Impact of Combined Light and Modified Atmosphere Packaging on Postharvest Quality and Carbohydrate Fluctuations of Kyoho Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples and Experimental Design
2.2. Visual Quality Assessment
2.3. Determination of Hardness, Electrical Conductivity and Malondialdehyde (MDA)
2.4. Determination of Vitamin C (VC), Fruiting Pedicel Water Content and Total Soluble Solid (TSS)
2.5. Determination of Polyphenol Oxidase (PPO), Superoxide Dismutase (SOD) and Phenylalanine Ammonia-Lyase (PAL)
2.6. Determination of Neutral Invertase (NI) and Acid Invertase (AI)
2.7. Determination of Sucrose Synthase (SS) and Sucrose Phosphate Synthase (SPS)
2.8. Determination of Glucose Content, Fructose Content and Sucrose Content
2.9. Statistical Analysis
3. Results and Discussion
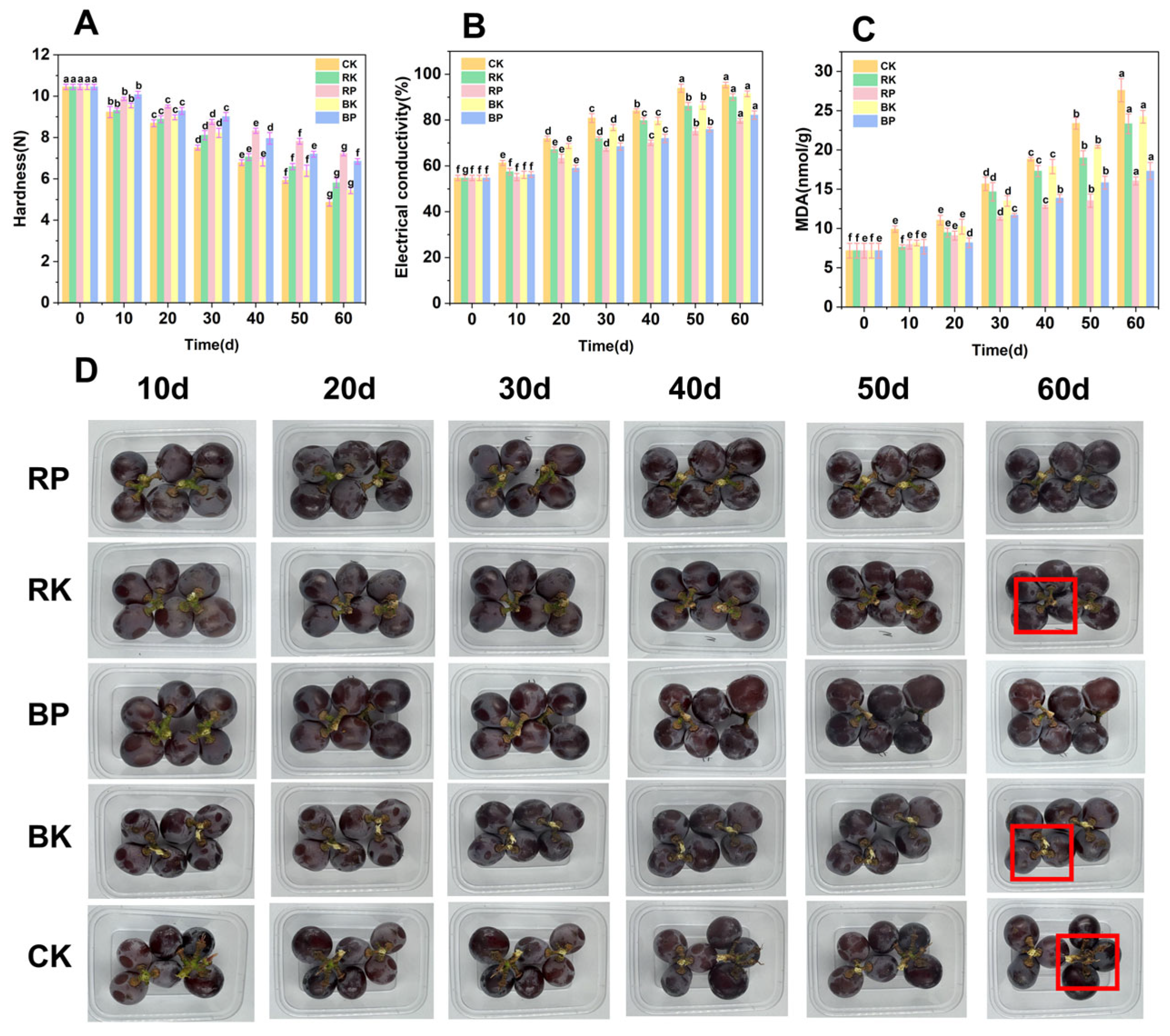
3.1. Analysis of Indicators Related to Postharvest Hardness of Grape Berries
3.2. Impact of Light Treatment Combined with MAP on Postharvest Fruit Quality
3.3. Impact of Light Treatment Combined with MAP on Postharvest Fruit Antioxidant Capacity
3.4. Improvement of Carbohydrate Metabolism-Related Enzyme Activities by Light Treatment Combined with MAP
3.5. Relationship Between Changes in Sugar Content and Activities of Carbohydrate Metabolism-Related Enzymes in Postharvest Grape Berries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, T.; Yi, W.G.; Wang, Y.; Cheng, P.; Dong, T.; Yun, X. Application of Poly(L-Lactic Acid)-Based Films for Equilibrium Modified Atmosphere Packaging of “Kyoho” Grapes and Its Favorable Protection for Anthocyanins. Food Chem. 2024, 452, 139573. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, J.; Tian, R.; Kuang, Y.; Wu, K.; Xiao, M.; Liu, Y.; Qian, H.; Jiang, F. Properties of Konjac Glucomannan/Curdlan-Based Emulsion Films Incorporating Camellia Oil and the Preservation Effect as Coatings on ‘Kyoho’ Grapes. Int. J. Biol. Macromol. 2024, 258, 128836. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, A.M.; Abdel-Sattar, M.; Aboukarima, A.M.; Eshra, D.H.; Górnik, K. Physico-Chemical Properties Prediction of Flame Seedless Grape Berries Using an Artificial Neural Network Model. Foods 2022, 11, 2766. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, X.; Yang, S.; Cheng, X.; Wang, W.; Zou, L.; Liu, S.; Yang, Y.; Liu, A.; He, L. Preparation of Composite Film with CoFe2O4/MXene/Chitosan for Extending the Shelf Life of Kyoho Grapes. Food Biosci. 2025, 64, 105900. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, L.; Dai, S.; Liu, M.; Guo, X.; Meng, F. Alginate-Based Edible Coating with Magnoliae Essential Oil/β-Cyclodextrin Inclusion Complex for Kyoho Grape Preservation. LWT 2024, 210, 116874. [Google Scholar] [CrossRef]
- Xia, S.; Zhao, Y.; Deng, Q.; Han, X.; Wang, X. VvRF2b Interacts with VvTOR and Influences VvTOR-Regulated Sugar Metabolism in Grape. Plant Sci. 2024, 349, 112276. [Google Scholar] [CrossRef]
- Huang, T.; Zheng, T.; Hong, P.; He, J.; Cheng, Y.; Yang, J.; Zhou, Y.; Wang, B.; Zhou, S.; Cheng, G.; et al. Sucrose Synthase 3 Improves Fruit Quality in Grape. Plant Physiol. Biochem. 2025, 221, 109590. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Zhang, J.; Li, W.; Chen, K.; Zhang, K.; Fang, Y. Supplementary Light with Different Wavelengths Improved the Monoterpenes Aroma and Quality Traits of ‘Shine Muscat’ Grape Berries under Facility Cultivation. Food Chem. 2025, 474, 143255. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.; Yang, M.; Shi, C.; Zheng, C. Studies of Postharvest Berry Abscission of ‘Kyoho’ Table Grapes during Cold Storage and High Oxygen Atmospheres. Postharvest Biol. Technol. 2007, 43, 95–101. [Google Scholar] [CrossRef]
- López-Díaz, A.S.; Antonio-Gutiérrez, O.; Palou, E.; Mani-López, E.; López-Malo, A.; Ramírez-Corona, N. Post-Harvest Quality Preservation of Red Globe Grapes Using Grape Juice-Based Edible Coatings Combined with UVC Treatment. Food Chem. 2025, 470, 142678. [Google Scholar] [CrossRef] [PubMed]
- Toyen, D.; Suppakul, P.; Siwayaprahm, P.; Thanomchat, P.; Sukatta, U.; Kosawatpat, P.; Banleng, T.; Saenboonruang, K. Optimizing UV-C Treatment for Postharvest Quality Preservation of Sea Grapes (Caulerpa lentillifera). Postharvest Biol. Technol. 2025, 227, 113602. [Google Scholar] [CrossRef]
- Hassan, M.; Ali, S. Carboxymethyl Cellulose Coating Delays Quality Deterioration in Harvested Table Grapes during Cold and Ambient Storage Conditions. Prog. Org. Coat. 2025, 200, 109031. [Google Scholar] [CrossRef]
- Huo, X.; Tian, X.; Liu, Z.; Wang, L.; Kong, Q.; Wang, D.; Ren, X. Combination of LED Blue Light with Peppermint Essential Oil Emulsion for the Postharvest Storage of Shine Muscat Grape to Control Aspergillus Carbonarius. Postharvest Biol. Technol. 2024, 218, 113175. [Google Scholar] [CrossRef]
- Zha, Q.; Zhong, H.; Tang, M.; Yin, X.; Sun, P.; Jiang, A.; Xi, X.; Wu, J. Effects of Temperature and Light during the Veraison Period on Grape Berry Growth. Plant Stress 2024, 14, 100642. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S.; Guo, Z. Preparation and Characterization of TiO2 Photocatalyst Films and Their Application for Preservation of Agaricus Bisporus in Combination with Blue-Violet LEDs. Food Biosci. 2024, 59, 103860. [Google Scholar] [CrossRef]
- Chong, L.; Ghate, V.; Zhou, W.; Yuk, H.G. Developing an LED Preservation Technology to Minimize Strawberry Quality Deterioration during Distribution. Food Chem. 2022, 366, 130566. [Google Scholar] [CrossRef]
- Yan, Z.; Yan, B.; Xu, D.; Yuan, S.; Xu, X.; Wu, C.; Shi, J.; Zuo, J.; Yue, X.; Wang, Q. Integrated Transcriptomic and MiRNA-Seq Analysis of Pak-Choi Provides Insight into the Molecular Mechanisms Involved in the Preservation of Postharvest Quality by White LED Light Irradiation. Postharvest Biol. Technol. 2025, 219, 113183. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Mujumdar, A.S.; Rui, L. Extending Shelf Life of Chilled Pork Pretreated with High-Voltage Electrostatic Field in Modified Atmosphere Packaging by Cinnamaldehyde Nanoemulsion at Non-Contact Mode. Meat Sci. 2025, 225, 109802. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.; Zhou, L.; Wang, W.; Liu, D.; Zhang, Y.; Bai, T.; Pan, D.; Zhang, L.; Pan, S.; et al. Investigation of Physicochemical Properties, Volatilome and Microbial Dynamics in Goat Meat under Modified Atmosphere and Vacuum Packaging. LWT 2025, 223, 117735. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B. High CO2-Modified Atmosphere to Preserve Sensory and Nutritional Quality of Organic Table Grape (Cv. ‘Italia’) During Storage and Shelf-Life. Eur. J. Hortic. Sci. 2016, 81, 197–203. [Google Scholar] [CrossRef]
- Souza, M.; Artés, F.; Jemni, M.; Artés–Hernández, F.; Martínez–Hernández, G.B. Combined Effect of UV–C and Passive Modified Atmosphere Packaging to Preserve the Physicochemical and Bioactive Quality of Fresh Figs During Storage. Postharvest Biol. Technol. 2022, 194, 112106. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Azhar, M.R.; Afrifa-Yamoah, E.; Woodward, A. Cinnamon Bark Oil Application and Modified Atmosphere Packaging Alleviates Chilling Injury, Ethylene Production and Maintains Antioxidant System in Persimmons. Food Chem. 2025, 144660. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Luo, Z.; Chen, Y.; Qi, Y.; Ye, M.; Chen, F.; Huang, H.; Dai, F. Combined Modified Atmosphere Package and Melatonin Treatments Delay the Senescence of Bitter Bamboo Shoots by Inhibiting the Cell Wall Changes after Harvest. LWT 2025, 219, 117558. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y. Effects of High CO2 and Low O2 Atmospheres on the Berry Drop of ‘Kyoho’ Grapes. Food Chem. 2007, 100, 768–773. [Google Scholar] [CrossRef]
- Dong, T.; Hao, T.; Zhang, P.; Hakeem, A.; Zhao, P.; Song, S.; Ren, Y.; Chen, Y.; Jia, H.; Fang, J. A Comprehensive Evaluation of Different Responses of Supplementary Light Qualities on Physiological and Biochemical Mechanisms of ‘Kyoho’ Grape. Sci. Hortic. 2024, 333, 113261. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Sarkhosh, A.; Richmond-Cosie, L.M.; Brecht, J.K. Physiological Responses and Quality Attributes of Muscadine Grape (Vitis rotundifolia Michx) to CO2-Enriched Atmosphere Storage. Postharvest Biol. Technol. 2021, 173, 111428. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Xie, J. Influence of Different Pre-Cooling Methods on the Postharvest Storage of ‘Kyoho’ Grapes (Vitis Labrusca × Vinifera ‘Kyoho’). Food Qual. Saf. 2024, 8. [Google Scholar] [CrossRef]
- Shuai, L.; Xue, P.Y.; Liao, L.; Liu, Y.; Song, M.; Shang, F.; Cai, W.; Yin, F.; Cai, J. Methyl Jasmonate Suppressed the Pericarp Browning in Postharvest Longan Fruit by Modulating Membrane Lipid and Energy Metabolisms. Postharvest Biol. Technol. 2024, 209, 112681. [Google Scholar] [CrossRef]
- Pérez-Pérez, C.A.; Gonzalez Viejo, C.; Fuentes, S.; Valiente-Banuet, J.I. Vineyard Proximal Sensing Using Multispectral Imaging to Evaluate Grape Ripening and Quality Traits Using Artificial Neural Networks Modeling. J. Agric. Food Res. 2025, 23, 102252. [Google Scholar] [CrossRef]
- Babajamali, A.; Gholami, M.; Baninasab, B. Drought Preconditioning Improves Freezing Tolerance in Drought-Tolerant and -Intolerant Grape Cultivars. Theor. Exp. Plant Physiol. 2022, 34, 395–407. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Iqbal, S.; Aucique-Perez, C.E.; Hussain, S.; Balal, R.M.; Charrier, G.; Mattia, M.; Chater, J.M.; Shahid, M.A. Drought-Stress Memory Confers Cold Hardiness in Grapefruit (Citrus paradisi) through Modulations in Antioxidant System, Osmolyte Production and Carbohydrate Metabolism. Plant Stress 2025, 15, 100801. [Google Scholar] [CrossRef]
- Shinomiya, R.; Fujishima, H.; Muramoto, K.; Shiraishi, M. Impact of Temperature and Sunlight on the Skin Coloration of the ‘Kyoho’ Table Grape. Sci. Hortic. 2015, 193, 77–83. [Google Scholar] [CrossRef]
- Zou, F.; Shinali, T.S.; Yang, M.; Zhong, Y.; Wu, J.; Wang, L.; Wang, H. Incorporation of Ascorbic Acid in Chitosan-Based Coating Combined with Plasma-Activated Water: A Technology for Quality Preservation of Red Grapes after Simulated Transportation. Int. J. Biol. Macromol. 2024, 270, 132366. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Belwal, T.; Javed, M.; Luo, Z. Influence of the Red LEDs Light Irradiation on the Quality and Chemical Attributes of Postharvest Table Grape (Vitis vinifera L.) During Storage. Food Bioproc. Tech. 2022, 15, 1436–1447. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Y.; Han, X.; Ji, H.; Su, X.; Yuan, Z.; Yang, K.; Jia, X. Inhibition Effect of Botrytis Cinerea and Quality Preservation of Grape by Bletilla Striata Polysaccharide. Physiol. Mol. Plant Pathol. 2025, 136, 102594. [Google Scholar] [CrossRef]
- Habiba, U.; Bajpai, A.; Shafi, Z.; Pandey, V.K.; Singh, R. Advancing Sustainability through Modified Atmosphere Packaging (MAP) for Fresh Food Preservation: A Critical Review. J. Stored Prod. Res. 2025, 112, 102657. [Google Scholar] [CrossRef]
- Zhang, L.; Diao, J.; Zhao, Z.; Zhang, X.; Lou, W. Cinnamon Essential Oil-Loaded Bagasse Cellulose/Hydroxypropyl-β-Cyclodextrin Microparticles with Sustained-Release Property and Its Application in Grapes Preservation. Int. J. Biol. Macromol. 2025, 304, 140972. [Google Scholar] [CrossRef] [PubMed]
- Nassarawa, S.S.; Luo, Z. Effect of Light Irradiation on Sugar, Phenolics, and GABA Metabolism on Postharvest Grape (Vitis vinifera L.) During Storage. Food Bioproc. Technol. 2022, 15, 2789–2802. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, H.; Yan, R.; Chen, Q.; Fu, M.; Xin, X. The Composite Edible Film of Sodium Alginate and Natamycin Maintained Color of Pedicel and Calyx of Tomato Fruit. Postharvest Biol. Technol. 2025, 222, 113413. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Feng, W.; Wan, S.; Bian, Y.; Xie, Z. Differential Regulation of Xylem and Phloem Differentiation in Grape Berries by GA3 and CPPU. Sci. Hortic. 2024, 337, 113582. [Google Scholar] [CrossRef]
- Ye, J.; Han, X.; Jiang, T. Effects of Active Modified Atmosphere Packaging on Postharvest Quality of Shiitake Mushrooms (Lentinula edodes) Stored at Cold Storage. J. Integr. Agric. 2012, 11, 474–482. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Liu, S.; Yu, Q.; Yin, M.; Huan, C.; Zheng, X.; Shen, S. Mechanisms of Blue Light-Mediated Inhibition of Chlorophyll Degradation in ‘Newhall’ Sweet Orange during Postharvest. Postharvest Biol. Technol. 2026, 231, 113895. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Yang, C.; Chen, J.; Yang, X.; Chen, C.; Kai, W.; Zeng, J.; Chen, M.; Gan, Z. Blue Light Inhibited Postharvest Softening of Kiwifruit by Modulating the Reactive Oxygen Species Scavenging System. Plant Physiol. Biochem. 2025, 227, 110182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Z.; Peng, C.; Zhang, H.; Zhang, L.; Zheng, S.; Chen, J. Roles of ROS in Physiological, Microbial and Metabolomic Alterations of Fresh–Cut Sugarcane under Red and Blue Light Irradiation. Food Chem. X 2025, 26, 102344. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Quarta, B.; Dri, A. Polyphenoloxidase Inactivation by Light Exposure in Model Systems and Apple Derivatives. Innov. Food Sci. Emerg. Technol. 2009, 10, 506–511. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Balic, I.; Sepúlveda, P.; Olmedo, P.; León, G.; Defilippi, B.G.; Blanco-Herrera, F.; Campos-Vargas, R. Effect of Modified Atmosphere Packaging (MAP) on Rachis Quality of ‘Red Globe’ Table Grape Variety. Postharvest Biol. Technol. 2016, 119, 33–40. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous White–Blue LED Light Exposition Delays Postharvest Senescence of Broccoli. LWT 2016, 65, 495–502. [Google Scholar] [CrossRef]
- Liu, J.; Pan, L.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Zhou, G.; Liu, C.; Wan, H. Evolution and Functional Roles of Neutral/Alkaline Invertases in Plant Growth, Development, and Stress Response. Plant Physiol. Biochem. 2025, 225, 110011. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Hu, J.; Xie, Q.; Chen, X.; Qi, X. Sucrose Synthase: An Enzyme with Multiple Roles in Plant Physiology. J. Plant Physiol. 2024, 303, 154352. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, L.C.; Li, Y.L.; Yang, Q.C.; Guo, W. zhong Alternating Red and Blue Irradiation Affects Carbohydrate Accumulation and Sucrose Metabolism in Butterhead Lettuce. Sci. Hortic. 2022, 302, 111177. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Zhang, W.; Li, L.; Yang, W.; Liu, Y.; Jiang, L.; Guo, M.; Chen, G. Melatonin Treatment Delayed Fruit Softening by Regulating Postharvest Carbohydrate Metabolism of Hami Melon. Plant Physiol. Biochem. 2025, 219, 109328. [Google Scholar] [CrossRef]
- Shi, L.; Cao, S.; Shao, J.; Chen, W.; Yang, Z.; Zheng, Y. Chinese Bayberry Fruit Treated with Blue Light after Harvest Exhibit Enhanced Sugar Production and Expression of Cryptochrome Genes. Postharvest Biol. Technol. 2016, 111, 197–204. [Google Scholar] [CrossRef]
- Shuai, L.; Xue, P.Y.; Liao, L.; Guo, X.; Liu, Y.; Song, M.; Cai, W.; Yin, F.; He, M. Methyl Jasmonate Improves Pulp Flavor by Modulating Sugar Metabolism in Postharvest Longan Fruit. Postharvest Biol. Technol. 2025, 219, 113268. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin Application Improves Berry Coloration, Sucrose Synthesis, and Nutrient Absorption in ‘Summer Black’ Grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Doud, M.S.; Luo, W.; Raithore, S.; Baldwin, E.A.; Zhao, W.; Plotto, A.; Bai, J.; Manthey, J.A.; Stover, E.; et al. Beneficial Horticultural Responses from the Application of Solar Thermotherapy to Mature Huanglongbing-Affected Citrus Trees. Hortic. Plant J. 2021, 7, 411–422. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Sun, X.; Li, J.; Chen, N. Inhibition of Sucrose and Galactosyl-Sucrose Oligosaccharide Metabolism in Leaves and Fruits of Melon (Cucumis melo L.) under Low Light Stress. Sci. Hortic. 2019, 244, 343–351. [Google Scholar] [CrossRef]
- Guan, J.; Liang, X.; Gao, G.; Yang, F.; Qi, H. The Interaction between CmPIF8 and CmACO1 under Postharvest Red Light Treatment Might Affect Fruit Ripening and Sucrose Accumulation in Oriental Melon Fruit. Postharvest Biol. Technol. 2024, 209, 112717. [Google Scholar] [CrossRef]



| Samples | Treatment |
|---|---|
| CK | sunlight-proofing |
| RK | Continuous red LED illumination (660 nm) |
| RP | Continuous red LED irradiation (660 nm) and Modified atmosphere packaging |
| BK | Continuous blue LED illumination (450 nm) |
| BP | Continuous blue LED irradiation (450 nm) and Modified atmosphere packaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Tao, S.; Ding, Z.; Xie, J. Impact of Combined Light and Modified Atmosphere Packaging on Postharvest Quality and Carbohydrate Fluctuations of Kyoho Grapes. Foods 2025, 14, 3308. https://doi.org/10.3390/foods14193308
Zhao K, Tao S, Ding Z, Xie J. Impact of Combined Light and Modified Atmosphere Packaging on Postharvest Quality and Carbohydrate Fluctuations of Kyoho Grapes. Foods. 2025; 14(19):3308. https://doi.org/10.3390/foods14193308
Chicago/Turabian StyleZhao, Kunpeng, Shaoyu Tao, Zhaoyang Ding, and Jing Xie. 2025. "Impact of Combined Light and Modified Atmosphere Packaging on Postharvest Quality and Carbohydrate Fluctuations of Kyoho Grapes" Foods 14, no. 19: 3308. https://doi.org/10.3390/foods14193308
APA StyleZhao, K., Tao, S., Ding, Z., & Xie, J. (2025). Impact of Combined Light and Modified Atmosphere Packaging on Postharvest Quality and Carbohydrate Fluctuations of Kyoho Grapes. Foods, 14(19), 3308. https://doi.org/10.3390/foods14193308









