, 10431–10436. [Google Scholar] [CrossRef] [PubMed]
- Peluola-Adeyemi, O.A.; Abdus-Salaam, R.B.; Obi, T.E.; Toyiemedo, N.C. Quality evaluation of bread produced from wheat flour using avocado (Persea americana) paste as substitute. J. Food Stab. 2021, 4, 1–12. [Google Scholar] [CrossRef]





| Samples | Chemical Parameters | Energy Value (kcal/100 g) | |||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Fat (%) | Protein (%) | Fiber (%) | Ash (%) | CRB (%) | ||
| WF | 12.74 ± 0.03 a | 1.56 ± 0.02 c | 12.89 ± 0.02 a | 3.41 ± 0.04 c | 0.64 ± 0.01 c | 72.17 ± 0.03 c | 354.28 ± 0.04 c |
| CF | 11.52 ± 0.02 b | 3.91 ± 0.03 b | 7.79 ± 0.04 b | 7.25 ± 0.06 b | 3.01 ± 0.02 b | 73.77 ± 0.04 b | 361.43 ± 0.05 b |
| RP | 7.26 ± 0.03 c | 4.87 ± 0.03 a | 6.49 ± 0.05 c | 56.09 ± 0.02 a | 4.49 ± 0.01 a | 76.89 ± 0.04 a | 377.35 ± 0.05 a |
| Samples | Chemical Parameters | Energy Value (kcal/100 g) | |||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Fat (%) | Protein (%) | Fiber (%) | Ash (%) | CRB (%) | ||
| BCF0 | 37.72 ± 0.03 a | 1.15 ± 0.02 e | 9.55 ± 0.03 a | 2.53 ± 0.06 e | 0.48 ± 0.02 e | 51.10 ± 0.03 a | 252.95 ± 0.05 e |
| BCF5 | 37.68 ± 0.03 a | 1.24 ± 0.04 d | 9.36 ± 0.04 b | 2.67 ± 0.05 d | 0.56 ± 0.05 d | 51.16 ± 0.04 a | 253.24 ± 0.04 d |
| BCF10 | 37.65 ± 0.03 a | 1.33 ± 0.03 c | 9.17 ± 0.03 c | 2.81 ± 0.06 c | 0.65 ± 0.03 c | 51.20 ± 0.03 a | 253.45 ± 0.05 c |
| BCF15 | 37.62 ± 0.03 a | 1.41 ± 0.02 b | 8.98 ± 0.04 d | 2.95 ± 0.04 b | 0.74 ± 0.04 b | 51.25 ± 0.04 a | 253.61 ± 0.04 b |
| BCF20 | 37.59 ± 0.02 a | 1.50 ± 0.04 a | 8.79 ± 0.03 e | 3.09 ± 0.05 a | 0.83 ± 0.02 a | 51.29 ± 0.04 a | 253.82 ± 0.03 a |
| BCF15 | 37.62 ± 0.02 a | 1.41 ± 0.02 a | 8.98 ± 0.04 a | 2.95 ± 0.06 e | 0.74 ± 0.04 a | 51.25 ± 0.04 a | 253.61 ± 0.04 a |
| BCF15RP0.5 | 37.62 ± 0.02 a | 1.43 ± 0.02 a | 8.96 ± 0.05 a | 3.14 ± 0.05 d | 0.75 ± 0.04 a | 51.24 ± 0.05 a | 253.67 ± 0.05 a |
| BCF15RP1 | 37.61 ± 0.02 a | 1.44 ± 0.03 a | 8.93 ± 0.06 a | 3.33 ± 0.03 c | 0.77 ± 0.03 a | 51.25 ± 0.03 a | 253.68 ± 0.04 a |
| BCF15RP2 | 37.60 ± 0.02 a | 1.46 ± 0.06 a | 8.89 ± 0.03 a | 3.72 ± 0.04 b | 0.80 ± 0.03 a | 51.25 ± 0.05 a | 253.71 ± 0.06 a |
| BCF15RP3 | 37.59 ± 0.02 a | 1.49 ± 0.03 a | 8.84 ± 0.03 a | 4.10 ± 0.06 a | 0.82 ± 0.03 a | 51.26 ± 0.04 a | 253.81 ± 0.05 a |
| Sample | FRAP (µM Fe2+/g d.w.) | DPPH (µM TE/g d.w.) | TPC (mg GAE/100 g d.w.) | TFC (mg QE/100 g d.w.) |
|---|---|---|---|---|
| WF | 2.23 ± 0.06 c | 2.84 ± 0.07 c | 50.84 ± 0.40 c | 33.78 ± 0.27 c |
| CF | 23.40 ± 1.26 b | 29.47 ± 1.43 b | 188.27 ± 1.47 b | 125.27 ± 1.69 b |
| RP | 515.86 ± 1.57 a | 592.85 ± 1.63 a | 2308.47 ± 1.96 a | 1410.37 ± 1.79 a |
| Sample | FRAP (µM Fe2+/g d.w.) | DPPH (µM TE/g d.w.) | TPC (mg GAE/100 g d.w.) | TFC (mg QE/100 g d.w.) |
|---|---|---|---|---|
| DCF0 | 2.11 ± 0.07 e | 2.61 ± 0.65 e | 50.52 ± 0.49 e | 33.27 ± 0.32 e |
| DCF5 | 2.97 ± 0.11 d | 3.72 ± 0.12 d | 56.32 ± 0.62 d | 37.10 ± 0.60 d |
| DCF10 | 3.79 ± 0.11 c | 4.87 ± 0.131 c | 62.08 ± 1.004 c | 41.15 ± 0.89 c |
| DCF15 | 4.56 ± 0.09 b | 6.09 ± 0.10 b | 67.54 ± 1.11 b | 45.02 ± 1.01 b |
| DCF20 | 5.41 ± 0.13 a | 7.11 ± 0.13 a | 73.40 ± 1.22 a | 48.63 ± 1.24 a |
| DCF15 | 4.56 ± 0.087 e | 6.085 ± 0.10 e | 67.54 ± 1.11 e | 45.02 ± 1.01 e |
| DCF15RP0.5 | 7.26 ± 0.079 d | 9.018 ± 0.09 d | 79.41 ± 1.08 d | 52.07 ± 0.95 d |
| DCF15RP1 | 10.02 ± 0.13 c | 12.032 ± 0.13 c | 91.42 ± 1.10 c | 59.51 ± 1.10 c |
| DCF15RP2 | 15.11 ± 0.15 b | 17.914 ± 0.14 b | 115.37 ± 1.22 b | 74.01 ± 1.21 b |
| DCF15RP3 | 20.28 ± 0.16 a | 23.707 ± 0.17 a | 138.89 ± 1.30 a | 88.18 ± 1.16 a |
| Samples | Elasticity (%) | Porosity (%) | Height/Diameter (H/D) |
|---|---|---|---|
| BCF0 | 90.95 ± 0.20 a | 77.44 ± 0.14 a | 0.58 ± 0.008 a |
| BCF5 | 87.22 ± 0.19 b | 75.97 ± 0.11 b | 0.56 ± 0.005 b |
| BCF10 | 84.55 ± 0.16 c | 74.89 ± 0.13 c | 0.55 ± 0.003 c |
| BCF15 | 80.97 ± 0.14 d | 73.70 ± 0.12 d | 0.54 ± 0.004 d |
| BCF20 | 77.43 ± 0.12 e | 72.32 ± 0.10 e | 0.53 ± 0.003 e |
| BCF15 | 80.97 ± 0.16 a | 73.70 ± 0.12 a | 0.54 ± 0.004 a |
| BCF15RP0.5 | 78.15 ± 0.14 b | 73.06 ± 0.11 b | 0.53 ± 0.005 a |
| BCF15RP1 | 76.88 ± 0.12 c | 72.39 ± 0.10 c | 0.52 ± 0.006 a |
| BCF15RP2 | 74.32 ± 0.13 d | 71.24 ± 0.12 d | 0.50 ± 0.003 b |
| BCF15RP3 | 72.70 ± 0.11 e | 70.16 ± 0.11 e | 0.49 ± 0.004 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, I.-A.; Dossa, S.; Stoin, D.; Neagu, C.; Moigradean, D.; Alexa, E.; Poiana, M.-A. Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour. Foods 2025, 14, 3343. https://doi.org/10.3390/foods14193343
Pop I-A, Dossa S, Stoin D, Neagu C, Moigradean D, Alexa E, Poiana M-A. Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour. Foods. 2025; 14(19):3343. https://doi.org/10.3390/foods14193343
Chicago/Turabian StylePop, Ioana-Alina, Sylvestre Dossa, Daniela Stoin, Christine Neagu, Diana Moigradean, Ersilia Alexa, and Mariana-Atena Poiana. 2025. "Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour" Foods 14, no. 19: 3343. https://doi.org/10.3390/foods14193343
APA StylePop, I.-A., Dossa, S., Stoin, D., Neagu, C., Moigradean, D., Alexa, E., & Poiana, M.-A. (2025). Nutritional, Rheological, and Functional Assessment in the Development of Bread Using Chestnut and Rosehip-Fortified Wheat Flour. Foods, 14(19), 3343. https://doi.org/10.3390/foods14193343










