Green Co-Extractant-Assisted Supercritical CO2 Extraction of Xanthones from Mangosteen Pericarp Using Tricaprylin and Tricaprin Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
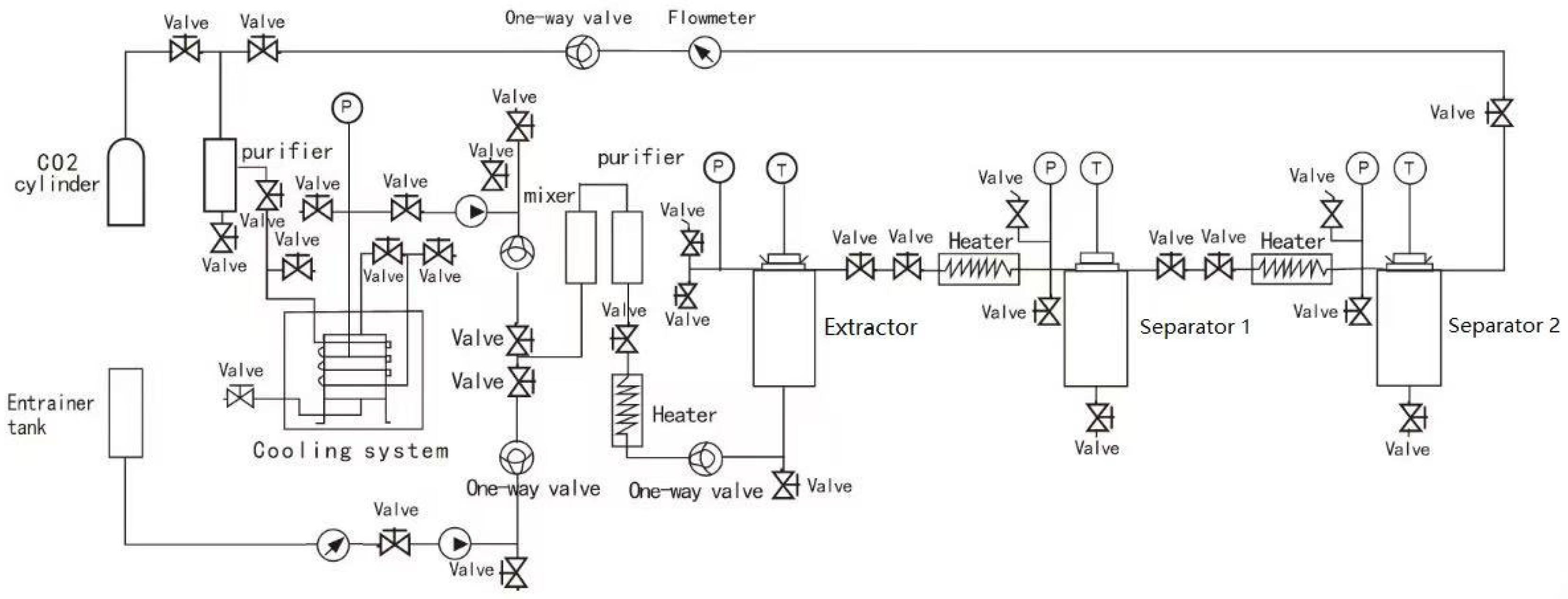
2.2. Supercritical Carbon Dioxide Extraction
2.3. Determination of Xanthones in Mangosteen Pericarp Extract
2.4. Viscosity Analysis
2.5. Extraction Kinetics Modelling and Generalization
2.5.1. Correlation with Pseudo-First-Order Kinetic Model
2.5.2. Development of Generalized Predictive Models
2.6. Statistical Analysis
3. Results and Discussion
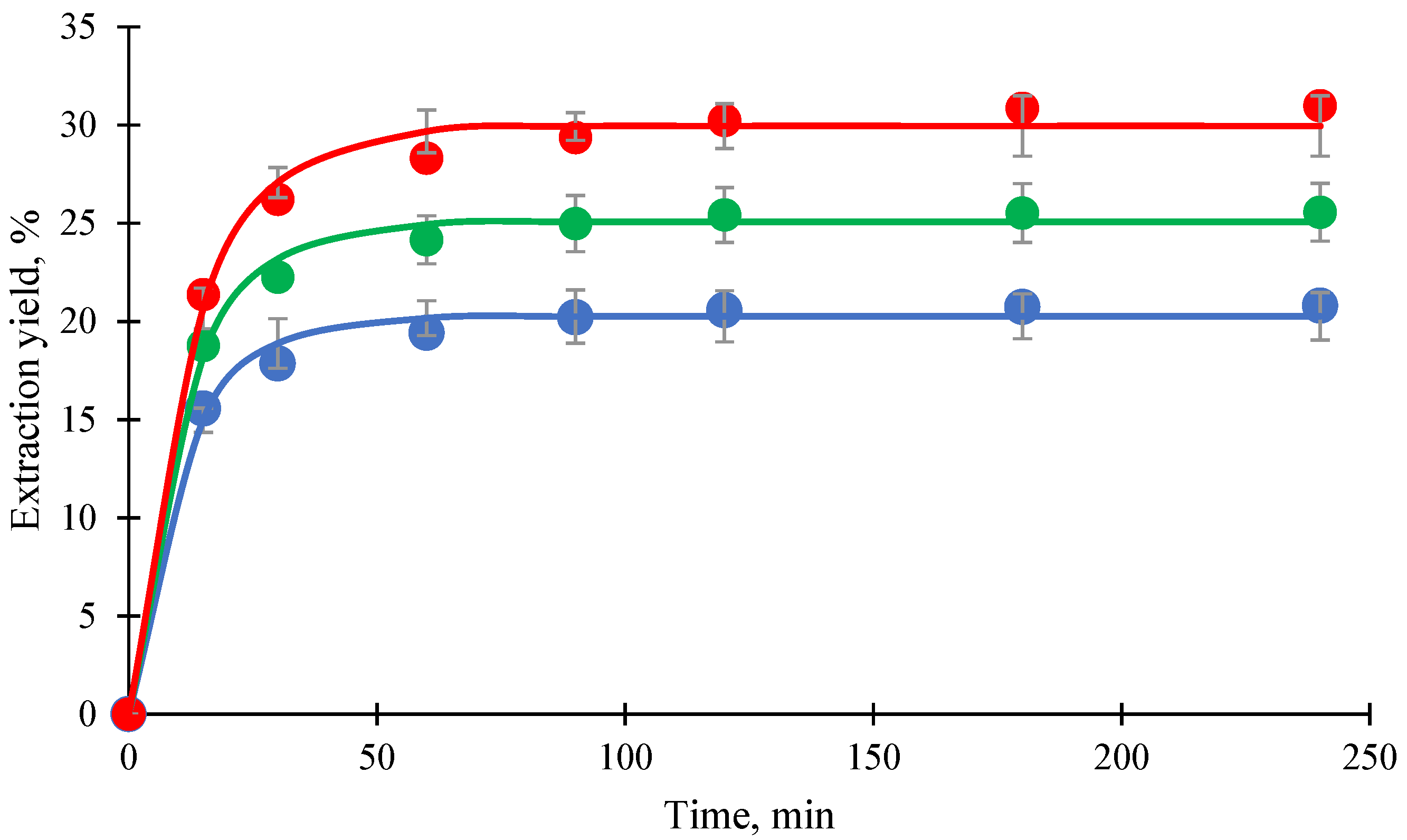
3.1. Effect of C8/C10 Co-Extractant Ratio to Mangosteen Pericarp on Xanthone Extraction
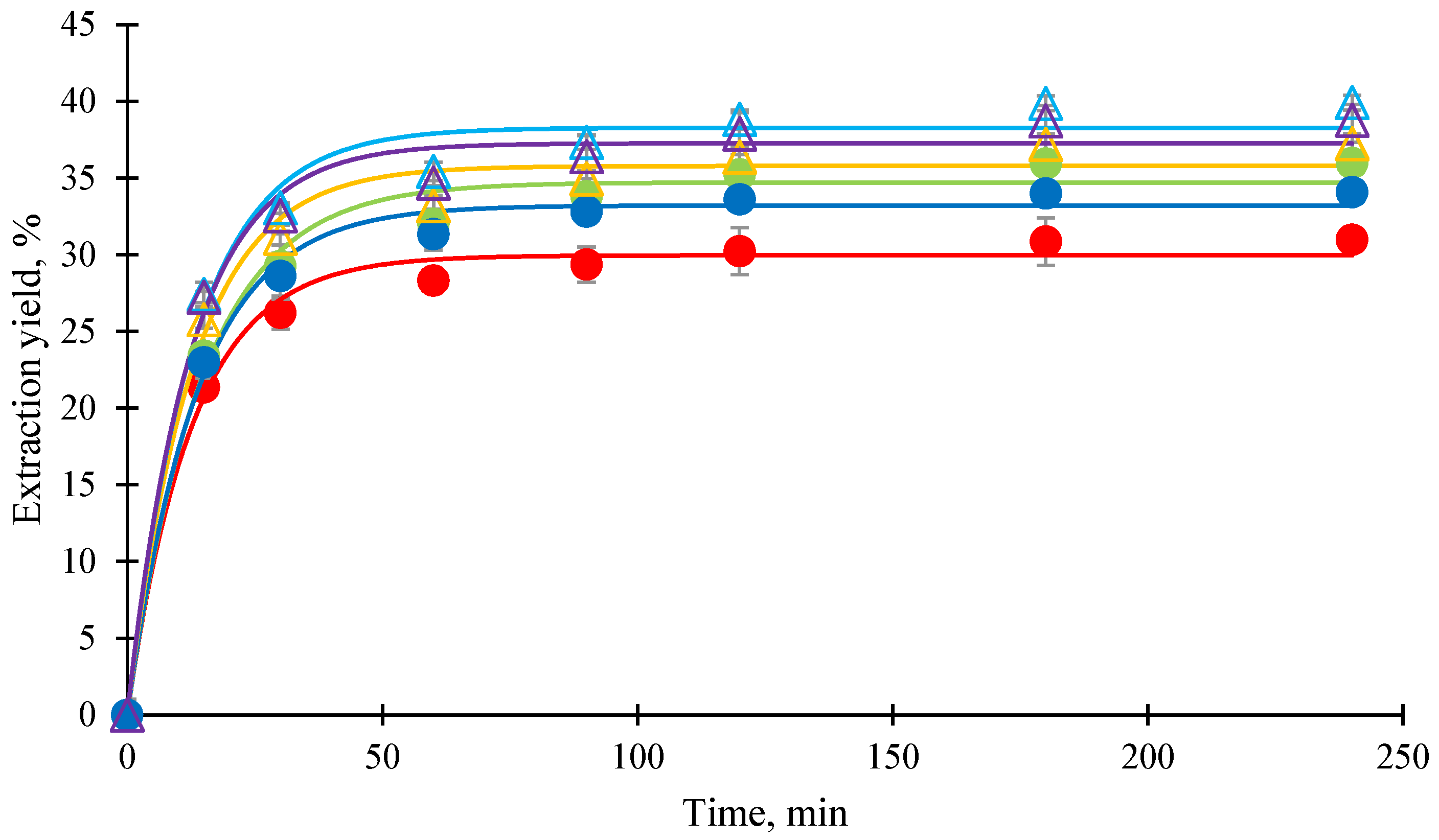
3.2. Effect of Type of Co-Extractant on Xanthone Extraction
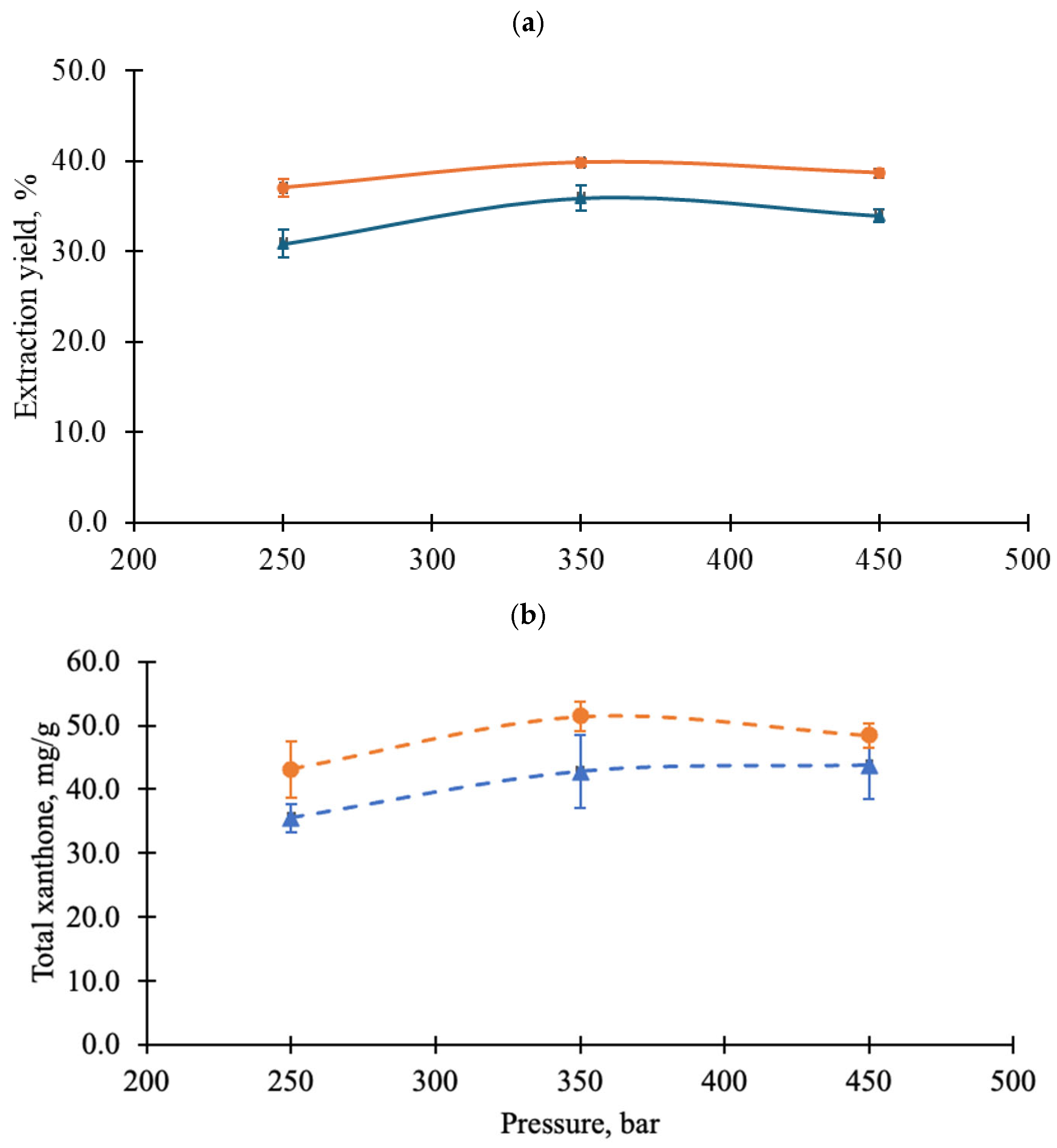
3.3. Effect of Different Supercritical Carbon Dioxide Conditions on Xanthone Extraction from Mangosteen Pericarp
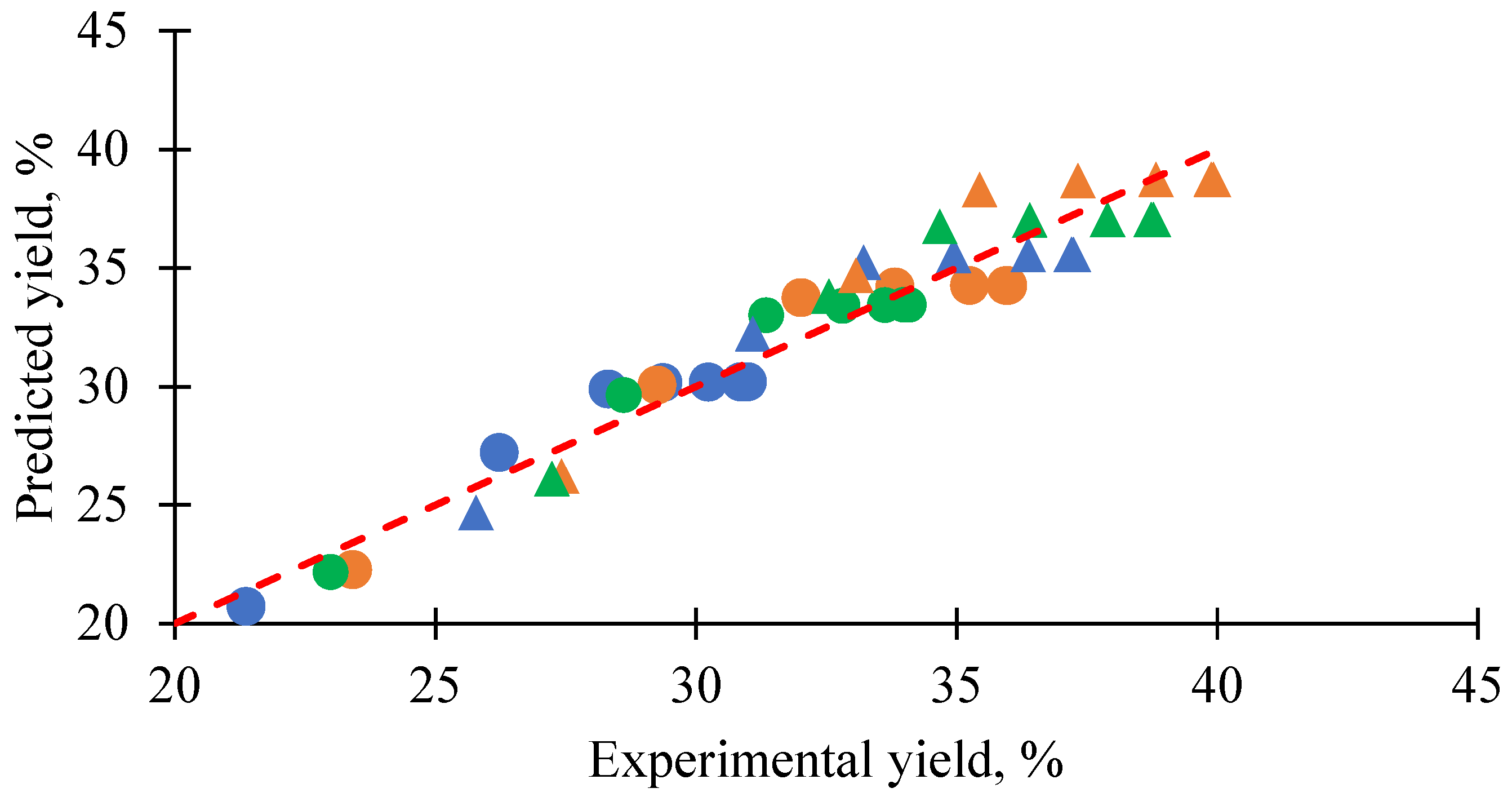
3.4. Generalized Predictive Model of Extraction Yield and Kinetics
3.5. Industry Relevance and Potential Expansion Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Soontornwat, A.; Pongsuttiyakorn, T.; Rakmae, S.; Sritham, E.; Sirisomboon, P.; Pun, U.K.; Krusong, W.; Pornchaloempong, P. Mangosteen pericarp processing technology to create economic value and reduce biowaste. Foods 2024, 13, 2286. [Google Scholar] [CrossRef]
- Cahya, B.; Nirmala, D.; Triantoro, B. Testing the antibacterial effectiveness of mangosteen peel extract (Garcinia mangostana L.) against Aeromonas hydrophila Bacterial infection in dumbo catfish (Clarias gariepinus). IOP Conf. Ser. Earth Environ. Sci. 2023, 1273, 012024. [Google Scholar] [CrossRef]
- Alfawaz, H.A.; Labban, R.S.M.; Bhat, R.S.; El-Ansary, A. Biological properties in relation to the health-promoting effects of independent and combined garcinia mangostana pericarp and curcuma in Lean Wistar Albino Rats. Appl. Sci. 2023, 13, 8567. [Google Scholar] [CrossRef]
- Brito, L.D.C.; Marques, A.M.; Da Cunha Camillo, F.; Figueiredo, M.R. Garcinia SPP: Products and by-products with potential pharmacological application in cancer. Food Biosci. 2022, 50, 102110. [Google Scholar] [CrossRef]
- Hendiani, I.; Famtahani, M.; Bawono, C.A.; Prasetyo, B.C. The Effectiveness of mangosteen peel (Garcinia mangostana L.) extract mouthwash on reducing gingival inflammation. J. Int. Dent. Med. Res. 2023, 16, 1206–1212. [Google Scholar]
- Janardhanan, S.; Mahendra, J.; Mahendra, L.; Devarajan, N. Cytotoxic effects of mangosteen pericarp extracts on oral cancer and cervical cancer cells, Asian Pac. J. Cancer Prev. 2020, 21, 2577–2583. [Google Scholar] [CrossRef]
- Kitikiew, S.; Chieng, Z.X.; Li, C.Y.; Kuo, H.W.; Cheng, W. Injection administration of mangosteen, Garcinia mangostana, husk extract enhances physiological and immune-neuroendocrinological regulation of Macrobrachium rosenbergii. Fish Shellfish Immunol. 2023, 141, 109019. [Google Scholar] [CrossRef]
- Octaviani, N.P.; Mooduto, L.; Sudirman, A. Antibacterial potency of mangosteen pericarp extracts (Garcinia mangostana L.) against Fusobacterium nucleatum. Conserv. Dent. J. 2020, 10, 44–47. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, B.; Liang, X.; Luo, Z.; Han, J.; Qu, D. Evaluation of the antioxidant activity and prebiotic properties of mangosteen peel proanthocyanidin extracts through simulated in vitro digestion and colonic fermentation. LWT 2024, 212, 116992. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Terro, T.M.; Ahari, S.H.; Abu-Yousef, I.A. α-Mangostin: A xanthone derivative in mangosteen with potent anti-cancer properties. Biomolecules 2024, 14, 1382. [Google Scholar] [CrossRef]
- Puripattanavong, J.; Khajorndetkun, W.; Chansathirapanich, W. Improved isolation of α-mangostin from the fruit hull of Garcinia mangostana and its antioxidant and antifungal activity. Planta Med. 2006, 72, P_328. [Google Scholar] [CrossRef]
- Wulandari, D.D.; Santoso, A.P.R.; Suciati, S.S.; Wulansari, D.D.; Andini, A.; Khanifah, F. Characterization and evaluation of the hepatoprotective activity of α-mangostin isolate in diabetic rats. Bali Med. J. 2023, 12, 3392–3398. [Google Scholar] [CrossRef]
- Amran, A.J.; Marizal, D.; Annafi, A.; Farahamida, D.; Rizqiawan, A. Effect of α-mangostin from Garcinia mangostana on Osteoblast cells in the wound healing process: A review. Res. J. Pharm. Technol. 2024, 17, 1885–1890. [Google Scholar] [CrossRef]
- Rattanachan, S.; Suksamrarn, A.; Pongpiriyadacha, Y. α-Mangostin Is a Xanthone Derivative from Mangosteen with Potent Immunomodulatory and Anti-Inflammatory Properties. Biomolecules 2025, 15, 681–695. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Jantrawut, P.; Pongpiriyadacha, Y. Optimization of Ethanol Concentration for Xanthone Extraction from Mangosteen Pericarp: A Comparative Study with Microwave-Assisted Extraction. Foods 2024, 13, 2134–2146. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef]
- Yuvanatemiya, V.; Srean, P.; Klangbud, W.K.; Venkatachalam, K.; Wongsa, J.; Parametthanuwat, T.; Charoenphun, N. A review of the influence of various extraction techniques and the biological effects of the xanthones from mangosteen (Garcinia mangostana L.) pericarps. Molecules 2022, 27, 8775. [Google Scholar] [CrossRef]
- Pattiram, P.D.; Abas, F.; Suleiman, N.; Mohamad Azman, E.; Chong, G.H. Edible oils as a co-extractant for the supercritical carbon dioxide extraction of flavonoids from propolis. PLoS ONE 2022, 17, e0266673. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Ultrasonic-assisted virgin coconut oil based extraction for maximizing polyphenol recovery and bioactivities of mangosteen peels. J. Food Sci. Technol. 2020, 57, 4032–4043. [Google Scholar] [CrossRef]
- Kok, S.L.; Lee, W.J.; Smith, R.L., Jr.; Suleiman, N.; Jom, K.N.; Vangnai, K.; Sharaai, A.H.B.; Chong, G.H. Role of virgin coconut oil (VCO) as co-extractant for obtaining xanthones from mangosteen (Garcinia mangostana) pericarp with supercritical carbon dioxide extraction. J. Supercrit. Fluids 2021, 176, 105305. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, C.C.; Ng, J.S.; Smith, R.L., Jr.; Kok, S.L.; Hee, Y.Y.; Lee, S.Y.; Tan, W.K.; Abidin, N.H.Z.; Lim, S.A.H.; et al. Supercritical carbon dioxide extraction of α-mangostin from mangosteen pericarp with virgin coconut oil as co-extractant and in-vitro bio-accessibility measurement. Process Biochem. 2019, 87, 213–220. [Google Scholar] [CrossRef]
- Liu, H.; Stanslas, J.; Ren, J.; Suleiman, N.B.; Chong, G.H. Exploring green solvent systems and enhanced solubility of xanthone in triglyceride-based tricaprin-tricaprylin mixtures with thermodynamic insights. BMC Chem. 2024, 18, 239. [Google Scholar] [CrossRef]
- Yodhnu, S.; Sirikatitham, A.; Wattanapiromsakul, C. Validation of LC for the determination of α-mangostin in mangosteen peel extract: A tool for quality assessment of Garcinia mangostana L. J. Chromatogr. Sci. 2009, 47, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.B. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef]
- Poole, C.F. Selectivity evaluation of extraction systems. J. Chromatogr. A 2023, 1695, 463939. [Google Scholar] [CrossRef]
- Zarena, A.; Sankar, K.U. Supercritical carbon dioxide extraction of xanthones with antioxidant activity from Garcinia mangostana: Characterization by HPLC/LC-ESI-MS. J. Supercrit. Fluids 2009, 49, 330–337. [Google Scholar] [CrossRef]
- Fasina, O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil. Chem. Soc. 2006, 83, 899–903. [Google Scholar] [CrossRef]
- Zarena, A.S.; Udaya Sankar, K. Supercritical carbon dioxide extraction of xanthones with co-solvents from Garcinia mangostana: Optimization by response surface methodology. J. Supercrit. Fluids 2012, 61, 206–211. [Google Scholar] [CrossRef]
- Ilieva, P.; Kilzer, A.; Weidner, E. Measurement of solubility, viscosity, density and interfacial tension of the systems tristearin and CO2 and rapeseed oil and CO2. J. Supercrit. Fluids 2016, 117, 40–49. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Madhu, B.; Dharmaraj, D.S.; Chandrasekar, V.; Shanmugasundaram, S. Quality assessment and authentication of virgin coconut oil: Recent technologies and prospects. In Preservation and Authentication of Coconut Products; Pandiselvam, R., Ramesh, S., Eds.; Springer: Cham, Switzerland, 2024; pp. 199–213. [Google Scholar]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’Afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Nosaka, N.; Kasai, M. Research on the nutritional characteristics of medium-chain fatty acids. J. Med. Investig. 2007, 54, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, X.; Rashedi, I.F.B.M.; Chong, G. Synergistic effect of a VCO-ethanol solvent mixture and ultrasound-assisted extraction in enhancing Xanthone extraction from mangosteen Pericarp. Food Bioprod. Process. 2025, 150, 350–359. [Google Scholar] [CrossRef]
- Xie, T.; He, H.B.; Gao, S.H. Synthetic progress of Polycyclic Xanthone. Chin. J. Org. Chem. 2020, 40, 551–562. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Tuning of Virgin Coconut Oil and Propylene Glycol Ratios for Maximizing the Polyphenol Recovery and In Vitro Bioactivities of Mangosteen (Garcinia mangostana L.) Pericarp. Process Biochem. 2019, 87, 179–186. [Google Scholar] [CrossRef]
- Phung, L.T.K.; Nam, H.M.; Tuyet, N.T.N. Supercritical fluid extraction of α-mangostin from mangosteen fruit rind (Garcinia mangostana Linn.). ASEAN Eng. J. 2015, 4, 6–15. [Google Scholar] [CrossRef]
- Lopez-Hortas, L.; Rodriguez, P.; Diaz-Reinoso, B.; Gaspar, M.C.; De Sousa, H.C.; Braga, M.E.; Dominguez, H. Supercritical fluid extraction as a suitable technology to recover bioactive compounds from flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- López-Cortés, I.Y.; Iglesias-Silva, G.A.; Ramos-Estrada, M.; Rivera-Rojas, J.L. A correlation for the viscosity of binary mixtures of ionic liquids with organic solvents and water. Fluid Phase Equilibria 2020, 514, 112543. [Google Scholar] [CrossRef]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications--A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and technological applications-a review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Fahrudin, F.I.; Phongthai, S.; Wirjantoro, T.I.; Intipunya, P. Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods 2025, 14, 2189. [Google Scholar] [CrossRef]
- Thompson, J.E.; Paluch, A.S. Revisiting the Clausius/Clapeyron Equation and the Cause of Linearity. Thermo 2023, 3, 412–423. [Google Scholar] [CrossRef]
- Zarena, A.; Manohar, B.; Udaya Sankar, K. Optimization of supercritical carbon dioxide extraction of xanthones from mangosteen pericarp by response surface methodology. Food Bioprocess Technol. 2012, 5, 1181–1188. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Medium-Chain Fatty Acids: Functional Lipids for the Prevention and Treatment of the Metabolic Syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef]
- Pootakham, K.; Pongpaibul, Y.; Tharavichitkul, P.; Ampasavate, C. HPLC Determination of Mangostin and Its Application to Storage Stability Study. CMU J. Nat. Sci. 2009, 8, 43–53. [Google Scholar]

| C8/C10–MP (%, w/w) | Yield (%) | Total Xanthones (mg/g) | Isomangostin (mg/g) | Garcinone C (mg/g) | Garcinone D (mg/g) | 8-Deoxygartanin (mg/g) | γ-Mangostin (mg/g) | Gartanin (mg/g) | α-Mangostin (mg/g) | β-Mangostin (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 20.80 ± 0.99 c | 22.61 ± 3.28 b | 0.03 ± 0.01 c | 0.19 ± 0.03 c | 0.28 ± 0.05 b | 0.84 ± 0.18 bc | 1.67 ± 0.21 b | 1.59 ± 0.34 ab | 17.44 ± 2.36 b | 0.58 ± 0.11 b |
| 30 | 25.56 ± 1.47 b | 30.09 ± 3.23 a | 0.05 ± 0.01 a | 0.59 ± 0.06 a | 0.49 ± 0.05 a | 1.00 ± 0.14 b | 3.26 ± 0.33 a | 1.87 ± 0.27 a | 22.14 ± 2.30 b | 0.69 ± 0.08 b |
| 40 | 30.90 ± ±1.52 a | 35.51 ± 2.21 a | 0.04 ± 0.00 b | 0.35 ± 0.02 b | 0.40 ± 0.02 a | 1.47 ± 0.10 a | 2.83 ± 0.19 a | 2.40 ± 0.15 a | 27.04 ± 1.67 a | 0.98 ± 0.06 a |
| 0 | 2.60 ± 0.04 d | 1.65 ± 0.16 c | n.d. | 0.01 ± 0.00 d | 0.01 ± 0.00 c | 0.15 ± 0.02 c | 0.06 ± 0.01 c | 0.30 ± 0.03 b | 1.03 ± 0.12 c | 0.07 ± 0.02 c |
| Co-Extractant Effectiveness (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C8/C10–MP (%, w/w) | Isomangostin | Garcinone C | Garcinone D | 8- Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| 20 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 30 | 192% | 304% | 174% | 119% | 196% | 118% | 127% | 119% |
| 40 | 146% | 181% | 145% | 176% | 169% | 151% | 155% | 169% |
| Selectivity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C8/C10–MP (%, w/w) | Isomangostin | Garcinone C | Garcinone D | 8- Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| 20 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 30 | 136% | 217% | 124% | 85% | 140% | 84% | 90% | 85% |
| 40 | 74% | 91% | 73% | 89% | 85% | 76% | 77% | 85% |
| C8/C10–MP | |||
|---|---|---|---|
| 20% | 30% | 40% | |
| Ymax (%) | 20.262 | 25.080 | 29.959 |
| k (min−1) | 0.090 | 0.086 | 0.078 |
| SSE | 2.590 | 2.367 | 5.358 |
| Co-Extractant | Yield (%) | Total Xanthones (mg/g) | Isomangostin (mg/g) | Garcinone C (mg/g) | Garcinone D (mg/g) | 8- Deoxygartanin (mg/g) | γ-Mangostin (mg/g) * | Gartanin (mg/g) | α-Mangostin (mg/g) | β-Mangostin (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| C8/10 | 30.90 ± 1.52 a | 35.51 ± 2.21 a | 0.04 ± 0.00 | 0.35 ± 0.02 b | 0.40 ± 0.02 c | 1.47 ± 0.10 b | 2.83 ± 0.19 a | 2.40 ± 0.15 b | 27.04 ± 1.67 a | 0.98 ± 0.06 a |
| C8 | 29.17 ± 0.98 ab | 27.52 ± 1.03 b | n.d. | 0.49 ± 0.01 a | 0.63 ± 0.01 a | 1.04 ± 0.05 c | 2.64 ± 0.07 a | 2.27 ± 0.12 bc | 19.84 ± 0.75 b | 0.62 ± 0.03 b |
| C10 | 28.36 ± 1.24 ab | 17.31 ± 0.63 d | n.d. | 0.28 ± 0.01 c | 0.48 ± 0.01 b | 0.43 ± 0.03 d | 1.71 ± 0.05 b | 1.00 ± 0.06 d | 13.18 ± 0.46 c | 0.24 ± 0.01 d |
| VCO | 26.92 ± 1.00 b | 30.18 ± 1.34 b | n.d. | 0.09 ± 0.01 e | 0.31 ± 0.02 d | 2.53 ± 0.11 a | 1.48 ± 0.07 b | 4.93 ± 0.23 a | 19.88 ± 0.87 b | 0.96 ± 0.05 a |
| Ethanol | 28.87 ± 1.44 ab | 23.74 ± 1.14 c | n.d. | 0.13 ± 0.02 d | 0.34 ± 0.02 d | 0.89 ± 0.05 c | 1.50 ± 0.09 b | 1.92 ± 0.10 c | 18.49 ± 0.89 b | 0.48 ± 0.02 c |
| Effectiveness (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Co-Extractant | Isomangostin | Garcinone C | Garcinone D | 8-Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| C8/10 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| C8 | 0% | 138% | 156% | 71% | 94% | 94% | 73% | 63% |
| C10 | 0% | 79% | 119% | 29% | 60% | 42% | 49% | 25% |
| VCO | 0% | 26% | 76% | 171% | 52% | 205% | 74% | 99% |
| Ethanol | 0% | 38% | 83% | 60% | 53% | 80% | 68% | 49% |
| Selectivity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C8/C10–MP (%, w/w) | Isomangostin | Garcinone C | Garcinone D | 8- Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| C8/10 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| C8 | 0% | 147% | 166% | 75% | 99% | 100% | 77% | 67% |
| C10 | 0% | 86% | 130% | 32% | 66% | 45% | 52% | 27% |
| VCO | 0% | 29% | 88% | 198% | 60% | 237% | 84% | 114% |
| Ethanol | 0% | 41% | 90% | 64% | 57% | 86% | 72% | 52% |
| Effectiveness (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pressure (bar) | Temperature (°C) | Isomangostin | Garcinone C | Garcinone D | 8-Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| 250 | 60 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 350 | 60 | 101% | 94% | 140% | 75% | 110% | 97% | 128% | 74% |
| 450 | 60 | 113% | 111% | 126% | 86% | 125% | 106% | 128% | 95% |
| 250 | 70 | 4% | 48% | 102% | 174% | 199% | 96% | 114% | 136% |
| 350 | 70 | 156% | 147% | 198% | 91% | 167% | 114% | 149% | 101% |
| 450 | 70 | 130% | 113% | 134% | 93% | 134% | 106% | 143% | 105% |
| Selectivity (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pressure (bar) | Temperature (°C) | Isomangostin | Garcinone C | Garcinone D | 8-Deoxygartanin | γ-Mangostin | Gartanin | α-Mangostin | β-Mangostin |
| 250 | 60 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 350 | 60 | 91% | 85% | 127% | 68% | 100% | 88% | 117% | 68% |
| 450 | 60 | 91% | 90% | 101% | 70% | 100% | 85% | 103% | 76% |
| 250 | 70 | 4% | 42% | 89% | 152% | 175% | 84% | 99% | 119% |
| 350 | 70 | 121% | 114% | 154% | 71% | 130% | 88% | 117% | 78% |
| 450 | 70 | 103% | 89% | 106% | 73% | 105% | 83% | 114% | 83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Stanslas, J.; Ren, J.; Suleiman, N.b.; Chong, G.H. Green Co-Extractant-Assisted Supercritical CO2 Extraction of Xanthones from Mangosteen Pericarp Using Tricaprylin and Tricaprin Mixtures. Foods 2025, 14, 2983. https://doi.org/10.3390/foods14172983
Liu H, Stanslas J, Ren J, Suleiman Nb, Chong GH. Green Co-Extractant-Assisted Supercritical CO2 Extraction of Xanthones from Mangosteen Pericarp Using Tricaprylin and Tricaprin Mixtures. Foods. 2025; 14(17):2983. https://doi.org/10.3390/foods14172983
Chicago/Turabian StyleLiu, Hua, Johnson Stanslas, Jiaoyan Ren, Norhidayah binti Suleiman, and Gun Hean Chong. 2025. "Green Co-Extractant-Assisted Supercritical CO2 Extraction of Xanthones from Mangosteen Pericarp Using Tricaprylin and Tricaprin Mixtures" Foods 14, no. 17: 2983. https://doi.org/10.3390/foods14172983
APA StyleLiu, H., Stanslas, J., Ren, J., Suleiman, N. b., & Chong, G. H. (2025). Green Co-Extractant-Assisted Supercritical CO2 Extraction of Xanthones from Mangosteen Pericarp Using Tricaprylin and Tricaprin Mixtures. Foods, 14(17), 2983. https://doi.org/10.3390/foods14172983







