UiO-66-NH2-Deposited Gold Nanoparticles Enable Enhanced Interference-Resistant Immunochromatographic Assay for Rapid Detection of Gentamicin in Animal-Derived Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Preparation of AuNPs and UiO-66-NH2@Au
2.3. Preparation of UiO-66-NH2@Au-Ab Probe
2.4. Sample Preparation
2.5. Assembly of ICA Strip

2.6. Performance Evaluation of UiO-66-NH2@Au-ICA and AuNPs-ICA
2.6.1. Sensitivity
2.6.2. The Selectivity, Accuracy, and Precision Evaluations
2.6.3. Confirmatory Test
Robustness Performance Analysis of UiO-66-NH2@Au-ICA and AuNPs-ICA
3. Results
3.1. Characteristics of UiO-66-NH2, UiO-66-NH2@Au and UiO-66-NH2@Au-Ab
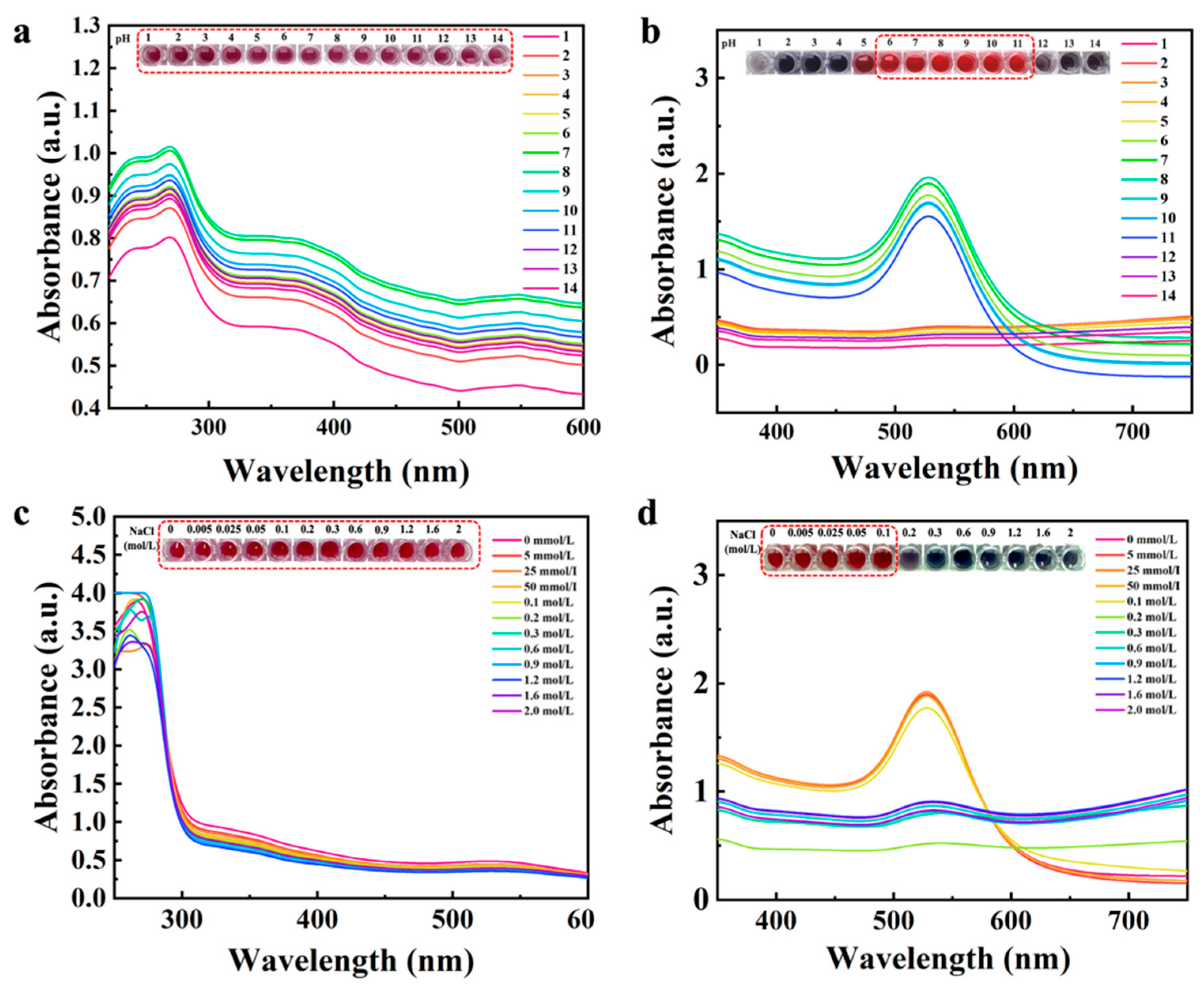
3.2. The Stability of the UiO-66-NH2@Au and AuNPs
3.3. Optimization of the UiO-66-NH2@Au-ICA
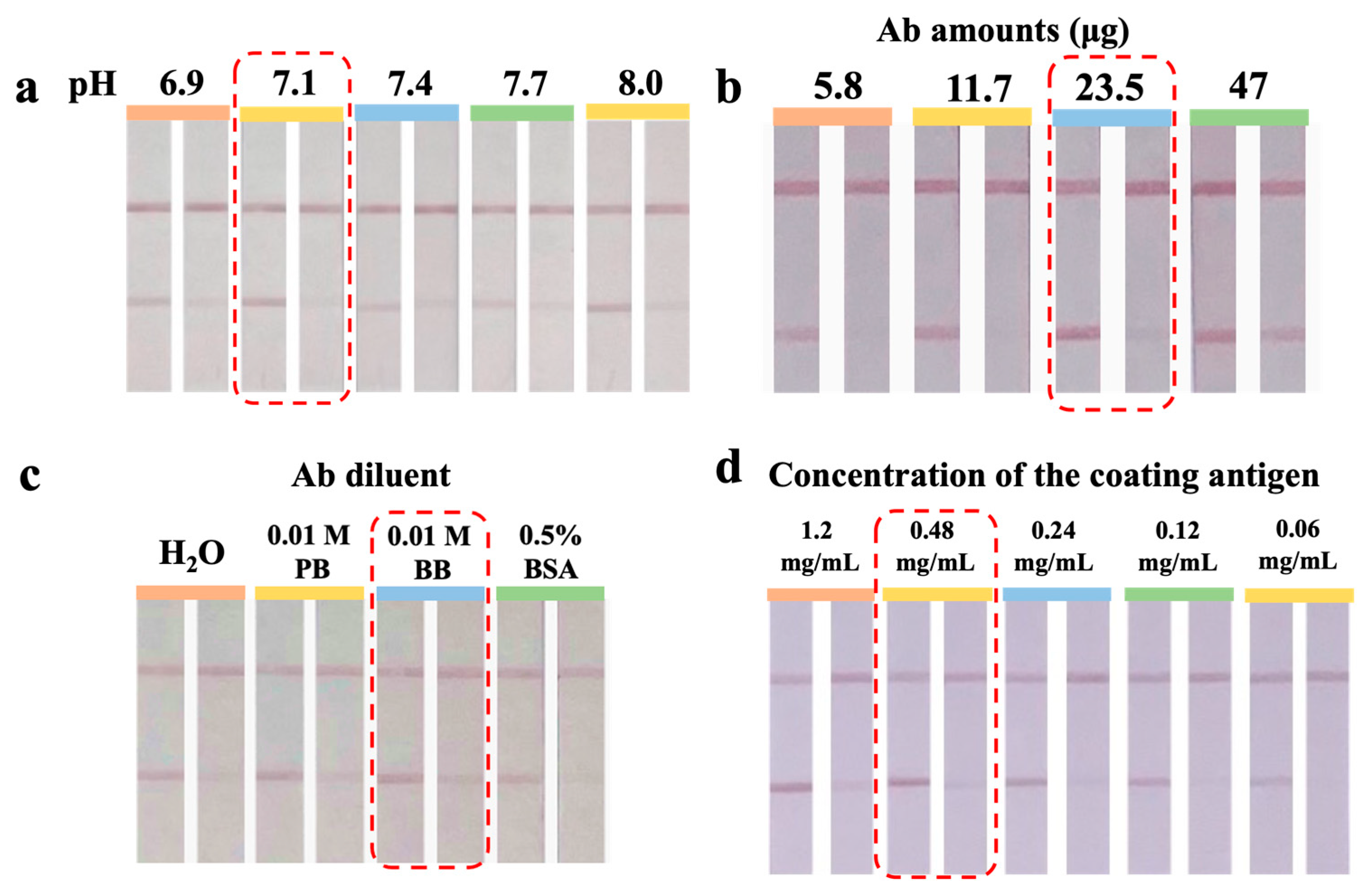
3.3.1. The Coupling pH Value
3.3.2. Optimization of Ab Amount
3.3.3. Optimization of Ab Diluent
3.3.4. Optimization of the Concentration of the Coating Antigen
3.4. Evaluation of UiO-66-NH2@Au-ICA and AuNPs-ICA
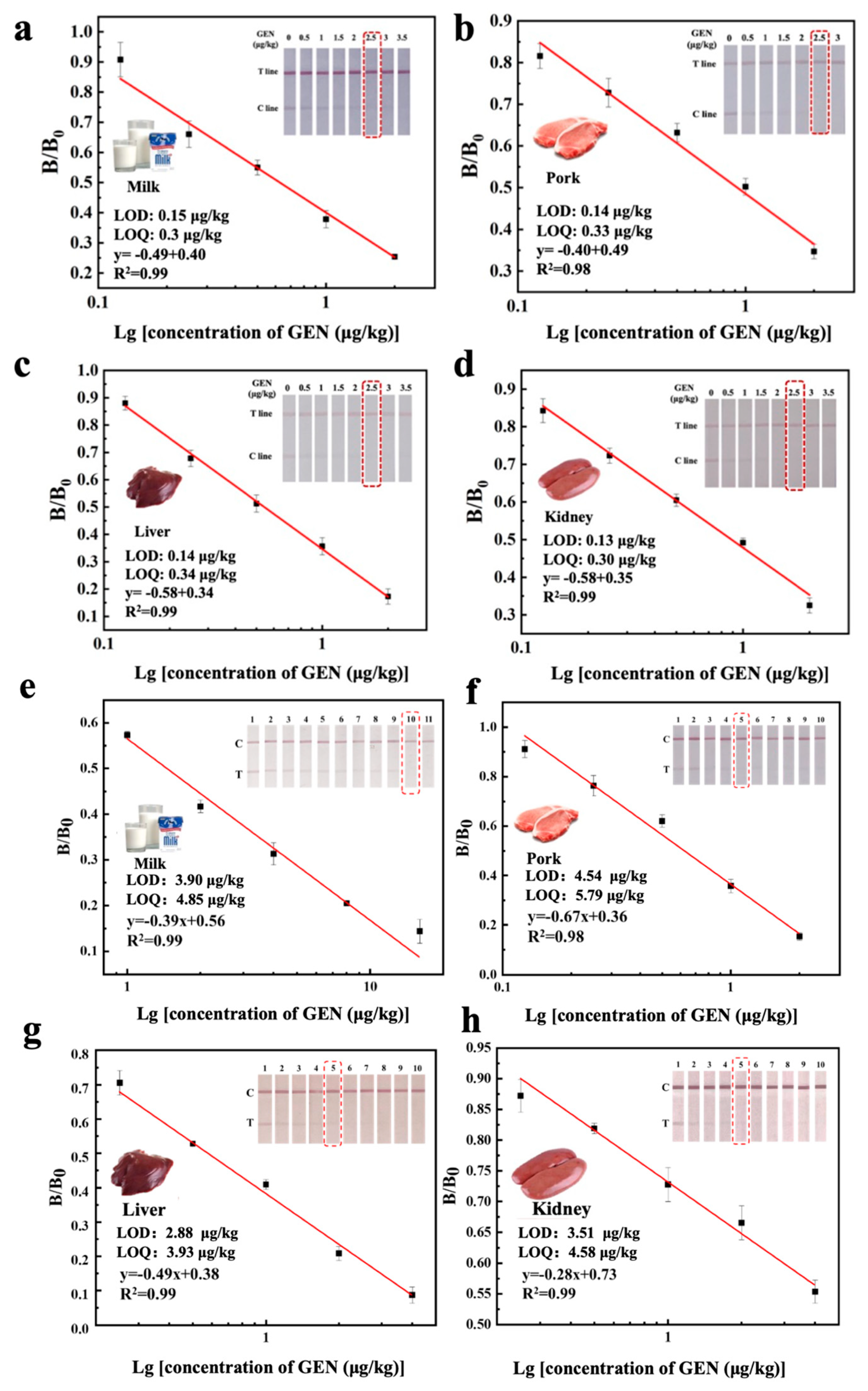
3.4.1. Analytical Sensitivity
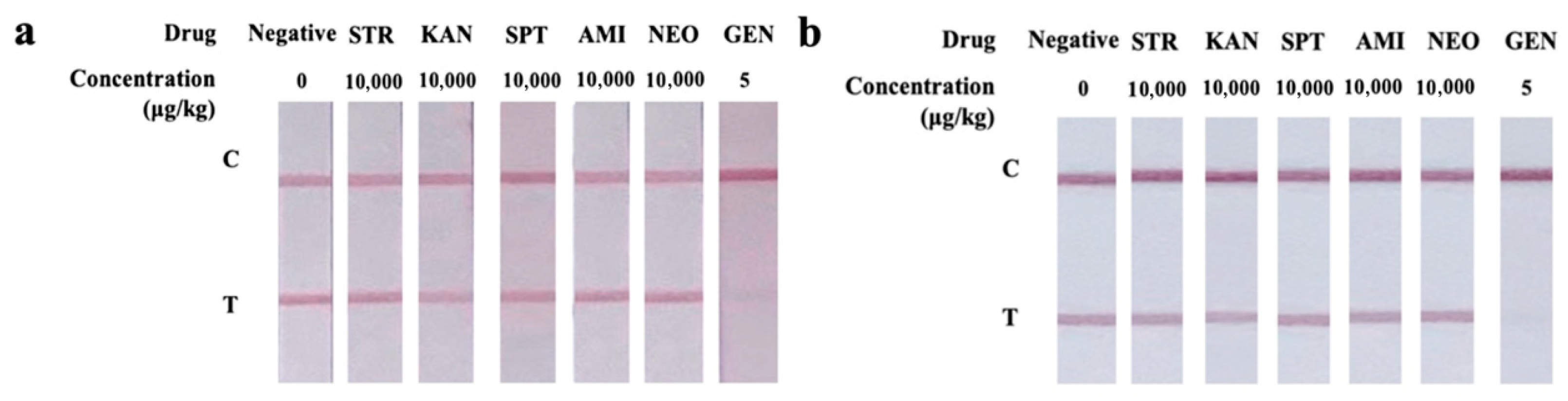
3.4.2. Cross-Reactivity Analysis
3.4.3. Accuracy and Precision
3.4.4. Confirmatory Test Results
3.5. Robustness Characteristics of UiO-66-NH2@Au-ICA Compared with AuNPs-ICA
3.6. Comparison of ICAs for GEN Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, A.; Rustum, A. Development and validation of a RP-HPLC method for the determination of gentamicin sulfate and its related substances in a pharmaceutical cream using a short pentafluorophenyl column and a charged aerosol detector. J. Pharm. Biomed. Anal. 2010, 51, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.; El-Aziz, G.M.A. A new approach for decreasing the detection limit of gentamicin ion-selective electrodes by incorporation of multiwall carbon nanotubes (MWCNTs)/lipophilic anionic additives. Electroanalysis 2017, 29, 566–577. [Google Scholar] [CrossRef]
- Ozaki, N.; Kato, H.; Ikeda, M.; Yano, Y.; Oishi, M. Identification of genes involved in gentamicin-induced nephrotoxicity in rats: A toxicogenomic investigation. Exp. Toxicol. Pathol. 2010, 62, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Chen, L.; Wang, C.; Xu, J.; Zhu, Y.; Wu, Y.; Wang, S. An enhanced immunochromatography assay based on colloidal gold-decorated polydopamine for rapid and sensitive determination of gentamicin in animal-derived food. Food Chem. 2022, 387, 132916. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Vigliani, G.A.; Fowler, V.G.; Abrutyn, E.; Corey, G.R.; Levine, D.P.; Rupp, M.E.; Chambers, H.F.; Karchmer, A.W.; Baddour, L.M. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin. Infect. Dis. 2009, 48, 713–721. [Google Scholar] [CrossRef]
- Health Canada. List of Maximum Residue Limits (MRLs) for Veterinary Drugs in Foods—Veterinary Drugs; Government of Canada—Health Products and Food Branch: Ottawa, ON, Canada, 2015.
- GB31650-2019; Veterinary Drug Residue Limit. National Standard of the People’s Republic of China: Beijing, China, 2019.
- Li, X.M.; Liu, X.; Zhang, L.; Wang, L.; Wang, Y.; Sun, J.; Zhang, D.; Wang, J. Residue depletion of gentamicin in swine tissues after intramuscular administration. J. Agric. Food Chem. 2009, 57, 7356–7362. [Google Scholar] [CrossRef]
- Zhao, B.X.; Wang, J.; Zhu, M.; Li, Y.; Wang, S. Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol. Sens. Actuators B Chem. 2018, 275, 223–229. [Google Scholar] [CrossRef]
- Ren, W.; Xu, Y.; Huang, Z.; Xu, L.; Zheng, J.; Wang, S. Plasmonic enhancement in lateral flow sensors for improved sensing of E. coli O157:H7. Biosens. Bioelectron. 2019, 126, 324–331. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Jeon, S. Enhanced sensitivity of lateral flow immunoassays by using water-soluble nanofibers and silver-enhancement reactions. Sens. Actuators B Chem. 2018, 273, 1323–1327. [Google Scholar] [CrossRef]
- Mortezaei, M.; Dadmehr, M.; Korouzhdehi, B.; Hakimi, M.; Ramshini, H. Colorimetric and label free detection of gelatinase positive bacteria and gelatinase activity based on aggregation and dissolution of gold nanoparticles. J. Microbiol. Methods 2021, 191, 105–111. [Google Scholar] [CrossRef]
- Shahi, S.C.; Dadmehr, M.; Korouzhdehi, B.; Tavassoli, A. A novel colorimetric biosensor for sensitive detection of aflatoxin mediated by bacterial enzymatic reaction in saffron samples. Nanotechnology 2021, 32, 505502. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, H.; Zhang, S.; Zhao, Y.; Gao, J.; Zheng, Y.; Zhao, P.; Zhang, Z.; Zaworotko, M.J.; Cheng, P.; et al. Antibodies@MOFs: An in vitro protective coating for preparation and storage of biopharmaceuticals. Adv. Mater. 2019, 31, 1805148. [Google Scholar] [CrossRef] [PubMed]
- Zhand, S.; Razmjou, A.; Azadi, S.; Bazaz, S.R.; Shrestha, J.; Jahromi, M.A.F.; Warkiani, M.E. Metal-organic framework-enhanced ELISA platform for ultrasensitive detection of PD-L1. ACS Appl. Bio Mater. 2020, 3, 4148–4158. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, X.; Zhou, Y.; Lin, T.; Leng, Y.; Huang, X.; Xiong, Y. “Three-in-One” multifunctional nanohybrids with colorimetric magnetic catalytic activities to enhance immunochromatographic diagnosis. ACS Nano 2022, 16, 3351–3361. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, S.; Fang, B.; Zhang, G.; Lai, X.; Su, L.; He, W.; Lai, W. Lateral flow immunoassay based on polydopamine-coated metal-organic framework for the visual detection of enrofloxacin in milk. Anal. Bioanal. Chem. 2021, 413, 7315–7323. [Google Scholar] [CrossRef]
- Yin, X.; Dou, L.; Yao, X.; Liu, S.; Zhang, L.; Zhao, M.; Su, L.; Sun, J.; Wang, J.; Zhang, D. Controllable assembly of metal-organic frameworks and gold nanoparticles composites for sensitive immunochromatographic assay. Food Chem. 2022, 367, 130738. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, Z.; Liu, X.; Shen, X.; Xu, Z.; Lei, H.; Zhao, H.; Li, X. A robust and sensitive enhanced immunochromatographic assay based on UiO-66-NH2@Au for simultaneous detection of carbofuran and 3-hydroxy-carbofuran in fruits and vegetables. Sens. Actuators B Chem. 2023, 397, 134633. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Xie, H.; Luo, P.; Li, X. An ultrasensitive lateral flow immunoassay based on metal-organic framework-decorated polydopamine for multiple sulfonylureas adulteration in functional foods. Foods 2023, 12, 539. [Google Scholar] [CrossRef]
- Liu, Z.; He, Z.; Wu, J.; Deng, Y.; Shen, X.; Lei, H.; Li, X. Facile immunochromatographic assay based on metal-organic framework-decorated polydopamine for the determination of hydrochlorothiazide adulteration in functional foods. Food Chem. 2023, 406, 135011. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Zhou, Z.; Shen, X.; Lei, H.; Li, X. Prussian blue immunochromatography with portable smartphone-based detection device for zearalenone in cereals. Food Chem. 2021, 369, 131008. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, Z.; Lin, Z.; Yang, Z.; Lin, M.; Xu, Z.; Lei, H.; Li, X. MXene bimetallic coating synergistic enhanced colorimetric-Raman dual signal-based immunochromatographic assay for advancing detection performance. Anal. Chem. 2024, 96, 19527–19536. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Z.; Wang, Y.; Xie, Y.; Xu, Z.; Lei, H.; Li, X. Post-synthetic modification fluorescence UiO-66-Eu immunochromatography for high-performance detection of sodium pentachlorophenoate. J. Hazard. Mater. 2024, 480, 135824. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wang, Y.; Wang, M.; Yang, Z.; Deng, Y.; Xu, Z.; Lei, H.; Li, X. Dual-mode immunochromatography based on Ti3C2Tx functionalized CdTe quantum dots for chloramphenicol detection in animal-derived foods. Chem. Eng. J. 2024, 499, 156393. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Tan, C.; Han, Z.; He, L. Cysteamine-modified gold nanoparticles as a colorimetric sensor for the rapid detection of gentamicin. J. Food Sci. 2018, 83, 1631–1638. [Google Scholar] [CrossRef]
- Tang, H.L.; Zhao, J.; Li, X. Study on rapid test method for aminoglycoside residues in raw milk. Chin. J. Health Lab. Technol. 2012, 22, 1415–1418. [Google Scholar]
- Li, X.M.; Wang, Z.H.; Xiao, X.L.; Wang, Z.P.; Wen, K.; Wu, X.P.; Jiang, H.Y. Development of a colloidal gold immunochromatographic technique for simultaneous detection of quinolones and gentamicin in milk. Sci. Agric. Sin. 2014, 47, 3883–3889. [Google Scholar]
- Wang, L.; Wang, L.; Yu, Z. Development of gentamicin semi-quantitative colloidal gold test strip. China Feed 2013, 24, 46–48. [Google Scholar]
- Beloglazova, N.V.; Shmelin, P.S.; Eremin, S.A. Sensitive immunochemical approaches for quantitative (FPIA) and qualitative (lateral flow tests) determination of gentamicin in milk. Talanta 2016, 149, 217–224. [Google Scholar] [CrossRef]

| Analyst | icELISA | UiO-66-NH2@Au-ICA | AuNPs-ICA | |||
|---|---|---|---|---|---|---|
| IC50 (μg/kg) | CR% | IC50 (μg/kg) | CR% | IC50 (μg/kg) | CR% | |
| Gentamicin | 3.26 | 100 | 2.44 | 100 | 2.85 | 100 |
| Streptomycin | >10,000 | <0.1 | >10,000 | <0.1 | >10,000 | <0.1 |
| Kanamycin | >10,000 | <0.1 | >10,000 | <0.1 | >10,000 | <0.1 |
| Spectinomycin | >10,000 | <0.1 | >10,000 | <0.1 | >10,000 | <0.1 |
| Amikacin | >10,000 | <0.1 | >10,000 | <0.1 | >10,000 | <0.1 |
| Neomycin | >10,000 | <0.1 | >10,000 | <0.1 | >10,000 | <0.1 |
| Sample | UiO-66-NH2@Au-ICA | AuNPs-ICA | ||||||
|---|---|---|---|---|---|---|---|---|
| Spiked Level (μg/kg) | Measured Level (μg/kg) | Recovery (%) | CV (%) | Spiked Level (μg/kg) | Measured Level (μg/kg) | Recovery (%) | CV (%) | |
| milk | 0.20 | 0.22 ± 0.03 | 112.0 | 11.70 | 1.50 | 1.88 ± 0.02 | 81.70 | 5.60 |
| 0.60 | 0.56 ± 0.02 | 93.80 | 6.80 | 7.50 | 9.01 ± 0.05 | 85.20 | 14.10 | |
| 1.50 | 1.34 ± 0.02 | 89.60 | 9.30 | 15.0 | 13.39 ± 0.05 | 113.50 | 14.80 | |
| pork | 0.20 | 0.21 ± 0.01 | 107.30 | 5.10 | 0.20 | 0.23 ± 0.03 | 87.40 | 2.80 |
| 0.60 | 0.66 ± 0.04 | 110.70 | 5.80 | 0.60 | 0.73 ± 0.04 | 81.40 | 2.00 | |
| 1.50 | 1.43 ± 0.05 | 95.80 | 4.50 | 1.50 | 1.76 ± 0.12 | 85.20 | 8.80 | |
| liver | 0.20 | 0.22 ± 0.07 | 109.70 | 1.30 | 0.30 | 0.37 ± 0.01 | 82.10 | 1.80 |
| 0.60 | 0.56 ± 0.04 | 93.60 | 2.50 | 1.50 | 1.69 ± 0.05 | 88.80 | 4.70 | |
| 1.50 | 1.63 ± 0.05 | 108.70 | 8.20 | 3.0 | 2.55 ± 0.09 | 118.30 | 12.90 | |
| kidney | 0.20 | 0.16 ± 0.11 | 80.10 | 5.50 | 0.30 | 0.32 ± 0.02 | 97.80 | 3.50 |
| 0.60 | 0.63 ± 0.03 | 106.10 | 4.40 | 1.50 | 1.58 ± 0.06 | 96.80 | 2.30 | |
| 1.50 | 1.58 ± 0.02 | 105.60 | 4.00 | 3.00 | 3.67 ± 0.12 | 82.40 | 6.40 | |
| Sample | Commercial ELISA Kit | UiO-66-NH2@Au-ICA | AuNPs-ICA | |||
|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| (μg/kg) | (μg/kg) | (μg/kg) | ||||
| Milk (n= 10) | ||||||
| Milk 1 | ND | - | ND | - | ND | - |
| Milk 2 | ND | - | ND | - | ND | - |
| Milk 3 | ND | - | ND | - | ND | - |
| Milk 4 | 2.34 ± 0.32 | 13.68% | 2.13 ± 0.25 | 11.74% | 2.12 ± 0.13 | 6.13% |
| Milk 5 | 2.59 ± 0.39 | 15.06% | 2.71 ± 0.32 | 11.81% | 2.71 ± 0.29 | 10.70% |
| Milk 6 | 5.53 ± 0.66 | 11.93% | 5.77 ± 0.28 | 4.85% | 5.27 ± 0.69 | 13.09% |
| Milk 7 | 5.76 ± 0.55 | 9.55% | 4.97 ± 0.31 | 6.24% | 4.99 ± 0.73 | 14.63% |
| Milk 8 | 10.91 ± 0.71 | 6.51% | 9.87 ± 0.34 | 3.44% | 9.57 ± 0.57 | 5.96% |
| Milk 9 | 99.6 ± 3.71 | 3.72% | 98.86 ± 3.34 | 3.38% | 100.45 ± 3.88 | 3.86% |
| Milk 10 | 95.98 ± 5.13 | 5.34% | 103.99 ± 4.12 | 3.96% | 99.4 ± 2.57 | 2.59% |
| Pork (n= 10) | ||||||
| Pork 1 | ND | - | ND | - | ND | - |
| Pork 2 | ND | - | ND | - | ND | - |
| Pork 3 | ND | - | ND | - | ND | - |
| Pork 4 | 2.42 ± 0.08 | 3.31% | 2.56 ± 0.35 | 13.67% | 2.66 ± 0.35 | 13.16% |
| Pork 5 | 2.76 ± 0.39 | 14.13% | 2.44 ± 0.27 | 11.07% | 2.68 ± 0.19 | 7.09% |
| Pork 6 | 5.82 ± 0.99 | 17.01% | 6.16 ± 0.87 | 14.12% | 6.12 ± 0.86 | 14.05% |
| Pork 7 | 5.45 ± 0.33 | 6.06% | 5.78 ± 0.45 | 7.79% | 4.98 ± 0.44 | 8.84% |
| Pork 8 | 10.24 ± 0.51 | 4.98% | 10.12 ± 0.98 | 9.68% | 10.22 ± 1.01 | 9.88% |
| Pork 9 | 50.8 ± 4.89 | 9.63% | 48.67 ± 4.89 | 10.05% | 49.77 ± 5.71 | 11.47% |
| Pork 10 | 101.22 ± 6.65 | 6.57% | 100.55 ± 7.18 | 7.14% | 104.24 ± 7.73 | 7.42% |
| Liver (n= 10) | ||||||
| Liver 1 | ND | - | ND | - | ND | - |
| Liver 2 | ND | - | ND | - | ND | - |
| Liver 3 | 5.32 ± 0.54 | 10.15% | 4.87 ± 0.65 | 13.35% | 6.01 ± 0.57 | 9.48% |
| Liver 4 | 5.19 ± 0.58 | 11.18% | 4.91 ± 0.24 | 4.89% | 4.94 ± 0.38 | 7.69% |
| Liver 5 | 10.16 ± 1.24 | 12.20% | 9.22 ± 0.45 | 4.88% | 12.05 ± 0.27 | 2.24% |
| Liver 6 | 10.02 ± 1.05 | 10.48% | 9.9 ± 0.95 | 9.60% | 9.99 ± 1.17 | 11.71% |
| Liver 7 | 99.07 ± 4.19 | 4.23% | 108.77 ± 10.81 | 9.94% | 105.43 ± 8.31 | 7.88% |
| Liver 8 | 105.19 ± 7.80 | 7.42% | 96.24 ± 4.73 | 4.91% | 110.96 ± 7.92 | 7.14% |
| Liver 9 | 2543.82 ± 208.23 | 8.19% | 2671.33 ± 180.56 | 6.76% | 2638.77 ± 199.48 | 7.56% |
| Liver 10 | 5051.11 ± 380.11 | 7.53% | 5489.12 ± 550.81 | 10.03% | 4985.9 ± 401.39 | 8.05% |
| Kidney (n= 10) | ||||||
| Kidney 1 | ND | - | ND | - | ND | - |
| Kidney 2 | ND | - | ND | - | ND | - |
| Kidney 3 | 5.2 ± 0.12 | 2.31% | 5.51 ± 0.37 | 6.72% | 5.12 ± 0.48 | 9.38% |
| Kidney 4 | 5.24 ± 0.32 | 6.11% | 4.92 ± 0.61 | 12.40% | 4.69 ± 0.67 | 14.29% |
| Kidney 5 | 9.11 ± 0.78 | 8.56% | 9.89 ± 1.23 | 12.44% | 10.05 ± 1.35 | 13.43% |
| Kidney 6 | 9.13 ± 0.73 | 8.00% | 11.7 ± 1.12 | 9.57% | 9.35 ± 1.31 | 14.01% |
| Kidney 7 | 101.95 ± 7.46 | 7.32% | 103.45 ± 12.01 | 11.61% | 100.26 ± 8.71 | 8.69% |
| Kidney 8 | 99.21 ± 6.79 | 6.84% | 101.11 ± 9.33 | 9.23% | 105.37 ± 7.38 | 7.00% |
| Kidney 9 | 1030.12 ± 109.90 | 10.67% | 1003.45 ± 120.92 | 12.05% | 980.76 ± 104.88 | 10.69% |
| Kidney 10 | 2070.65 ± 170.67 | 8.24% | 2019.12 ± 183.45 | 9.09% | 1994.35 ± 150.46 | 7.54% |
| Method | Sample | Pretreatment | Assay Time | Cut-Off Value (μg/kg) | LOD | Robustness | Reference |
|---|---|---|---|---|---|---|---|
| (min) | (μg/kg) | ||||||
| AuNPs-ICA | Milk | Centrifuge after organic extraction | 15 | 139.0 | - | - | [26] |
| AuNPs-ICA | Milk | Dilution | 10 | 100.0 | - | - | [27] |
| AuNPs-ICA | Milk | Direct detection | - | 20.0 | - | - | [28] |
| AuNPs-ICA | Milk | - | - | 20.0 | - | - | [29] |
| QDs-ICA | Milk | Dilution | - | 10.0 | 5.0 | - | [30] |
| CG@PDA | Milk | Direct detection | 8 | 5.0 | 1.51 | - | [4] |
| pork | centrifugation (pork, liver, kidney) | 5.0 | 1.49 | - | |||
| liver | 5.0 | 1.61 | - | ||||
| kidney | 5.0 | 1.75 | - | ||||
| AuNPs-ICA | Milk | Direct detection centrifugation | 8 | 10.0 | 3.90 | pH range: 6 to 8 NaCl concentration (0–0.3 M) Methanol content: 0–20% (v/v) | Present study |
| pork | 5.0 | 4.54 | |||||
| liver | 5.0 | 2.88 | |||||
| kidney | 5.0 | 3.51 | |||||
| UIO-66-NH2@Au-ICA | Milk | Direct detection (milk); centrifugation (pork, liver, kidney) | 8 | 2.50 | 0.15 | pH range: 4 to 10 NaCl concentration: (0–1.2 M) Methanol content: 0–40% (v/v) | Present study |
| pork | 2.50 | 0.14 | |||||
| liver | 2.50 | 0.14 | |||||
| kidney | 2.50 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Yang, Z.; Liu, X.; Shen, X.; Lei, H.; Li, X. UiO-66-NH2-Deposited Gold Nanoparticles Enable Enhanced Interference-Resistant Immunochromatographic Assay for Rapid Detection of Gentamicin in Animal-Derived Foods. Foods 2025, 14, 3264. https://doi.org/10.3390/foods14183264
Pang Y, Yang Z, Liu X, Shen X, Lei H, Li X. UiO-66-NH2-Deposited Gold Nanoparticles Enable Enhanced Interference-Resistant Immunochromatographic Assay for Rapid Detection of Gentamicin in Animal-Derived Foods. Foods. 2025; 14(18):3264. https://doi.org/10.3390/foods14183264
Chicago/Turabian StylePang, Yimeng, Zehao Yang, Xiaohua Liu, Xing Shen, Hongtao Lei, and Xiangmei Li. 2025. "UiO-66-NH2-Deposited Gold Nanoparticles Enable Enhanced Interference-Resistant Immunochromatographic Assay for Rapid Detection of Gentamicin in Animal-Derived Foods" Foods 14, no. 18: 3264. https://doi.org/10.3390/foods14183264
APA StylePang, Y., Yang, Z., Liu, X., Shen, X., Lei, H., & Li, X. (2025). UiO-66-NH2-Deposited Gold Nanoparticles Enable Enhanced Interference-Resistant Immunochromatographic Assay for Rapid Detection of Gentamicin in Animal-Derived Foods. Foods, 14(18), 3264. https://doi.org/10.3390/foods14183264







