Inorganic Contaminants in Rapadura from Latin America
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Solutions
2.3. Determination of Inorganic Contaminants in Rapadura
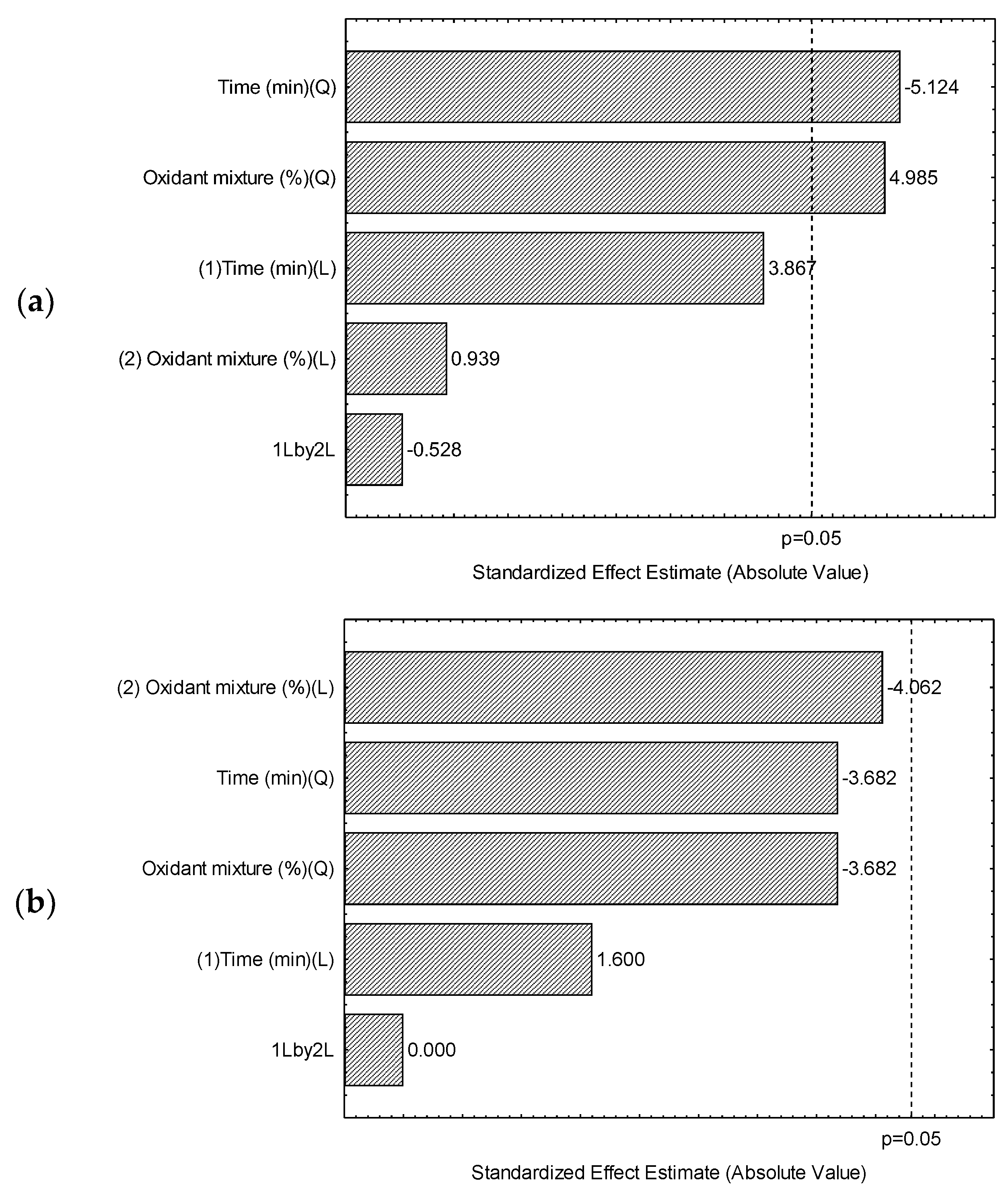
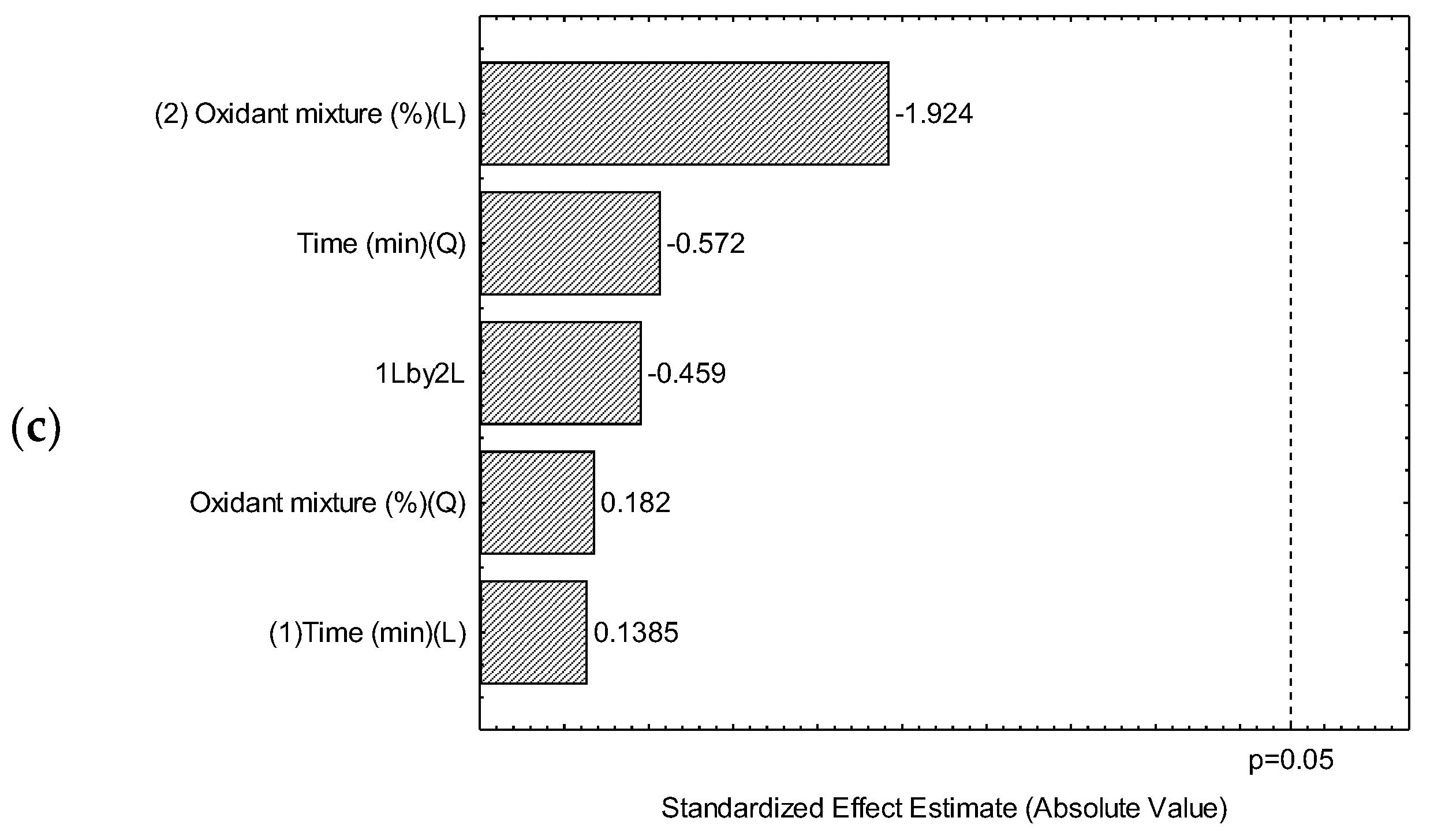
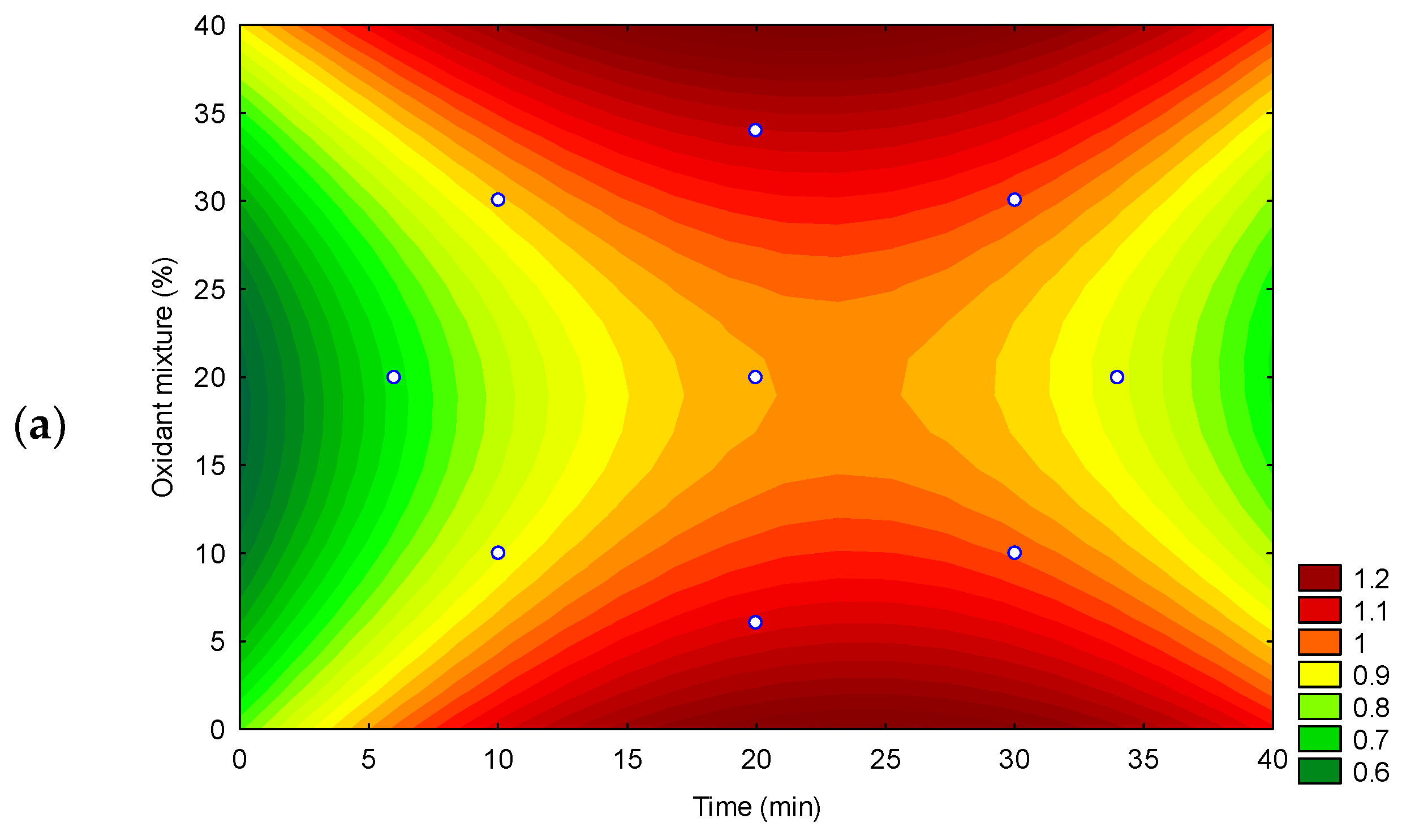
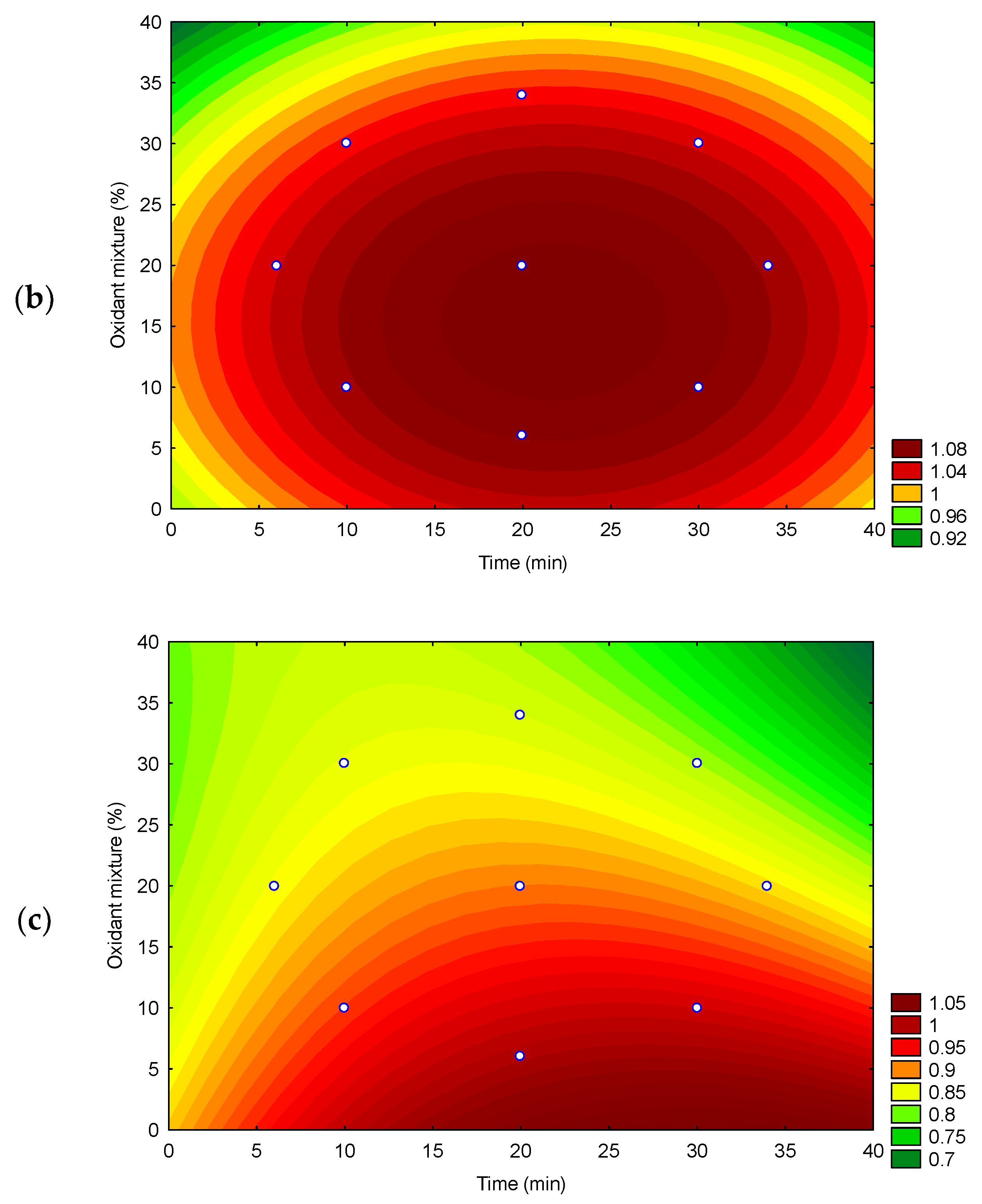
2.3.1. Optimization of the Extraction Method
2.3.2. Inorganic Contaminants in Rapadura by ICP OES
2.4. Quality Control and Statistical Analysis
2.5. Exposure Assessment and Risk Characterization
3. Results and Discussion
3.1. Optimization of an Ultrasound Acid Digestion Method for Inorganic Contaminants in Rapadura by ICP OES
3.2. Occurrence of Arsenic, Cadmium, and Lead in NCS Samples from Latin America
3.3. Exposure and Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffé, W.R. Nutritional and Functional Components of Non Centrifugal Cane Sugar: A Compilation of the Data from the Analytical Literature. J. Food Compos. Anal. 2015, 43, 194–202. [Google Scholar] [CrossRef]
- Baqueta, M.R.; Pizano, F.P.; Villani, J.D.; Toro, S.J.H.; Bragotto, A.P.A.; Valderrama, P.; Pallone, J.A.L. Kurtosis-Based Projection Pursuit Analysis to Evaluate South American Rapadura. Food Chem. 2022, 368, 130731. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, W.R. Health Effects of Non-Centrifugal Sugar (NCS): A Review. Sugar Tech 2012, 14, 87–94. [Google Scholar] [CrossRef]
- Silva, F.S.; Cristale, J.; Ribeiro, M.L.; Marchi, M.R.R. de Polycyclic Aromatic Hydrocarbons (PAHs) in Raw Cane Sugar (Rapadura) in Brazil. J. Food Compos. Anal. 2011, 24, 346–350. [Google Scholar] [CrossRef]
- FAO–Food and Agriculture Organization of the United Nations. Procesados de Productos Diversos. Fichas Técnicas. 2014, 2014, 1. [Google Scholar]
- Wojtczak, M.; Antczak, A.; Lisik, K. Contamination of Commercial Cane Sugars by Some Organic Acids and Some Inorganic Anions. Food Chem. 2013, 136, 193–198. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2024; FAO: Rome, Italy, 2024. [Google Scholar]
- Lee, J.S.; Ramalingam, S.; Jo, I.G.; Kwon, Y.S.; Bahuguna, A.; Oh, Y.S.; Kwon, O.J.; Kim, M. Comparative Study of the Physicochemical, Nutritional, and Antioxidant Properties of Some Commercial Refined and Non-Centrifugal Sugars. Food Res. Int. 2018, 109, 614–625. [Google Scholar] [CrossRef]
- FAO/WHO CF/17 INF—Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. 17th Session 15–19 April 2024 (Panama City). Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF (Prepared By Japan and The Netherlands). 1 April 2024. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/jp/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-17%252FWorking%2Bdocuments%252Fcf17_inf1x.pdf (accessed on 8 August 2025).
- Morgano, M.A.; Teixeira Martins, M.C.; Rabonato, L.C.; Milani, R.F.; Yotsuyanagi, K.; Rodriguez-Amaya, D.B. Inorganic Contaminants in Bee Pollen from Southeastern Brazil. J. Agric. Food Chem. 2010, 58, 6876–6883. [Google Scholar] [CrossRef]
- Alcívar, M.; Vinueza, E.; Pernía, B.; Álvarez-Montero, X.; Gallardo, A. Contamination by Cadmium and Lead in Sugarcane and Its Derived Products in Ecuador. Agriculture 2024, 14, 2121. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Cadmium Dietary Exposure in the European Population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Mekassa, B.; Etana, E.; Merga, L.B. Proximate Analysis, Levels of Trace Heavy Metals and Associated Human Health Risk Assessments of Ethiopian White Sugars. J. Agric. Food Res. 2024, 16, 101086. [Google Scholar] [CrossRef]
- Brazil; ANVISA—Agência Nacional de Vigilância Sanitária-Resolução Da Diretoria Colegiada—RDC No 722/22. Dispõe Sobre os Limites Máximos Tolerados (LMT) de Contaminantes em Alimentos, os Princípios Gerais para o seu Estabelecimento e os Métodos de Análise para fins de Avaliação de Conformidade. Available online: https://anvisalegis.datalegis.net/action/UrlPublicasAction.php?acao=abrirAtoPublico&num_ato=00000722&sgl_tipo=RDC&sgl_orgao=RDC/DC/ANVISA/MS&vlr_ano=2022&seq_ato=002&cod_modulo=134&cod_menu=1696 (accessed on 8 August 2025).
- MERCOSUR—GMC/RES Nº 12/11. Regulamento Técnico Mercosul Sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos (Revogação das RES. GMC Nº 102/94 e Nº 35/96). Available online: https://normas.mercosur.int/public/normativas/2474 (accessed on 8 August 2025).
- European Union Commission Regulation (EU) 2023/915; 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. European Union Commission Regulation (EU): Brussels, Belgium, 2023; pp. 1–77.
- Waheed, S.; Rahman, S.; Gill, K.P. INAA and AAS of Different Products from Sugar Cane Industry in Pakistan: Toxic Trace Elements for Nutritional Safety. J. Radioanal. Nucl. Chem. 2009, 279, 725–731. [Google Scholar] [CrossRef]
- dos Santos, J.M.; Quináia, S.P.; Felsner, M.L. Fast and Direct Analysis of Cr, Cd and Pb in Brown Sugar by GF AAS. Food Chem 2018, 260, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, R.M.; Sherif, M.K.; Mohamed, A.E.; Grass, F. Determination of Trace Elements in Egyptian Cane Sugar (Deshna Factories) by Neutron Activation, Atomic Absorption Spectrophotometric and Inductively Coupled Plasma-Atomic Emission Spectrometric Analysis. J. Radioanal. Nucl. Chem. Artic. 1986, 98, 49–64. [Google Scholar] [CrossRef]
- Abdel-Rahman, G.N.; Nassar, N.R.A.; Heikal, Y.A.; Abou-Donia, M.A.M.; Naguib, M.M.; Fadel, M. Effect of Different Treatments on Heavy Metal Concentration in Sugar Cane Molasses. Int. J. Agric. Biosyst. Eng. 2016, 10, 43–48. [Google Scholar]
- Salles, P.M.B.; Menezes, M.Â.d.B.C.; Jaćimović, R.; Campos, T.P.R. Inorganic Elements in Sugar Samples Consumed in Several Countries. J. Radioanal. Nucl. Chem. 2016, 308, 485–493. [Google Scholar] [CrossRef]
- Oppong, A.; Azanu, D.; Ofori, L.A. Assessment of Sugarcane Grown in Wetlands Polluted with Wastewater. Cogent. Env. Sci. 2018, 4, 1455277. [Google Scholar] [CrossRef]
- Souza, S.O.; Costa, S.S.L.; Brum, B.C.T.; Santos, S.H.; Garcia, C.A.B.; Araujo, R.G.O. Determination of Nutrients in Sugarcane Juice Using Slurry Sampling and Detection by ICP OES. Food Chem. 2019, 273, 57–63. [Google Scholar] [CrossRef]
- Milani, R.F.; Morgano, M.A.; Diego Quintaes, K. Rapid Elemental Analysis of Sugarcane Spirits by Inductively Coupled Plasma: Optical Emission Spectrometry. Anal. Lett. 2019, 52, 526–538. [Google Scholar] [CrossRef]
- Milani, R.F.; Morgano, M.A.; Cadore, S. A Simple and Reliable Method to Determine 16 Trace Elements by ICP OES in Ready to Drink Beverages. Food Anal. Methods 2018, 11, 1763–1772. [Google Scholar] [CrossRef]
- INMETRO—Instituto Nacional de Metrologia, N. e Q.I. Coordenação Geral de Acreditação; National Institute of Metrology, Standardization and Industrial Quality (INMETRO): Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Cantwell, H. Eurachem Guide: The Fitness for Purpose of Analytical Methods A Laboratory Guide to Method Validation and Related Topics, 3rd ed.; Eurachem: Uppsala, Sweden, 2025. [Google Scholar]
- European Community Commission Regulation (EC) No 333/2007; 28 March 2007 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)Pyrene in Foodstuffs. European Union Commission Regulation (EU): Brussels, Belgium, 2007.
- WHO—World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- EFSA—European Food Safety Authority. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic Dietary Exposure to Inorganic Arsenic. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Grindlay, G.; Gras, L.; Mora, J.; de Loos-Vollebregt, M.T.C. Carbon-Related Matrix Effects in Inductively Coupled Plasma Atomic Emission Spectrometry. Spectrochim Acta Part B Spectrosc 2008, 63, 234–243. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- De Sousa, R.A.; Ribeiro, A.S.; Vieira, M.A.; Curtius, A.J.; Baccan, N.; Cadore, S. Determination of Trace Elements in Liquid Aspartame Sweeteners by ICP OES and ICP-MS Following Acid Digestion. Microchim. Acta 2007, 159, 241–246. [Google Scholar] [CrossRef]
| Country | N | Origin |
|---|---|---|
| Brazil | 16 | Espírito Santo (n = 1), Minas Gerais (n = 13), and Rio Grande do Sul (n = 2). |
| Colombia | 36 | Boyacá (n = 1), Cundinamarca (n = 1), Nariño (n = 3), Santander (n = 1), and Valle del Cauca (n = 30) |
| Ecuador | 12 | Milagro (n = 2), Quito (n = 6), Vilcabamba (n = 1), and not available (n = 3). |
| Mexico | 3 | Not available (n = 3) |
| Peru | 5 | La Victoria (n = 1), Lima (n = 3), and not available (n = 1) |
| Experiment | Parameters | Response (Recovery, %) | |||
|---|---|---|---|---|---|
| Time (min) | Oxidant Mixture (%) | As | Cd | Pb | |
| 1 | 10 (−1) | 10 (−1) | 82 | 103 | 90 |
| 2 | 10 (−1) | 30 (+1) | 96 | 102 | 83 |
| 3 | 30 (+1) | 10 (−1) | 92 | 107 | 95 |
| 4 | 30 (+1) | 30 (+1) | 102 | 106 | 80 |
| 5 | 6 (−√2) | 20 (0) | 71 | 106 | 87 |
| 6 | 34 (+√2) | 20 (0) | 89 | 104 | 88 |
| 7 | 20 (0) | 6 (−√2) | 117 | 109 | 102 |
| 8 | 20 (0) | 34 (+√2) | 107 | 101 | 84 |
| 9 | 20 (0) | 20 (0) | 93 | 109 | 100 |
| 10 | 20 (0) | 20 (0) | 92 | 107 | 84 |
| 11 | 20 (0) | 20 (0) | 99 | 109 | 86 |
| Figure of Merit | Inorganic Contaminant | |||
|---|---|---|---|---|
| Arsenic | Cadmium | Lead | ||
| LOD (n = 7) (mg kg−1) | 0.012 | 0.005 | 0.012 | |
| LOQ (n = 7) (mg kg−1) | 0.037 | 0.015 | 0.039 | |
| Precision—repeatability (n = 7) (CV, %) | 15 | 3 | 3 | |
| Spiked experiments (n = 3) (Trueness—recovery, %) | Level 1 (10 µg kg−1) | 104 ± 3 | 108 ± 1 | 97 ± 5 |
| Level 2 (20 µg kg−1) | 100 ± 7 | 105 ± 3 | 94 ± 4 | |
| Level 3 (50 µg kg−1) | 96 ± 2 | 104 ± 1 | 101 ± 1 | |
| Level 4 (100 µg kg−1) | 102 ± 1 | 107 ± 2 | 105 ± 1 | |
| CRM (n = 3) | Certified value (mg kg−1) | 0.106 ± 0.021 | 0.0302 ± 0.0040 | 1.78 ± 0.24 |
| Obtained value (mg kg−1) | 0.099 ± 0.011 | 0.028 ± 0.002 | 1.66 ± 0.48 | |
| Recovery (%) | 93 ± 10 | 93 ± 6 | 93 ± 3 | |
| Country | Inorganic Contaminant (mg kg−1) | |||
|---|---|---|---|---|
| Arsenic | Cadmium | Lead | ||
| Brazil (n = 16) | Mean | <LOQ | <LOQ | 0.085 ab |
| Range | <LOQ–<LOQ | <LOQ–<LOQ | 0.065–0.118 | |
| Colombia (n = 36) | Mean | <LOQ | 0.001 b | 0.074 b |
| Range | <LOQ–<LOQ | <LOQ–0.043 | 0.067–0.092 | |
| Ecuador (n = 12) | Mean | <LOQ | <LOQ | 0.096 a |
| Range | <LOQ–<LOQ | <LOQ–<LOQ | 0.078–0.108 | |
| Mexico (n = 3) | Mean | <LOQ | 0.005 a | 0.101 a |
| Range | <LOQ–<LOQ | <LOQ–0.015 | 0.081–0.122 | |
| Peru (n = 5) | Mean | <LOQ | 0.018 a | 0.107 a |
| Range | <LOQ–<LOQ | <LOQ–0.045 | 0.085–0.119 | |
| Inorganic Contaminant | Population Group | Highest Exposure, mg kg−1 bw day−1 (Contribution to the HBGV) | ||
|---|---|---|---|---|
| Portion: 20 g | Portion: 40 g | Sample Region | ||
| Cadmium | Children | 0.000060 (0.2% PTMI; 2.4% TWI) | 0.000120 (0.5% PTMI; 4.8% TWI) | Peru |
| Adults | 0.000015 (0.1% PTMI; 0.6% TWI) | 0.000030 (0.1% PTMI; 1.2% TWI) | ||
| Lead | Children | 0.00016 (1.3% BMDL0.1) | 0.00033 (2.8% BMDL0.1) | Mexico |
| Adults | 0.00004 (0.3% BMDL10) | 0.00008 (0.5% BMDL10) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, R.F.; Rodrigues, J.L.; Toro, S.J.H.; Mauri, A.A.; Bragotto, A.P.A.; Morgano, M.A. Inorganic Contaminants in Rapadura from Latin America. Foods 2025, 14, 3285. https://doi.org/10.3390/foods14193285
Milani RF, Rodrigues JL, Toro SJH, Mauri AA, Bragotto APA, Morgano MA. Inorganic Contaminants in Rapadura from Latin America. Foods. 2025; 14(19):3285. https://doi.org/10.3390/foods14193285
Chicago/Turabian StyleMilani, Raquel Fernanda, Juliana Lopes Rodrigues, Sandra Julieth Henao Toro, Adriana Aparecida Mauri, Adriana Pavesi Arisseto Bragotto, and Marcelo Antonio Morgano. 2025. "Inorganic Contaminants in Rapadura from Latin America" Foods 14, no. 19: 3285. https://doi.org/10.3390/foods14193285
APA StyleMilani, R. F., Rodrigues, J. L., Toro, S. J. H., Mauri, A. A., Bragotto, A. P. A., & Morgano, M. A. (2025). Inorganic Contaminants in Rapadura from Latin America. Foods, 14(19), 3285. https://doi.org/10.3390/foods14193285







