Synergistic Protective Effects of Oleaster Fruit and Sophora japonica L. Fruit Extracts Against IL-1β-Induced Inflammation in Human Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleaster Fruit Extract (OE), S. japonica L. Fruit Extract (SJE), and Their Mixtures
2.3. Cell Culture and Protective Effect
2.4. Measurement of TNF-α, IL-6, and NO Levels
2.5. Measurement of MMP-9, MMP-13, and Hyaluronan
2.6. Evaluation of Synergistic Effect
2.7. Statistical Analysis
3. Results and Discussions
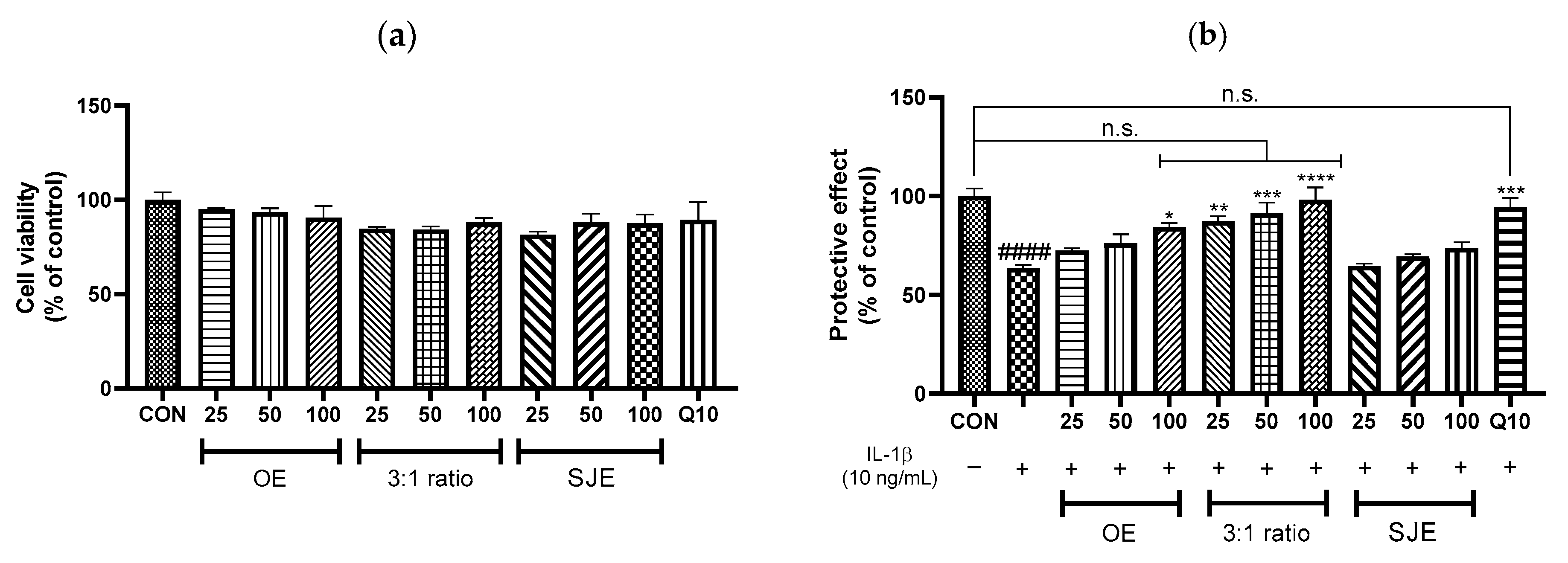
3.1. Protective Effects of OE, SJE, and the OE:SJE Combinations Against IL-1β-Induced Inflammation in Human Chondrocytes
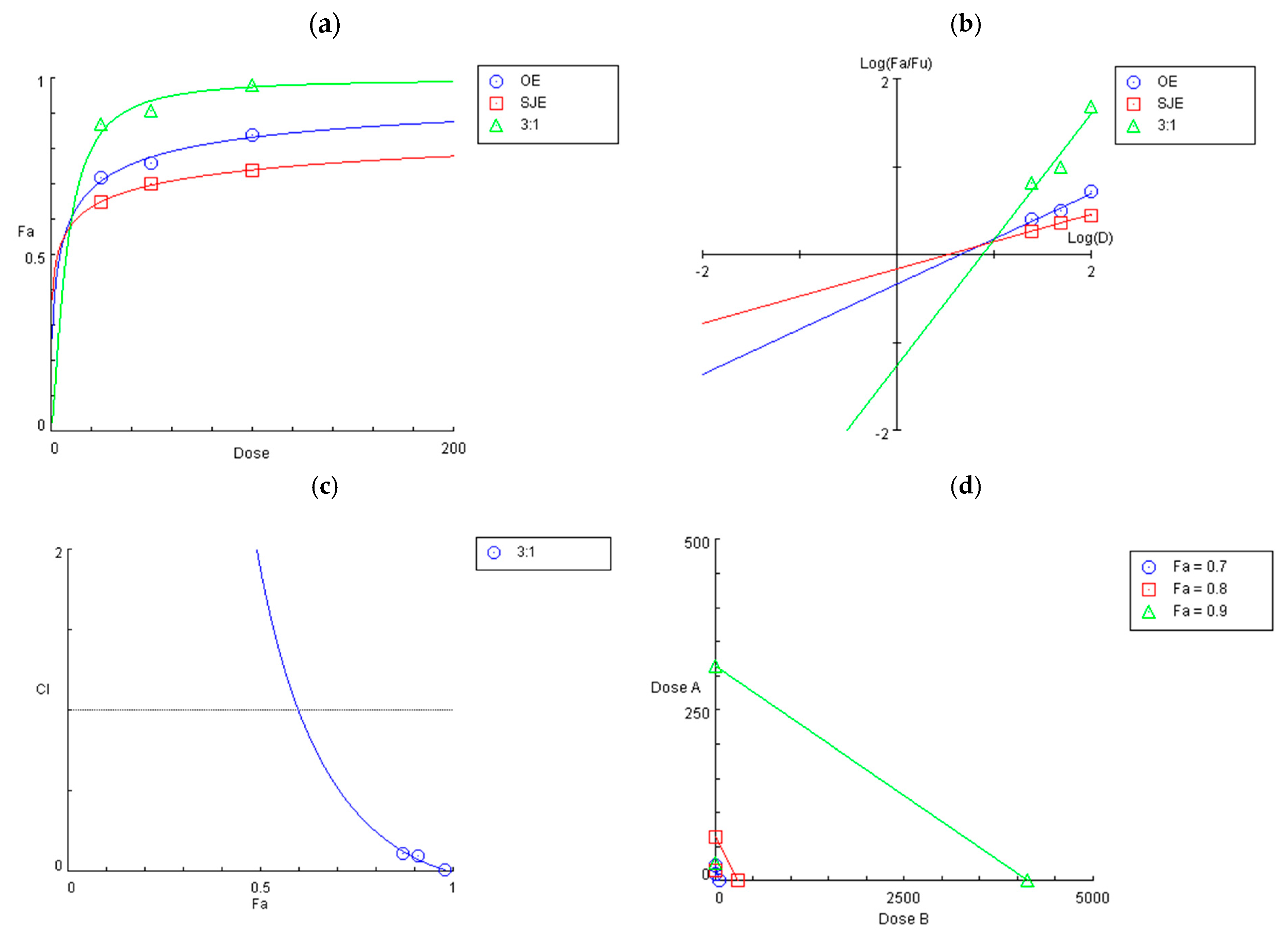
3.2. Synergistic Effect of the 3:1 OE:SJE Combination Against IL-1β-Induced Inflammation in Human Chondrocytes
3.3. Inhibitory Effects of the 3:1 OE:SJE Combination on Inflammatory and Cartilage Matrix Degradation Markers in IL-1β-Induced Human Chondrocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bijlsma, J.W.J.; Berenbaum, F.; Lafeber, F.P.J.G. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2, 14002. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Feng, Y.; Li, C.; Yang, K.; Sun, T.; Xu, L.; Chen, Y.; Yan, C.-H.; Lu, W.W.; Chiu, K.-Y. Magnoflorine with hyaluronic acid gel promotes subchondral bone regeneration and attenuates cartilage degeneration in early osteoarthritis. Bone 2018, 116, 266–278. [Google Scholar] [CrossRef]
- Faramarz, S.; Dehghan, G.; Jahanban-Esfahlan, A. Antioxidants in different parts of oleaster as a function of genotype. Bioimpacts 2015, 5, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M.; Shahlari, N.; Hamidpour, R. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J. Tradit. Complement. Med. 2017, 7, 24–29. [Google Scholar] [CrossRef]
- Heydari Nasrabadi, M.; Parsivand, M.; Mohammadi, N.; Asghari Moghaddam, N. Comparison of Elaeagnus angustifolia L. extract and quercetin on mouse model of knee osteoarthritis. J. Ayurveda Integr. Med. 2022, 13, 100529. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Karimifar, M.; Soltani, R.; Hajhashemi, V.; Sarrafchi, S. Evaluation of the effect of Elaeagnus angustifolia alone and combined with Boswellia thurifera compared with ibuprofen in patients with knee osteoarthritis: A randomized double-blind controlled clinical trial. Clin. Rheumatol. 2017, 36, 1849–1853. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Bayat, N.; Hosseini, S.M.; Sahebkar, A. Efficacy of Elaeagnus angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016, 15, 203–210. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Li, J.Y.; Zhou, S.Z.; Zhao, P.; Fan, M.T. Four flavonoid glycosides from the pulps of Elaeagnus angustifolia and their antioxidant activities. Adv. Mater. Res. 2013, 756–759, 16–20. [Google Scholar] [CrossRef]
- Natanzi, M.M.; Pasalar, P.; Kamalinejad, M.; Dehpour, A.R.; Tavangar, S.M.; Sharifi, R.; Ghanadian, N.; Rahimi-Balaei, M.; Gerayesh-Nejad, S. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med. Iran. 2012, 50, 589–596. [Google Scholar]
- Fu, Y.; Ruan, J.; Zhang, P.; Zhang, W.; Yang, D.; Zhang, Y.; Dang, Z.; Zhang, Y.; Wang, T. The bioactive constituents from the fruits of Elaeagnus angustifolia L. Phytochemistry 2025, 230, 114334. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kang, H.-J. Fructus sophorae attenuates secretion of proinflammatory mediators and cytokines through the modulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW 264.7 macrophages. Gen. Physiol. Biophys. 2016, 35, 323–331. [Google Scholar] [CrossRef]
- Joo, S.-S.; Won, T.-J.; Kang, H.-C.; Lee, D.-I. Isoflavones extracted from sophorae fructus upregulate IGF-I and TGF-beta and inhibit osteoclastogenesis in rat bone marrow cells. Arch. Pharm. Res. 2004, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chakuleska, L.; Shkondrov, A.; Popov, G.; Zlateva-Panayotova, N.; Petrova, R.; Atanasova, M.; Krasteva, I.; Doytchinova, I.; Simeonova, R. Beneficial effects of the fructus sophorae extract on experimentally induced osteoporosis in New Zealand white rabbits. Acta Pharm. 2022, 72, 289–302. [Google Scholar] [CrossRef]
- Han, H.-M.; Hong, S.-H.; Park, H.-S.; Jung, J.-C.; Kim, J.-S.; Lee, Y.-T.; Lee, E.-W.; Choi, Y.-H.; Kim, B.-W.; Kim, C.-M.; et al. Protective effects of fructus sophorae extract on collagen-induced arthritis in BALB/c mice. Exp. Ther. Med. 2017, 13, 146–154. [Google Scholar] [CrossRef]
- Shim, J.G.; Yeom, S.H.; Kim, H.J.; Choi, Y.W.; Lee, D.I.; Song, K.Y.; Kwon, S.H.; Lee, M.W. Bone loss preventing effect of sophorae fructus on ovariectomized rats. Arch. Pharm. Res. 2005, 28, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, L.; Wang, L.; Li, J. Flos sophorae immaturus: Phytochemistry, bioactivities, and its potential applications. Food Rev. Int. 2023, 39, 3185–3203. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Maksimenko, E.V.; Borisenko, S.N.; Lekar, A.V.; Borisenko, N.I.; Minkin, V.I. Extraction of rutin and quercetin antioxidants from the buds of Sophora japonica L. by subcritical water. Russ. J. Phys. Chem. B 2017, 11, 1202–1206. [Google Scholar] [CrossRef]
- Kim, M.; Heo, H.; Hong, S.; Lee, J.; Lee, H. Synergistic effect of madecassoside and rosmarinic acid against ultraviolet B-induced photoaging in human skin fibroblasts. J. Med. Food 2023, 26, 919–926. [Google Scholar] [CrossRef]
- Baik, S.; Heo, H.; Hong, S.; Jeong, H.S.; Lee, J.; Lee, H. Combination of nicotinamide and Agastache rugosa extract: A potent strategy for protecting Hs68 cells from UVB-induced photoaging. Prev. Nutr. Food Sci. 2024, 29, 162–169. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic effects of Chinese herbal medicine: A comprehensive review of methodology and current research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Liang, X.; Chen, X.; Liang, Q.; Zhang, H.; Hu, P.; Wang, Y.; Luo, G. Metabonomic study of Chinese medicine shuanglong formula as an effective treatment for myocardial infarction in rats. J. Proteome Res. 2011, 10, 790–799. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Wang, P.; Sun, H.; Wu, G.; Sun, W.; Lv, H.; Jiao, G.; Xu, H.; Yuan, Y.; et al. Metabolomics coupled with proteomics advancing drug discovery toward more agile development of targeted combination therapies. Mol. Cell Proteomics 2013, 12, 1226–1238. [Google Scholar] [CrossRef]
- Chou, T.-C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 2018, 7, 49–50. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–111. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- He, Y.; Moqbel, S.A.A.; Xu, L.; Ran, J.; Ma, C.; Xu, K.; Bao, J.; Jiang, L.; Chen, W.; Xiong, Y.; et al. Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NF-κB and Wnt/β-catenin signaling pathways. Mol. Med. Rep. 2019, 20, 312–322. [Google Scholar] [CrossRef]
- Shi, P.; Liao, J.; Duan, T.; Wu, Q.; Huang, X.; Pei, X.; Wang, C. Chemical composition and pharmacological properties of flos sophorae immaturus, flos sophorae and fructus sophorae: A review. J. Future Foods 2023, 3, 330–339. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, X.; Wang, C.; Li, Q.; Xu, M.; Guan, X.; Lan, Z.; Ni, Y.; Zhang, Y. Widely targeted metabolomics analysis of enriched secondary metabolites and determination of their corresponding antioxidant activities in Elaeagnus angustifolia var. orientalis (L.) Kuntze fruit juice enhanced by Bifidobacterium animalis subsp. lactis HN-3 fermentation. Food Chem. 2022, 374, 131568. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Xie, W.-P.; Bi, Y.-F.; Wang, B.-A.; Song, H.-B.; Wang, S.-L.; Bi, R.-X. Quercetin suppresses apoptosis of chondrocytes induced by IL-1β via inactivation of p38 MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Dilek Tepe, H.; Doyuk, F. Determination of phytochemical content by chromatographic methods and antioxidant capacity in methanolic extract of jujube (Zizyphus jujuba Mill.) and oleaster (Elaeagnus angustifolia L.). Int. J. Fruit Sci. 2020, 20, S1876–S1890. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, M.-K.; Shim, J.-G.; Yeom, S.-H.; Kwon, S.-H.; Lee, M.-W. Anti-oxidative phenolic compounds from sophorae fructus. Nat. Prod. Sci. 2004, 10, 330–334. [Google Scholar]
- Wang, H.; Yan, Y.; Pathak, J.L.; Hong, W.; Zeng, J.; Qian, D.; Hao, B.; Li, H.; Gu, J.; Jaspers, R.T.; et al. Quercetin prevents osteoarthritis progression possibly via regulation of local and systemic inflammatory cascades. J. Cell. Mol. Med. 2023, 27, 515–528. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Xu, W.; Zou, L.; Ye, J.; Liu, Y.; Xiao, Y.; Luo, J. Rutin inhibited the advanced glycation end products-stimulated inflammatory response and extra-cellular matrix degeneration via targeting TRAF-6 and BCL-2 proteins in mouse model of osteoarthritis. Aging 2021, 13, 22134–22147. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Anti-inflammatory effects of dietary polyphenols through inhibitory activity against metalloproteinases. Molecules 2023, 28, 5426. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Starowicz, M.; Wronkowska, M.; Zieliński, H. Multifaceted biological activity of rutin, quercetin, and quercetin’s glucosides. Molecules 2025, 30, 2555. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Guo, Q.; Wang, P.; Li, P.; Du, Q.; Xu, H.; Yu, Q.; Zhao, X.; Zhang, W.; et al. Sophoricoside reduces inflammation in type II collagen-induced arthritis by downregulating NLRP3 signaling. Biochem. Biophys. Rep. 2024, 40, 101867. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lim, J.; Oh, M.; Lee, J. Synergistic Protective Effects of Oleaster Fruit and Sophora japonica L. Fruit Extracts Against IL-1β-Induced Inflammation in Human Chondrocytes. Foods 2025, 14, 3099. https://doi.org/10.3390/foods14173099
Lee H, Lim J, Oh M, Lee J. Synergistic Protective Effects of Oleaster Fruit and Sophora japonica L. Fruit Extracts Against IL-1β-Induced Inflammation in Human Chondrocytes. Foods. 2025; 14(17):3099. https://doi.org/10.3390/foods14173099
Chicago/Turabian StyleLee, Hana, Jinyeong Lim, Myeonghwan Oh, and Junsoo Lee. 2025. "Synergistic Protective Effects of Oleaster Fruit and Sophora japonica L. Fruit Extracts Against IL-1β-Induced Inflammation in Human Chondrocytes" Foods 14, no. 17: 3099. https://doi.org/10.3390/foods14173099
APA StyleLee, H., Lim, J., Oh, M., & Lee, J. (2025). Synergistic Protective Effects of Oleaster Fruit and Sophora japonica L. Fruit Extracts Against IL-1β-Induced Inflammation in Human Chondrocytes. Foods, 14(17), 3099. https://doi.org/10.3390/foods14173099






